INTRODUCTION
One recurring theme within parasite ecology is the relative stability of parasite populations in domestic and wild animal hosts (Anderson and May, Reference Anderson and May1978; Tompkins and Hudson, Reference Tompkins and Hudson1999), which suggests that some form of regulatory mechanism must be ensuring population stability. The majority of these mechanisms are driven by parasite density, i.e. are a function of parasite burden within individual hosts; thus acting on infra-populations as opposed to populations as a whole. Indeed, density-dependent regulatory mechanisms act on many aspects of the parasite lifecycle, such as parasite establishment, growth, fecundity, development and maturation times, and adult survival (Walker et al. Reference Walker, Hall, Anderson and Basanez2009). Growth and fecundity for instance, being the two most common aspects of the life cycle regulated by such mechanisms in helminth populations (Tompkins and Hudson, Reference Tompkins and Hudson1999), are particularly important at regulating the abundance of the ‘free-living’, infectious stages within the environment, and therefore determining the extent of future infections. These density-dependent mechanisms are important for regulating and stabilizing transmission dynamics, and therefore the parasite–host relationship.
Despite knowledge of the existence of such regulation, the mechanisms underlying density dependence are poorly understood, as it is difficult to disentangle host and parasite responses to increasing parasite challenge (Paterson and Viney, Reference Paterson and Viney2002). Host immune responses have been demonstrated to reduce establishment, survivability and fecundity of parasitic nematodes, and it is hypothesized that innate and adaptive immune responses, whose response to infection increases with increasing parasite density, are responsible for the manifestation of density dependence (Paterson and Viney, Reference Paterson and Viney2002). Similarly, intraspecific competition for space and resources once inside the host has also been implicated as a driver of density-dependent regulation. Indeed, Michael and Dunby (Reference Michael and Dunby1989), hypothesized that parasite-mediated competition was responsible for Trichuris muris establishment in the mouse, owing to the finite carrying-capacity of the caecum.
Syngamus trachea is a parasitic nematode occurring in a wide range of avian hosts (Lewis, Reference Lewis1928; Morgan and Clapham, Reference Morgan and Clapham1934; Goble and Kutz, Reference Goble and Kutz1945). The non-specific nature of this parasite makes it possible to study differences in host-mediated responses to a natural challenge of S. trachea. In a previous paper by Gethings et al. (Reference Gethings, Sage and Leather2015), we highlighted the fact that despite increased opportunities for transmission of S. trachea within pheasant release pens, relatively low numbers of adult worms are consistently recovered upon post-mortem investigation. This is often the case in experimental infections using large numbers of infective larvae (Olivier, Reference Olivier1944; Guilford and Herrick, Reference Guilford and Herrick1954). Despite these previous studies finding relationships between S. trachea establishment and host immunity, no such work has been conducted in semi-naturally occurring pheasant populations using natural infections of S. trachea. The aims of the present study were to determine firstly, whether worm length is a good indicator of fecundity within S. trachea populations, and secondly, to determine whether fecundity is impaired in response to increasing worm burden in two host species.
MATERIALS AND METHOD
Study sites
The two study sites were selected due to their large size and annual occurrence of clinical Syngamosis. Site 1 was located approximately at grid reference ST 97502 39837 and consisted of seven release pens. Site 2 was situated approximately at grid reference SU 17769 30326 and similarly consisted of seven release pens. Site 2 undertook rigorous corvid control as part of a game management programme with the use of Larsen traps, whereas site 1 used traps sporadically.
Carcass recovery
Male and female ring-necked pheasants (Phasianus colchicus) were recovered from two pheasant estates in the South West of England from January to November 2015. All birds were either obtained during the shooting season or were found dead on the estates at various times of the year. Crows (Corvus carone) were opportunistically sampled throughout the season, as the sites were undertaking Corvid control via the use of Larsen traps. Crows and Rooks are known to be commonly infected with S. trachea, and any density-dependent effects would likely be more apparent as worm burdens tend to be larger than in pheasants. Crows were primarily recovered from site 2, as their corvid control programme was more consistent. Age was roughly estimated by the presence/absence and size of the bursa of fabricius, which has usually atrophied by 6 months (Williams and Newton, Reference Williams and Newton1969); however, no formal analysis of the effects of age on either parasite burdens or length was undertaken during this study.
Adult worm recovery
Adult S. trachea worms were recovered from the trachea of pheasants and crows. Adult worms are sexually dioecious, and show marked sexual dimorphism from 7 to 9 days post infection (dpi) with female worms being considerably larger than males; female length = ≳13 mm, male length = >4 mm at 14 dpi (Fernando et al. Reference Fernando, Stockdale and Remmler1971). The trachea was first resected from the underlying connective tissue and transected slightly above the proximal bifurcation of the bronchi. The trachea was then incised longitudinally through the tracheal cartilage and the worms recovered using fine-tipped forceps. Adult worms were distinguished from juvenile (L4) worms by observation under a microscope at varying magnifications in order to detect the presence of fertilized ova. Adult worms from both species were assessed according to Lewis (Reference Lewis1928), which confirmed that these worms were indeed S. trachea and that the worms were identical between species justifying the between-species comparisons.
Worm length and fecundity
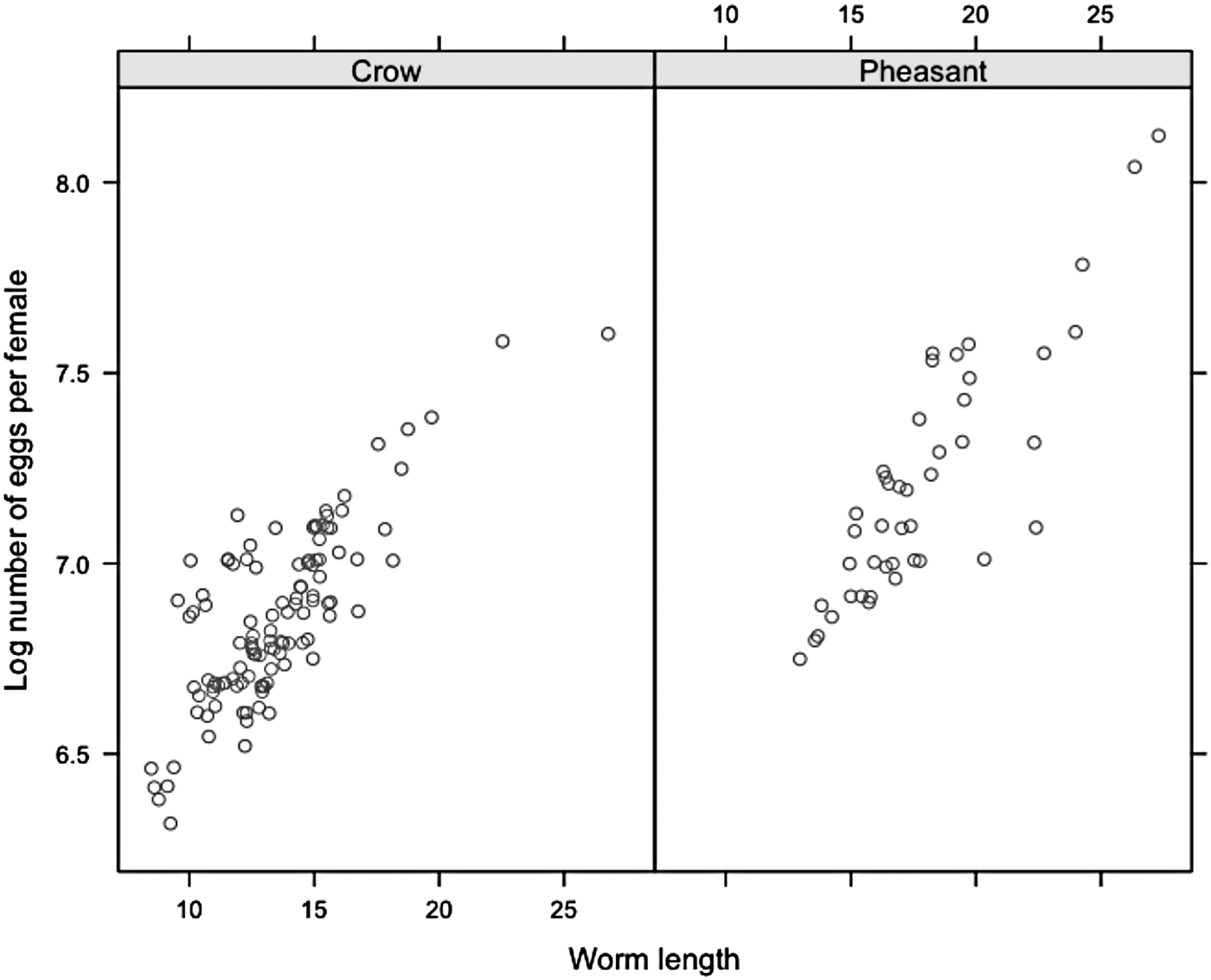
Fernando et al. (Reference Fernando, Stockdale and Remmler1971) conducted in-depth pathogenetic examinations detailing adult worm length at various stages of development, and determined the number of dpi to the production of fertilized ova. Female S. trachea worms are fertile by 14 dpi, with minimum female length at the adult stage generally averaging 10–15 mm. Once fertile, Guilford and Herrick (Reference Guilford and Herrick1954) found there is no relationship between dpi and female worm length, so we concluded that the number of dpi was not a significant confounding factor within this study. As several authors have demonstrated that worm length is significantly correlated with worm fecundity (Michael and Dunby, Reference Michael and Dunby1989; Stear et al. Reference Stear, Bairden, Duncan, Holmes, McKellar, Park, Strain, Murray, Bishop and Gettinby1997; Stear and Bishop, Reference Stear and Bishop1999; Tompkins and Hudson, Reference Tompkins and Hudson1999; Walker et al. Reference Walker, Hall, Anderson and Basanez2009), the same principle was applied in this study. A total of 157 female worms were recovered using systematic sampling from ten randomly selected pheasants and crows in order to estimate the effect of length on the number of eggs per worm. Although adult worms were recovered from 130 birds, in utero eggs were counted in female worms recovered from every seventh (130/20 = 6·5 rounded to 7) bird, providing it was infected. Female worm was measured using a digital calliper (accuracy to 0·01 mm) and the number of eggs was counted using a stereomicroscope. Genital tubes were liberated from female worms and each egg was counted in situ. To ensure egg viability, eggs were recovered from each worm and maintained in the laboratory at 24 °C (Wehr, Reference Wehr1937). Eggs were cultured to the infective stage (L3) and manually hatched by applying light pressure between two cover slips. Male worms were not measured during this study (Fig. 1).
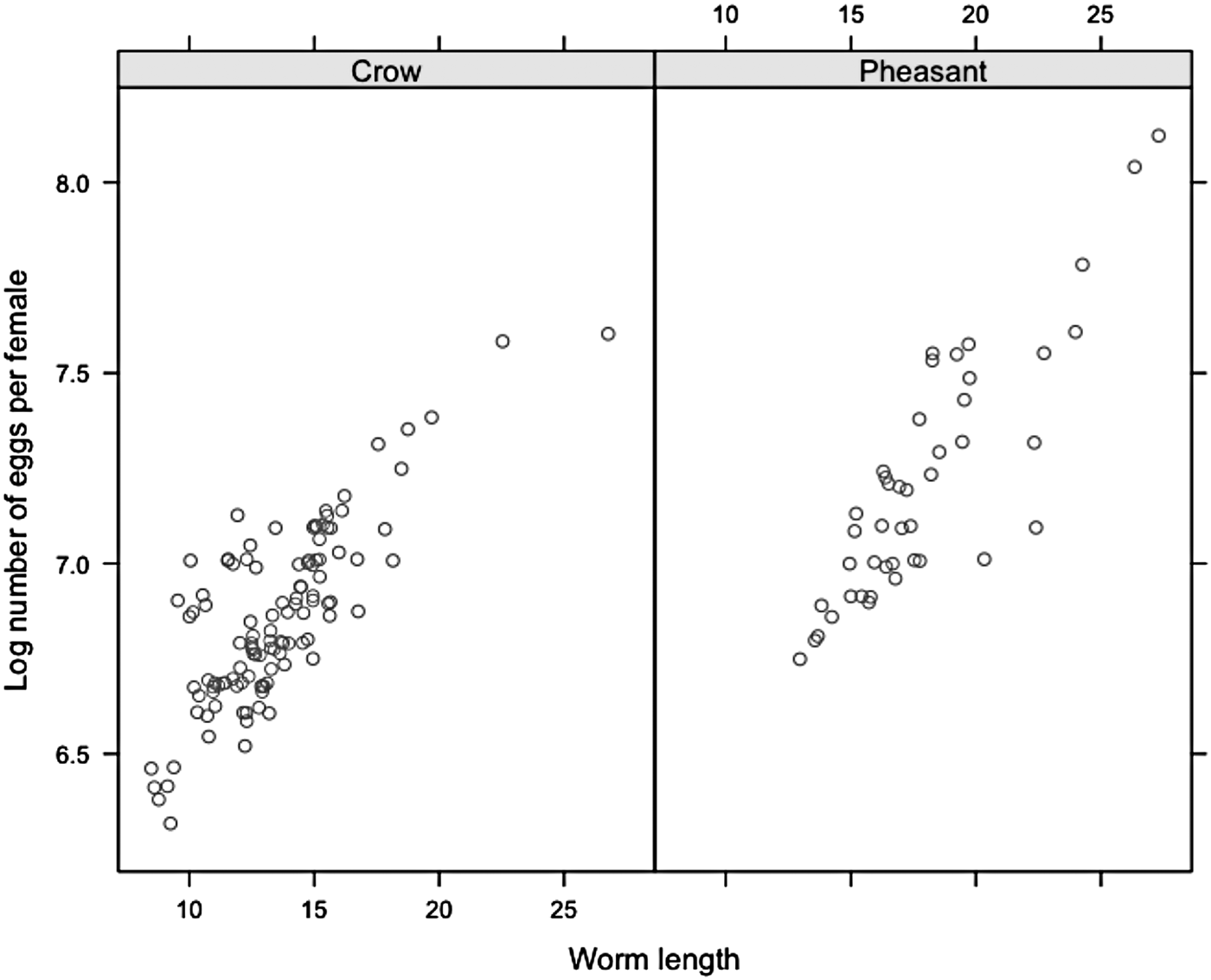
Fig. 1. Relationship between worm length and the number of eggs per worm (fecundity) for both species.
Condition of the trachea
It has been demonstrated that prolonged infections with S. trachea result in the formation of hyperplastic tracheal cartilage in which the adult male worms are deeply embedded (Clapham, Reference Clapham1935). These nodules begin to form between 26 and 37 dpi and generally remain indefinitely; meaning previous exposure and duration of current infection can be determined. These nodules form at the point of attachment, so if no worms are found within these nodules, it can be concluded that the bird has been exposed to S. trachea previously, thus conferring some degree of immunity. To assess whether previous exposure influenced mean worm length or mean worm burden in subsequent infections, pheasant tracheas were examined for the present of nodules. These nodules do not form at the point of attachment in corvids, so previous exposure cannot be determined. Therefore, crows were excluded from this part of analysis.
Retrospective data analysis
Guilford and Herrick (Reference Guilford and Herrick1954) experimentally infected pheasants with S. trachea larvae and counted the number of worms upon post-mortem examination, measured their length and noted the presence/absence of nodules. These data (n = 32) were combined with data recovered in this study (n = 21) to evaluate whether physiological evidence of previous exposure influenced parasite intensity and/or parasite length.
Statistical analysis
All data were analysed using the R statistical package for Macintosh. Differences in the mean number of worms and mean worm length between species were assessed using Welch's t-test for unequal samples. Unless stated otherwise, all regression analyses were conducted using generalized linear models with negative binomial error distributions and log link functions (glm.nb available in the MASS package). Data were assessed for negative-binomial model fit by comparing with models with Poisson error distributions by maximum likelihood using the pchisq function in R. Significance levels were calculated using χ 2 tests from the deviance explained by each factor and pseudo R 2 values were calculated for each model (1 – residual deviance/null deviance). The effect of parasite intensity on mean parasite length was analysed using log-transformed mean counts for parasite intensity in the 37 pheasants and 92 crows sampled (Tompkins and Hudson, Reference Tompkins and Hudson1999; Ives, Reference Ives2015). Non-constant error variance was assessed using the Breusch–Pagan test (B–P test) and ‘length’ data were transformed to the appropriate power transformation (ŷ−0·02). The B–P test was then conducted on the transformed data, which confirmed constant variance [χ 2 = 0·30, d.f. = 1, P = 0·57]. In order to ensure that worm length was a reliable indicator of fecundity, the effect of parasite length on the number of eggs per adult female was assessed using raw count data of 157 adult female worms recovered from ten pheasants and ten crows. In order to determine the minimum parasite density at which negative effects are observable, iterative backwards-stepwise deletion of the highest parasite densities was conducted until the regression was no longer significant at the P ⩽ 0·05 level.
RESULTS
The trachea of 37 pheasants and 92 crows were recovered and examined for the presence of adult S. trachea worms, of which 1314 pairs were recovered.
Parasite fecundity
Model comparisons suggested the negative binomial model best fit the data (χ 2 = 3281·27, d.f. = 1, P ⩽ 0·001) so data were analysed with negative binomial error distributions. In utero egg counts were performed on 157 pooled adult female worms recovered from ten crows (113 worms) and ten pheasants (44 worms), with an average (±s.e.m.) of 1066 (±41·5) eggs per worm. Worm length (coef = 0·06, deviance = 590·47, d.f. = 1, P ⩽ 0·001), after accounting for within-host variation in worm burden (coef = −0·05, deviance = 7·77, d.f. = 1, P = 0·006), was significantly correlated with the number of eggs per female worm; explaining 79% of the deviance (residual deviance = 157·05, d.f. = 154, P ⩽ 0·001).
Worm length and parasite intensity
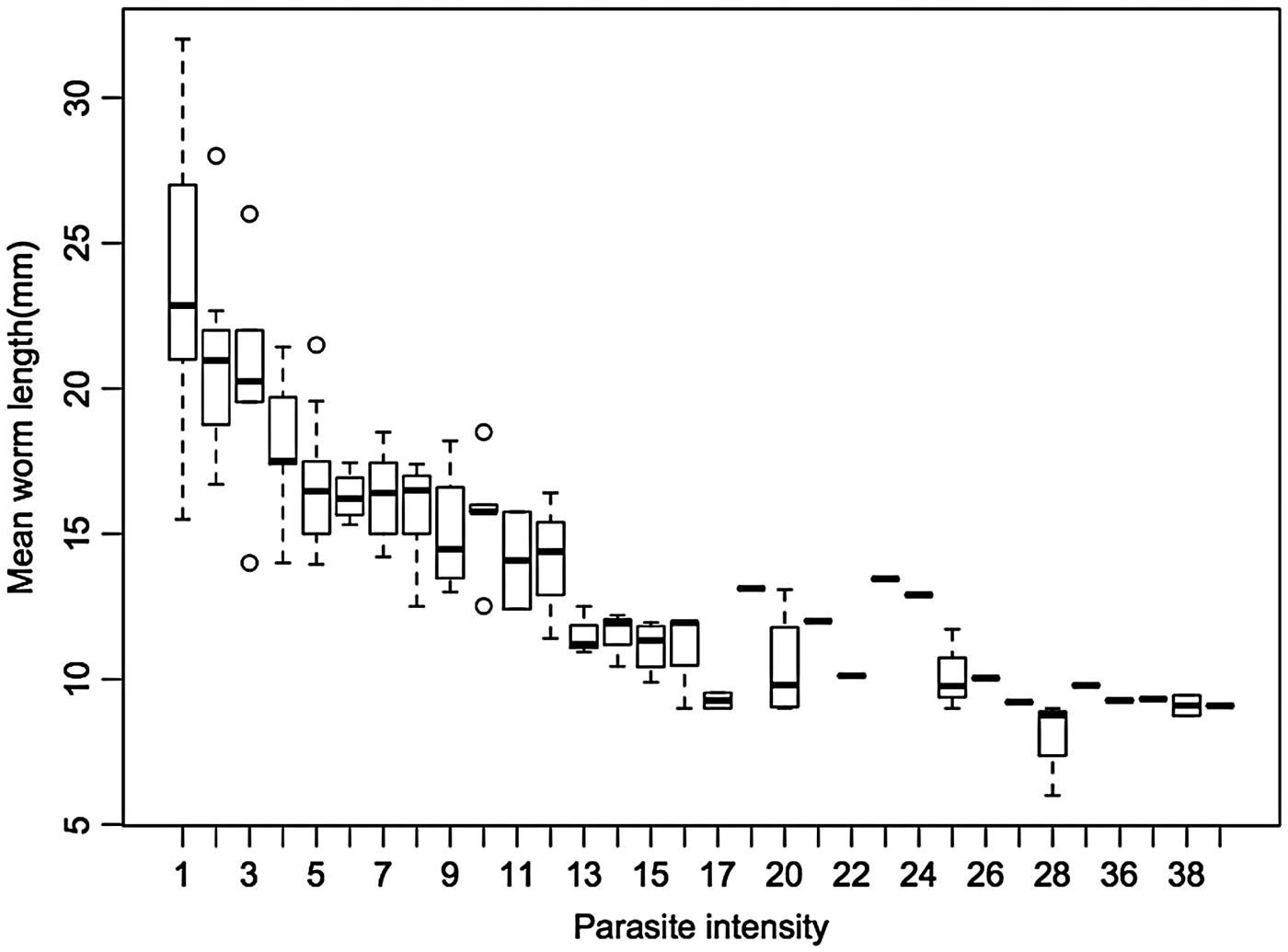
A total of 286 and 1028 adult S. trachea pairs were recovered from the trachea of 37 pheasants and 92 crows respectively. Mean worm length was significantly correlated with mean parasite density for pheasants and crows, with a significant reduction in mean worm length at higher parasite densities (F 1,127 = 393·3, R 2 = 0·759, P ⩽ 0·001) (Fig. 2). For individual species, there was a stronger effect of mean worm burden on mean worm length for crows (F 1,90 = 340·2, R 2 = 0·79, P ⩽ 0·001) than for pheasants (F 1,35 = 64·21, R 2 = 0·64, P ⩽ 0·001). Stepwise data-point deletion of the highest parasite densities revealed that density-dependent effects begin to manifest above four worms per bird for pheasants, and two worms per bird for crows, with the regression model not reaching the significance level of P < 0·05 below these thresholds.
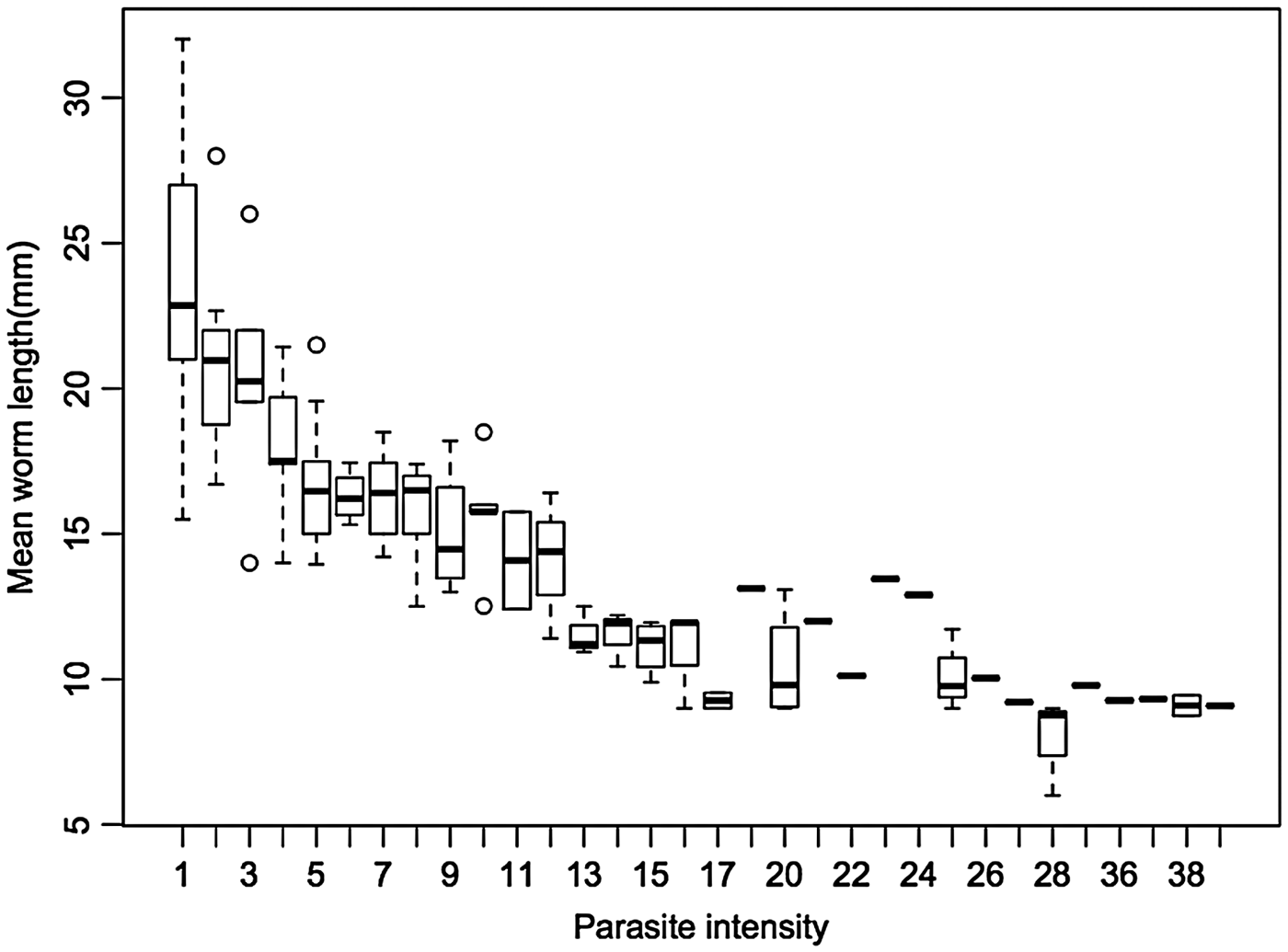
Fig. 2. Mean worm length (mm) for varying parasite intensities (n = 1314 worms across 129 hosts).
Presence of nodules, mean worm length and number of adult worms
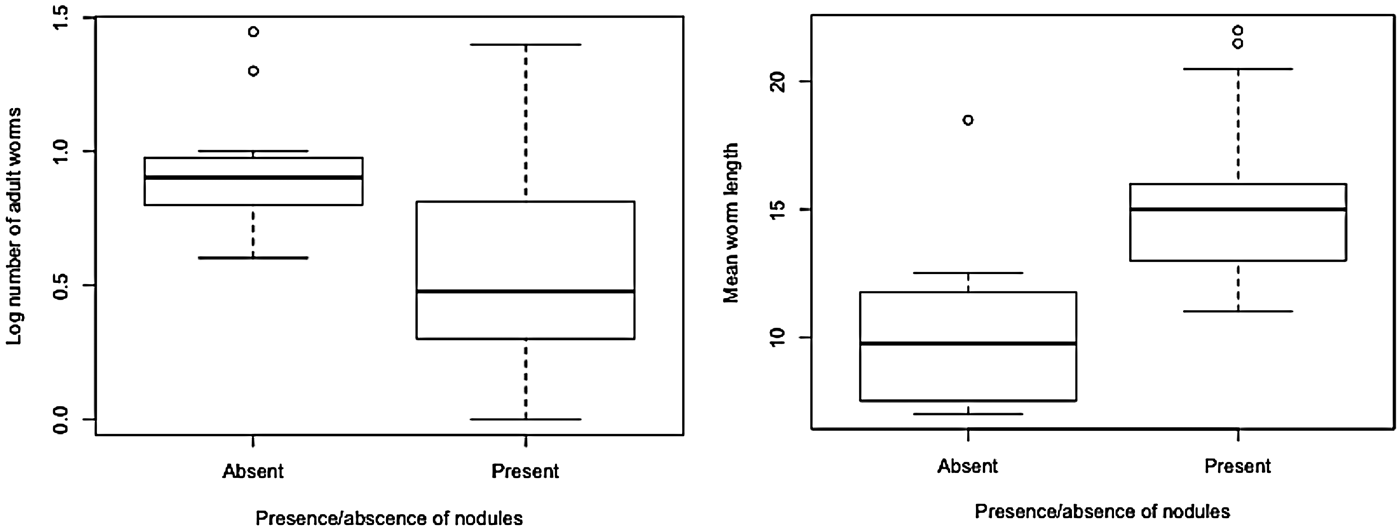
Crows were excluded from this part of analysis, so the results are not reported. Retrospective analysis of the Guilford and Herrick (Reference Guilford and Herrick1954) data, and trachea condition in the present study revealed that pheasants with hyperplastic tracheal nodules had fewer adult worms present in the trachea (mean ± s.e.m. = 5·26 ± 1·01 worms per bird) than birds without nodules (mean ± s.e.m. = 11·12 ± 1·68 worms per bird) (n = 51, t 46 = 2·97, P = 0·004). Female worms in birds with the evidence of previous exposure were also longer (mean ± s.e.m. = 16·5 mm ± 1·51) when compared with worms in birds that had no evidence of previous exposure (mean ± s.e.m. = 13·01 mm ± 0·53) (n = 51, t 21·3 = −2·10, P = 0·04) (Fig. 3).

Fig. 3. Relationship between the presence of nodules and mean worm burden and mean worm length in pheasants.
Mean worm length and mean worm burden between species
The mean number of adult worms per trachea differed significantly between species, with crows having a mean worm burden of 11·17 (±s.e.m. = 0·10) and pheasants having an average of 7·72 (±s.e.m. = 1·39) worms per trachea (t 72·14 = 2·02, P = 0·04). Similarly, mean worm length differed significantly between species, with pheasants having a mean worm length of 17·97 mm (±s.e.m. = 0·85) and crows having a mean worm length of 15·55 mm (±s.e.m. = 0·55) (t 66·58 = 2·34, P = 0·02).
DISCUSSION
Density-dependent reductions in worm size and fecundity have been reported in a large number of studies (Michael and Dunby, Reference Michael and Dunby1989; Stear et al. Reference Stear, Bairden, Duncan, Holmes, McKellar, Park, Strain, Murray, Bishop and Gettinby1997; Stear and Bishop, Reference Stear and Bishop1999; Tompkins and Hudson, Reference Tompkins and Hudson1999; Walker et al. Reference Walker, Hall, Anderson and Basanez2009), and density-dependent reductions in worm length, but not necessarily fecundity, are reported in the vast majority of nematode species studied (Mossinger and Wenk, Reference Mossinger and Wenk1986; Szalai and Dick, Reference Szalai and Dick1989; Sinniah and Subramaniam, Reference Sinniah and Subramaniam1991; Skorping et al. Reference Skorping, Read and Keymer1991; Marcogliese, Reference Marcogliese1997; Irvine et al. Reference Irvine, Stien, Dallas, Halvorsen, Langvatn and Albon2001; Richards and Lewis, Reference Richards and Lewis2001; Dezfuli et al. Reference Dezfuli, Volponi, Beltrami and Poulin2002). A vast majority of studies concerning density dependence have been laboratory-based experimental infections, which do not accurately represent conditions facing free-living wild animal populations in terms of parasite load and encounter rates. The present study provides reliable information concerning apparent density-dependent regulation of fecundity in both an intensively-managed pheasant population, and a free-living wild population of corvids. Although the fact that immune status is responsible for regulating the establishment of S. trachea in ring-necked pheasants is not novel, this is first mention of both parasite and host-mediated factors regulating S. trachea populations in any bird species.
In agreement with previous studies (Olivier, Reference Olivier1944; Guilford and Herrick, Reference Guilford and Herrick1954), immune function appears to be predominantly responsible for the establishment of S. trachea within the ring-necked pheasant; however, other factors such as exposure rates and larval viability were not taken into account in this study. This is demonstrated by a reduction in parasite abundance in birds that had evidence of previous exposure. There were, however, a greater number of adult worms in crows, which suggests that worm establishment is not constrained by size or length of the trachea, and therefore overall host size, however, is perhaps a function of host immunity. Indeed, Olivier (Reference Olivier1944) found that S. trachea establishment was dose dependent. He found that the number of worms establishing was inversely proportional to the size of the infective dose, and attributed this to the strength of the immune response (Olivier, Reference Olivier1944). This result is in stark contrast to the findings of Michael and Dunby (Reference Michael and Dunby1989), who found that T. muris establishment in the murine host is believed to be regulated by density-dependent infraspecific competition, owing to the finite space in the caecum. It is unlikely however that S. trachea establishment is regulated in a similar manner as more worms have been found in crows with a shorter trachea. This apparent immune-mediated inhibition on worm establishment has also been identified for S. trachea in chickens, with a lower mean worm burden generally identified in older, previously exposed chickens (Crawford, Reference Crawford1940). If establishment was merely a result of parasite-mediated competition, worm establishment, and therefore burden, would be similar in both immunological naïve and previously exposed birds (Luong et al. Reference Luong, Vigliotti and Hudson2011).
One reason to explain the trend for higher worm abundance in crows is acquired-immunity. Pheasants are known to develop moderate immunity to S. trachea; however, no such work has been conducted in wild crow populations. Being a known reservoir for S. trachea, it may be that crows have a higher parasite threshold for the stimulation of an immune response or they do not develop significant immunity to subsequent infections. Indeed, pheasants appear to be more susceptible to infection early on in the rearing process, whereas S. trachea adults have been recovered from crows of varying ages (personal unpublished data). Further work is however required in order to determine whether wild crows develop any immunity to S. trachea.
Although density-dependent reduction of worm fecundity was present in both species, the fact that the effect of crowding on mean worm length was more profound within the crow population is interesting. Mean worm burden explained 82% of the variation in mean worm length in crows, compared with 64% in pheasants, with crows having a tendency for a greater mean worm burden when compared with pheasants. Even so, the fact that worms tended to be shorter in crows, in response to higher mean worm burdens, suggests that these effects are indeed density-dependent. The negative association between worm length and worm burden was present in both species, and appears to be a result of parasite-mediated competition, for either space or resources. Indeed, these effects were even observed in pheasants with no history of previous exposure. Similarly, as there were a vast number of birds of different ages, it is unlikely that age-dependent acquired-immunity was responsible for the manifestation of density dependence within these birds, as the effects were identified in juveniles, as well as adult birds, with little to no acquired immunity. Conversely, Paterson and Viney (Reference Paterson and Viney2002), observed the absence of density-dependent mechanisms at regulating survivability and fecundity of Strongyloides ratti infra-populations in immuno-compromised hosts. These mechanisms were however, present in later primary infections, suggesting that host-mediated heterogeneities in immuno-competence are regulating population dynamics before intraspecific competition for space and nutrients ever occurs in experimentally infected rats (Paterson and Viney, Reference Paterson and Viney2002). Alternatively, worm length has been shown to be related to levels of local parasite-specific immunoglobulin A (Stear et al. Reference Stear, Bairden, Duncan, Holmes, McKellar, Park, Strain, Murray, Bishop and Gettinby1997). These responses are however, often absent in immunologically naïve animals, and only generally manifest in animals that have been previously exposed (Craig et al. Reference Craig, Smith, Pate, Morrison and Knight2014) so it is unlikely to be occurring within these study populations.
The parasite threshold for the manifestation of density dependence within this study was low compared with other studies. For instance, the threshold for density-dependent reductions in fecundity in the caecal nematode, Heterakis gallinarum, in pheasants is 96 worms (Tompkins and Hudson, Reference Tompkins and Hudson1999). Similarly, this threshold for Tricostrongylus colubriformis in sheep is around 3000 worms per host (Dobson et al. Reference Dobson, Waller and Donald1990). It is generally believed that density-dependent effects are of greater importance for parasites that are large compared with their host (Poulin and Morand, Reference Poulin and Morand2000). Indeed, S. trachea adults can grow up to ~33 mm in length in an 80–100 mm long trachea (Crow). In comparison, mean worm length of H. gallinarum adults in the caecae of pheasants is about 9·64 mm (±0·11) (Tompkins and Hudson, Reference Tompkins and Hudson1999), in caecae ranging from 240·11 for male and 213·84 for female pheasants, respectively. Similarly, Pterygodermatites peromysci, a nematode parasite of mice, is regulated by tight density-dependent restrictions on the number and length of adult worms in the small intestine (Luong et al. Reference Luong, Vigliotti and Hudson2011). Similarly to S. trachea, P. peromysci can grow up to 33 mm in a 250 mm mouse intestine (Luong et al. Reference Luong, Vigliotti and Hudson2011).
The findings of the present study are in agreement with previous work that pheasants do indeed develop immunity to S. trachea (Olivier, Reference Olivier1944; Guilford and Herrick, Reference Guilford and Herrick1954). However, nematode length and fecundity appear to be a function of parasite density, and therefore parasite-mediated competition and not host-mediated heterogeneities in immunocompetence.
ACKNOWLEDGEMENTS
The authors would like to express their thanks to the estate owners and the Gamekeepers for use of the sites and the Game and Wildlife Conservation Trust for use of facilities. Special thanks to Dr Eric Morgan, of the University of Bristol, and to an anonymous referee for assistance with statistical analyses.
FINANCIAL SUPPORT
This study is part of an on-going Ph.D. project and is funded by the BBSRC [Grant code – BB/K012770/1 (O. J. G)].