1. Introduction
1.1. Non-addictive drug use
The use of drugs is a widespread phenomenon in many societies of the world, even though there are cultural differences influencing the kinds of drugs used and the ways in which drugs are taken (Heath Reference Heath2000; Kuntsche et al. Reference Kuntsche, Knibbe, Gmel and Engels2006). The U.S. National Survey on Drug Use and Health (NSDUH 2007) revealed that approximately 19.9 million Americans (8% of the population) aged 12 or older consumed at least one illicit drug such as marijuana/hashish, cocaine, heroin, hallucinogens, solvents, or prescription-type psychotherapeutics. More than 50% of Americans aged 12 or older reported they were current drinkers of alcohol, and more than 28.6% of Americans aged 12 or older used tobacco products. European surveys revealed that in the general population of the 15-to-64-year-olds, about 324 million people (84%) drank alcohol daily. A recent survey (EMCDDA 2009) estimating illicit drug use by response to a question about “last month's use” determined current cannabis users at 12.5 million people (3.7%) and cocaine users at 2.0 million people (0.5%).
It has been established beyond any doubt that drug addiction is a major psychiatric disorder that causes harm to the individual, to the social environment, and to society (American Psychiatric Association 1994). Much research is devoted to understanding and curing drug addiction. Epidemiological data show, however, that the majority of people who consume psychoactive drugs with an addiction potential are not addicts and will never become addicted (Glynn et al. Reference Glynn, LoCastro, Hermos and Bosse1983; O'Malley & Johnston Reference O'Malley and Johnston2002; Zinberg & Jacobson Reference Zinberg and Jacobson1976; Zinberg et al. Reference Zinberg, Harding, Stelmack and Marblestone1978). Of those people who are classified as current alcohol drinkers in the United States, 14.9% are diagnosed as addicts based on the SAMHSA (2005) report. Among the 20.4 million current users (use in the previous month) of illicit drugs in the United States – which include marijuana, cocaine, heroin, hallucinogens, solvents, and prescription-type drugs – 34.3% have been estimated to be addicts (SAMHSA 2005). European Union estimates are similar. In the European Union, about 7.1% of the daily drinkers of alcohol are alcohol dependent (Anderson & Baumberg Reference Anderson and Baumberg2006), whereas about 32% of the current cannabis consumers show a problematic consumption (EMCDDA 2009). In a U.S. National Comorbidity Survey, the cumulative risk until the age of 54 to fulfill criteria for dependence to marijuana is 10%; for cocaine, up to 21% (until age 40); and for alcohol, about 20% (Chen & Anthony Reference Chen and Anthony2004; Wagner & Anthony Reference Wagner and Anthony2002). From surveys of this kind, it is clear that the majority of psychoactive drug users are not and will never be drug addicts (Heyman Reference Heyman1996). Although drug addiction is an undeniable, major burden to society, to a considerable degree, use of psychoactive drugs is unrelated to addiction.
1.2. Psychoactive drug use
In this article, we regard a drug (a single chemical compound with unique structure) as psychoactive when it (a) interacts with the function of the central nervous system (CNS) and (b) changes subjective experience, behavior, or both. Whereas considerable research effort has been made to understand drug addiction and how it develops (Hill & Newlin Reference Hill and Newlin2002; West Reference West2006), an adaptive role or beneficial effect for psychoactive drugs is often categorically denied (e.g., Sullivan et al. Reference Sullivan, Hagen and Hammerstein2008). Without an account of non-addictive drug use, conceptualizing the transition between non-addictive to addicted drug use is difficult. In this article, we suggest that people use psychoactive drugs not because their reward systems have been “hijacked,” but to advance specific behaviors relevant for their own “fitness.”
2. Widening the explanatory scope for drug use
The consumption of psychoactive drugs is usually considered a maladaptation, particularly in people with genetic or environmental risks that make them prone to addiction (e.g., Campbell et al. Reference Campbell, Szumlinski and Kippin2009; Schumann Reference Schumann2007; Schumann et al. Reference Schumann, Rujescu, Kissling, Soyka, Dahmen, Preuss, Wieman, Depner, Wellek, Lascorz, Bondy, Giegling, Anghelescu, Cowen, Poustka, Spanagel, Mann, Henn and Szegedi2003; Reference Schumann, Johann, Frank, Preuss, Dahmen, Laucht, Rietschel, Rujescu, Lourdusamy, Clarke, Krause, Dyer, Depner, Wellek, Treutlein, Szegedi, Giegling, Cichon, Blomeyer, Heinz, Heath, Lathrop, Wodarz, Soyka, Spanagel and Mann2008). Many drugs that humans consume are plant toxins, such as nicotine, cocaine, or cannabis, which serve as plant defenses and prevent plant consumption. The widely accepted evolutionary adaptation of these toxins for plants is to deter herbivores (e.g., Nathanson et al. Reference Nathanson, Hunnicutt, Kantham and Scavone1993). Why certain plants develop substances that reinforce plant consumption and why any organism should have a mechanism that reinforces the toxin consumption are therefore a puzzle. This apparent evolutionary contradiction has been termed the “paradox of drug reward” (Hagen et al. Reference Hagen, Sullivan, Schmidt, Morris, Kempter and Hammerstein2009; Sullivan & Hagen Reference Sullivan and Hagen2002; Sullivan et al. Reference Sullivan, Hagen and Hammerstein2008).
Dosage is one aspect of the resolution of this paradox. Drugs like cocaine induce euphoria only at low to medium doses. At higher doses, cocaine induces highly aversive paranoia and behavioral stereotypes (Gawin Reference Gawin1991; Gawin & Kleber Reference Gawin and Kleber1986; Kramer et al. Reference Kramer, Fischman and Littlefield1967). Drugs with a low euphoria component, such as nicotine or caffeine, are voluntarily consumed, usually at low, non-toxic doses (Cauli & Morelli Reference Cauli and Morelli2005). It is important to realize that the doses in which humans and animals voluntarily consume psychoactive drugs are usually below the acute toxic range (Gable Reference Gable2004; Hagen et al. Reference Hagen, Sullivan, Schmidt, Morris, Kempter and Hammerstein2009). As such, the general “paradox of drug reward” may be resolved at the dose-response level: In a low- to medium-dose range, the drug effect is not toxic in the sense of being an immediate threat to life. In the range of medium to low doses, therefore, a role for drugs in functional adaptation can reasonably be considered (Chisholm Reference Chisholm1999).
A number of alternative views seek to explain the development and persistence of psychoactive drug consumption. The behavior may, hence, be based on (1) a fitness-irrelevant hijacking of generic motivational systems, (2) a fitness-irrelevant but subjectively perceived improvement in a variety of fitness-relevant motivational states specific to important goals, or (3) an actual improvement in success in these same motivational states.
-
1. Nesse and Berridge (Reference Nesse and Berridge1997) have argued that those psychoactive drugs that induce positive emotions might provide at the same time a false fitness benefit signal, which in turn “hijacks” incentive salience mechanisms, such as “wanting” and “liking” in the brain. For positive as well as negative emotions, a clear fitness benefit can be identified, in that these emotions can be conceptualized as “specialized states shaped to cope with situations that involve the opportunities or gains and a great number of different kinds of situations that involve threats or losses” (Nesse & Berridge Reference Nesse and Berridge1997). This evolutionary approach expanded the reinforcement-centered explanations for drug taking by an emotional perspective in that drugs are consumed to change emotions in a broad sense, not just for relatively narrow euphoria/hedonia. Although this can be done safely in some circumstances, it might leave the organism with less fitness when the natural function of emotion is constantly circumvented or “hijacked” (Nesse & Berridge Reference Nesse and Berridge1997; Panksepp et al. Reference Panksepp, Knutson and Burgdorf2002).
-
2. Newlin (Reference Newlin2002) suggested that drugs are taken to “inflate” the “self-perceived survival ability and reproduction fitness” (SPFit). SPFit is a concept for the mammalian motivation to enhance and protect survival and reproductive fitness, much related to the feelings of personal power and sexual attractiveness. Indeed, it could be shown that common drugs like alcohol, if taken in moderate amounts, increase the subjective perception of power in humans (Wilsnack Reference Wilsnack1974). Although it was argued that drugs enhance this subjective feeling, Newlin (Reference Newlin2002) denies a potential evolutionary benefit.
-
3. Some authors have at least raised the possibility of actual short- (Lende & Smith Reference Lende and Smith2002) and long-term beneficial effects of drug use (Hagen et al. Reference Hagen, Sullivan, Schmidt, Morris, Kempter and Hammerstein2009). Viewing drug use as an evolutionary adaptation, Lende and Smith (Reference Lende and Smith2002) argued that the adaptive function of drug use is to provide an individual with a predictable short-term pleasure in an unsafe environment, where the pursuit of natural reinforcers can only be poorly established. Drug addiction is then seen as a maladaptation based on a missing built-in regulatory function in the salience signaling mesolimbic dopamine (DA) system (Lende & Smith Reference Lende and Smith2002). Lende and colleagues (2007) analyzed the behavioral function of methamphetamine in a population of heavy users in Atlanta, Georgia (United States). They interviewed users for their perceived functions of use and identified three main categories: (1) enhanced function, (2) increased productivity, and (3) functioning normally. This study revealed an important detail: methamphetamine users did not perceive their drug use to impair their daily functioning, but rather to enhance it (Lende et al. Reference Lende, Leonard, Sterk and Elifson2007).
Several authors have acknowledged the subjectively perceived psychological benefits of drug consumption (Baum-Baicker Reference Baum-Baicker1985; Chick Reference Chick1999; Peele & Brodsky Reference Peele and Brodsky2000). These subjectively reported and objectively measured benefits are related to a moderate and non-compulsive– that is, non-addictive – consumption of alcohol and comprise fields such as subjective health, mood enhancement, stress reduction, sociability, mental health, long-term cognitive functioning, and work performance (Chick Reference Chick1999; Molnar et al. Reference Molnar, Busseri, Perrier and Sadava2009; Peele & Brodsky Reference Peele and Brodsky2000). In addition, there is evidence for beneficial effects of methamphetamine use on everyday function (Lende Reference Lende, Wenda Trevathan, Smith and McKenna2007; Lende et al. Reference Lende, Leonard, Sterk and Elifson2007).
Overall, several researchers have recognized subjectively reported beneficial effects of certain consumption patterns and have reviewed in much detail the evidence for it. However, a systematic analysis of drug taking as either a functional adaptation or, alternatively, as a beneficial effect of current adaptations is still in its infancy. As such, a general principle for non-addictive psychoactive drug consumption has yet to emerge. The presented functional analysis of non-addictive psychoactive drug consumption suggests that psychoactive drug use does indeed result in an improvement of fitness-relevant behavior. It also suggests that humans are able to subjectively perceive or cognitively reflect – or both – not only the improved outcome of behavior, but also the rather systematic use of the behavior “psychoactive drug consumption.”
3. Why do human beings consume psychoactive drugs? A drug instrumentalization framework
We propose that the large majority of non-addicted humans who consume psychoactive drugs as a normal part of their lives, take drugs because the drugs' effects are useful for their personal goals. Psychoactive drugs can be instrumentalized. We refer to drug instrumentalization as a two-step behavioral process: (1) the seeking and consumption of a psychoactive drug in order to change the present mental state into a previously learned mental state, which then allows for (2) better performance of other, previously established behaviors and better goal achievement.
An instrument may be defined as something that helps to achieve a goal that would not be achievable or which would require a higher workload without the use of the instrument. As such, behavior itself can be an elaborate instrument (Frolov & Pavlova Reference Frolov and Pavlova2003; Skinner Reference Skinner1938). A goal is referred to here as the outcome of an already established behavior. If a behavioral goal is, for example, to socialize and to maintain a social network, instrumental behaviors would be seeking a place where other people are to be found and starting social interaction with them.
How can a psychoactive drug be considered an “instrument”? The instrument is, in this case, the effect of the drug on the organism's mental state. The nervous system of human beings and other vertebrates displays different modes of action, which can be referred to as mental states (also termed internal or affective states). Mental states are the brain's working modes that are held stable over longer periods of time (minutes to hours) during which they provide the functional setting for fast computational processes in the millisecond-to-minutes range. Mental states govern an organism's subjective perception, memory retrieval, and autonomic and behavioral responses (White Reference White1996). It is suggested that the brain's mental states are determined by the different functional states of the modulatory transmitter systems, such as the dopaminergic, serotonergic (5-HT), acetylcholinergic (ACh), noradrenergic (NA), and various neuropeptidergic systems, which control the information processing in diencephalic and telencephalic target regions of the brain (Castren Reference Castren2005). These systems display different modes of basal activity depending on various external factors, such as time of day, season, or environment, as well as on various internal factors, such as glucose, oxygen, or hormone levels in the blood (e.g., Aston-Jones et al. Reference Aston-Jones, Rajkowski and Cohen1999; Jacobs & Fornal Reference Jacobs, Fornal, Müller and Jacobs2010; Sarter & Bruno Reference Sarter and Bruno1997; Schultz Reference Schultz2000; Steriade et al. Reference Steriade, Datta, Pare, Oakson and Curro Dossi1990). Under different tonic activity modes, environmental stimuli can elicit very different phasic responses. Tonic, as well as stimulation-dependent phasic, responses determine stimulus processing and behavioral responses generated by the brain.
These mental states predispose an organism's responses to the options that its environment offers. These responses, in turn, determine an organism's success in performing previously established instrumental behaviors and, hence, how effectively the organism can reach its goals. As such, an organism's mental state essentially determines if a previously established behavior will be performed to reach a certain goal. Furthermore, if an organism pursues a goal, there is a particular mental state that allows the organism to most effectively perform the behavior with respect to the outcome. For example, if the goal is to get from place A to B by the behavior “driving a car,” an organism can perform this action best in an attentive mental state and less well in a tired and distracted state.
By definition, all psychoactive drugs change an organism's mental state (e.g., Fischman & Schuster Reference Fischman and Schuster1982; Post et al. Reference Post, Kotin and Goodwin1974). However, this would be a trivial explanation for drug-taking behavior and does not acknowledge the full extent of the behavioral complex involved in non-addictive drug consumption. Here, we argue that the set of the organism, the surrounding settings, and the subsequent behaviors that follow the change in mental state are pivotal (Zinberg Reference Zinberg1984) for a full appreciation of drug seeking and consumption, and the resulting mental state change. On the one hand, drug consumption arises in a particular environment and in particular mental states. Drug-unrelated behaviors are performed, however, when the drug is on board and the drug-induced change in mental state is in full swing. These behaviors can be viewed as drug-independent, in that they were established independently from drug use and could be performed without anteceding drug use and mental state change. For example, most adults can drive a car from A to B undrugged. After a long working day, however, having a last coffee and a subsequently “refreshed” and attentive mind may enable the driver to better drive home. In this example, the effects of caffeine on the mental state are the instrument. The A process of psychoactive drug instrumentalization would be the “coffee preparing and drinking,” whereas the B process would be “driving the car.” The individual instrumentalization goal would be “driving home,” which might belong to the goal class of “improving cognitive performance and counteracting fatigue.” A superior goal achievement would outweigh the additional effort of seeking and consuming a psychoactive drug before performing the behavior, for example, an instrumental behavior (Heyman Reference Heyman1996).
4. Psychoactive drug use from an evolutionary perspective
In an evolutionary approach to non-addictive psychoactive drug consumption, we discuss the evidence for “drug instrumentalization” at four different levels of behavioral analysis: (1) its evolutionary history, (2) in its adaptive function for reproduction and survival, (3) the proximate causation of the behavior, and (4) the ontogeny of the behavior – that is, its development in the life history of a single individual (Hill & Newlin Reference Hill and Newlin2002; Nesse Reference Nesse2002; Tinbergen Reference Tinbergen1963). A distinct behavior, to be acknowledged as a true adaptation, needs to solve an adaptational problem that would not be solved by chance without specific selection pressure (Miller Reference Miller2000). Is there any adaptational problem to which drug consumption could reasonably offer a solution? We suggest that the adaptational problem is the occurrence of multiple distinct microenvironments for single individuals who must make fast transitions among those microenvironments (Bronfenbrenner Reference Bronfenbrenner1994).
Microadaptations as specific adaptations to each microenvironment may be supported best by behaviors that are under opposing selection pressures (Cosmides & Tooby Reference Cosmides, Tooby, Hirschfeld and Gelman1994; Crawford Reference Crawford2000). Static behavioral traits that are constant over a developmental period, or even over the whole life span, may appear as less advantageous than a mechanism that allows flexibly adjusting behavioral traits according to each microenvironment (Cosmides & Tooby Reference Cosmides, Tooby, Hirschfeld and Gelman1994; Tooby & Cosmides Reference Tooby, Cosmides, Barkow, Cosmides and Tooby1992). An example for this may be “social disinhibition” as a behavioral trait in humans. Although certainly appropriate and rewarded in a close social setting, it is inappropriate and even punished in a professional work environment. An adaptation to flexibly shift between enhanced and suppressed “social disinhibition” when changing microenvironments may provide an optimal net adaptation.
4.1. The ultimate cause of psychoactive drug use
Evolutionary psychologists suggest that many of our current behaviors can be viewed as adaptations to our ancestral environment. Furthermore, these historical adaptations should have solved a problem ultimately enhancing lifetime reproduction of self or kin (Cosmides & Tooby Reference Cosmides, Tooby, Hirschfeld and Gelman1994; Reference Cosmides and Tooby1999). The consumption of psychoactive plants has occurred for at least 10,000 years, according to oldest human records (Abel 1980; Dudley Reference Dudley2002; Heath Reference Heath2000; Seefelder Reference Seefelder1996; Streatfeild Reference Streatfeild2001). One origin of psychoactive drug instrumentalization may be found in the selective acquisition and preparation of food. The selective consumption of psychoactive compounds may be based on selective food seeking and consumption behavior and its flexible modification by psychological learning processes (Dudley Reference Dudley2000; Lozano Reference Lozano1998). Seeking and consumption of a particular type of food can be very specific depending on nutritional needs. Phylogenetically old learning mechanisms associate sensory parameters such as taste and visual cues of foods with their ingredients and physiological effects. The lack of a particular nutrient can trigger a focused search and consummatory behavior for a particular type of food. Based on whether the consumption maintained or failed to maintain homeostasis, this particular food will either be searched out and consumed in the future or avoided (Johnson et al. Reference Johnson, Beaton and Hall1975, Lozano Reference Lozano1998; Rozin & Kalat Reference Rozin and Kalat1971).
Ethological research in chimpanzees has shown that the choice of food may be guided not only by the nutrient content, but also by non-nutritional properties of plant compounds, in particular secondary plant metabolites (Robles et al. Reference Robles, Aregullin, West and Rodriguez1995). Wild chimpanzees selectively consume plants to self-medicate for infections, gastrointestinal problems, and other physically stressful conditions (Glander Reference Glander and Etkin1994; Page et al. Reference Page, Balza, Nishida and Towers1992; Rodriguez et al. Reference Rodriguez, Aregullin, Nishida, Uehara, Wrangham, Abramowski, Finlayson and Towers1985; Wrangham & Nishida Reference Wrangham and Nishida1983), referred to as ‘“zoopharmacognosis” (Huffman Reference Huffman2003; Rodriguez & Wrangham Reference Rodriguez and Wrangham1993). Zoopharmacognosis appears to be learned as much as food preference or avoidance (Lozano Reference Lozano1998). Depending on the physical state of the body, self-medication can be a conditional behavior in mammals – that is, consuming a particular food only when stressed, but less in recovery (Lisonbee et al. Reference Lisonbee, Villalba, Provenza and Hall2009; Villalba et al. Reference Villalba, Provenza, Hall and Lisonbee2010). Consummatory choices are made either as a prophylactic/preventive self-medication, which reduces the risk of physical distress, or as therapeutic/curative self-medication, which may reduce the physical stress once it occurred (Kester & Barbosa Reference Kester and Barbosa1994; Hagen et al. Reference Hagen, Sullivan, Schmidt, Morris, Kempter and Hammerstein2009; Singer et al. Reference Singer, Mace and Bernays2009; Sullivan et al. Reference Sullivan, Hagen and Hammerstein2008). The ability to dynamically adapt food choice according to the organism's physical state based on a learning mechanism may hence be a basic adaptive trait in mammals, enhancing survival and reproduction (Clayton & Wolfe Reference Clayton and Wolfe1993; Hagen et al. Reference Hagen, Sullivan, Schmidt, Morris, Kempter and Hammerstein2009; Sullivan et al. Reference Sullivan, Hagen and Hammerstein2008). We speculate that those learning systems, which can dynamically adapt individual food choice for nutritional needs and self-medication, are the same as those involved in choosing food to change mental state.
We also suggest that two major changes between our ancestral environment and modern environments have taken place, and these changes are crucial for understanding current drug-taking behavior (Lende Reference Lende, Wenda Trevathan, Smith and McKenna2007):
-
1. Only recently, with the isolation and purification of natural compounds and with the advent of synthetic chemistry, psychoactive drugs became available in pure (e.g., cocaine, amphetamine) or highly concentrated form (e.g., alcohol). For other drugs, selective breeding of the crops increased their drug content significantly (e.g., Δ9-THC in cannabis plants). Recently available purified psychoactive substances may represent a new “niche” we have constructed; and this niche has the potential to modify the future basis of natural selection (Laland et al. Reference Laland, Odling-Smee and Feldman2000; Reference Laland, Odling-Smee and Myles2010). Psychoactive drug consumption is now considered a polygenetically determined behavior (Stacey et al. Reference Stacey, Clarke and Schumann2009) influenced by environment and culture (Blomeyer et al. Reference Blomeyer, Treutlein, Esser, Schmidt, Schumann and Laucht2008; Clarke & Schumann Reference Clarke and Schumann2009; Laland et al. Reference Laland, Odling-Smee and Myles2010). The availability of purified psychoactive substances is now part of the environment in many societies (Lende Reference Lende, Wenda Trevathan, Smith and McKenna2007), which may then interact with a genetically determined predisposition for drug use and drug addiction (Bierut et al. Reference Bierut, Dinwiddie, Begleiter, Crowe, Hesselbrock, Nurnberger, Porjesz, Schuckit and Reich1998; Kendler et al. Reference Kendler, Prescott, Myers and Neale2003a; Reference Kendler, Jacobson, Prescott and Neale2003b; Schumann Reference Schumann2007).
Psychoactive drug seeking and consumption can be observed in fruit flies (Devineni & Heberlein Reference Devineni and Heberlein2009), rodents (Arroyo et al. Reference Arroyo, Markou, Robbins and Everitt1998; Witkin et al. Reference Witkin, Savtchenko, Mashkovsky, Beekman, Munzar, Gasior, Goldberg, Ungard, Kim, Shippenberg and Chefer1999; Yokel & Pickens Reference Yokel and Pickens1973), dogs (Risner & Jones Reference Risner and Jones1980), and monkeys (Fantegrossi et al. Reference Fantegrossi, Woolverton, Kilbourn, Sherman, Yuan, Hatzidimitriou, Ricaurte, Woods and Winger2004; Howell & Byrd Reference Howell and Byrd1995; Johanson et al. Reference Johanson, Balster and Bonese1976; Ritz & Kuhar Reference Ritz and Kuhar1989) when given access to a drug. Although the availability of purified psychoactive drugs is a new environmental feature (Nesse Reference Nesse2002), the behavioral capacity to consume drugs – the learning mechanisms – developed much earlier in evolution.
-
2. In industrialized societies, an individual's workload is so high that a person may need to perform many different behaviors with contrasting types of effort. It is speculative whether single behaviors need to be performed with more effort now than in the past when considering available resources (there are more tools now) and relative outcomes (tool-supported behaviors are usually more effective). One may, nevertheless, reasonably guess that the modern environment contains more and stronger differentiated microenvironments. This may become evident, for example, by the availability of technical tools that can now be very specific for a microenvironment and yet require a high degree of training and effort in their use (e.g., a computer for work or a bicycle for spare time). We can also guess that transitions between these settings occur at a much faster rate than they did for our pre-agricultural ancestors. This may put a selection pressure not only on single behaviors, but even more on behavioral flexibility – that is, the transition from one behavior to another.
We suggest that non-addictive psychoactive drug instrumentalization helps to solve an adaptational problem, employing species-general learning mechanisms that dynamically adapt the search for and consumption of plants and plant compounds. In a modern environment, however, the problem changed together with the emergence of supportive ‘“instruments.” As for many functional adaptations (Cosmides & Tooby Reference Cosmides and Tooby1999; Wakefield Reference Wakefield1999), psychoactive drug use behavior may, under these relatively recently occurring environmental changes, have led a minority of individuals to evolutionary dysfunctional behaviors, one of which is drug addiction (American Psychiatric Association 1994).
4.2. Proximate mechanisms of psychoactive drug use
A consideration of the evolution of psychoactive drug consumption suggests a number of different proximate mechanisms that provide unique adaptations to particular microenvironments (Lende Reference Lende, Wenda Trevathan, Smith and McKenna2007). An organism's environment can therefore be considered the sum of its microenvironments (Bronfenbrenner Reference Bronfenbrenner1994), in which distinct behavioral flexibility is the best adaptation. Each of these behaviors may be seen as a microadaptation. In fast-changing microenvironments, short transition times between mental states may be advantageous because they allow behavioral flexibility. We suggest the proximate adaptive problem that may be solved by psychoactive drug use is (a) to facilitate the transition between different mental states and (b) to enhance the magnitude or duration – or both – of an ongoing mental state.
This generally stated hypothesis can be quickly made empirical by asking non-addicts why and under which circumstances they consume psychoactive drugs. Epidemiological data indicate that people give a wide range of different answers on the question why they consume psychoactive drugs (e.g., Brown Reference Brown1985; Brown et al. Reference Brown, Goldman, Inn and Anderson1980; Cooper et al. Reference Cooper, Frone, Russell and Mudar1995; Maloff et al. Reference Maloff, Becker, Fonaroff, Rodin, Zinberg and Harding1981). Baum-Baicker (Reference Baum-Baicker1985) has summarized five areas of benefit for alcohol consumption: (1) stress reduction, (2) mood enhancement, (3) cognitive performance, (4) reduced clinical symptoms of depression, and (5) improved function in the elderly. Reviews of the growing experimental evidence by Chick (Reference Chick1999), Heath (Reference Heath2000), and Peele and Brodsky (Reference Peele and Brodsky2000) and a functional analysis of methamphetamine use by Lende et al. (Reference Lende, Leonard, Sterk and Elifson2007) confirmed these earlier observations. Of course, not all motivations for consumption are consciously accessible and reportable (Davies Reference Davies1997; O'Brien et al. Reference O'Brien, Childress, Ehrman and Robbins1998; Skog Reference Skog2000). Overall, these themes of drug consumption may ultimately serve efforts directed to reproduction or efforts directed toward one's own survival. Therefore, psychoactive drug use should, like any important human behavior, be considered with regard to these two life themes raised by behavioral ecologists when considering the continuous trade-off between allocations of finite human resources (Hill & Chow Reference Hill and Chow2002).
In the following paragraphs, we describe different proximal mechanisms of psychoactive drug use in terms of unique instrumentalization goals. Their general functions for reproduction or survival and maintenance (or both) are discussed. To substantiate these views, we suggest plausible neuropharmacological mechanisms for improved functioning.
4.2.1. Improved social interaction
The establishment and maintenance of social groups and networks is essential for many species (Axelrod & Hamilton Reference Axelrod and Hamilton1981; Hamilton Reference Hamilton1964). For obligatorily social animals, including humans, social interaction becomes an incentive by itself (e.g., Matthews et al. Reference Matthews, Abdelbaky and Pfaff2005; Panksepp & Lahvis Reference Panksepp and Lahvis2007). In modern societies, most adults spend a great deal of their time in training- or work-related microenvironments. A large body of explicit and implicit rules governs interactions between people in these microenvironments. Although social encounters are manifold, private social interactions are systematically suppressed in order to enhance work performance. Professional interactions require a high degree of attention and cognitive effort, as well as a suppression of overt emotional responses. These microenvironments appear to make the establishment of social bonds deliberately difficult. The formation of social bonds is rather facilitated by a state of emotional openness and accessibility and some display of private individuality. Transitory periods between professional and private microenvironments, such as after-school or after-work gatherings or activities, are where peer groups and social networks are formed and maintained.
Interestingly, a number of drugs change mental states in a way that appears to facilitate the transition from a professional to a private behavioral repertoire. It is important to note that it is not the pharmacological effect of the drug alone that enhances social behavior; rather, it is the interaction with the social environment. In a drug-free state, social settings alone induce social behavior, but perhaps less effectively and more briefly.
Psychoactive drugs that can facilitate social behavior under various circumstances are alcohol (Bradizza et al. Reference Bradizza, Reifman and Barnes1999; Glynn et al. Reference Glynn, LoCastro, Hermos and Bosse1983; Kuntsche et al. Reference Kuntsche, Knibbe, Gmel and Engels2005), marijuana (Bonn-Miller et al. Reference Bonn-Miller, Zvolensky and Bernstein2007; Zvolensky et al. Reference Zvolensky, Vujanovic, Bernstein, Bonn-Miller, Marshall and Leyro2007), cocaine (Lende Reference Lende2005; O'Malley et al. Reference O'Malley, Johnston and Bachman1985), and other psychostimulants (Davey et al. Reference Davey, Richards and Freeman2007; O'Malley et al. Reference O'Malley, Johnston and Bachman1985), when used in a low- to medium-dose range (Boys & Marsden Reference Boys and Marsden2003; Boys et al. Reference Boys, Marsden, Griffiths, Fountain, Stillwell and Strang1999; Reference Boys, Marsden and Strang2001; Cato Reference Cato1992; Segal Reference Segal1985; Simons et al. Reference Simons, Correia and Carey2000). Also, some effects of nicotine and caffeine may be useful to maintain social interactions (Cauli & Morelli Reference Cauli and Morelli2005; Eissenberg & Balster Reference Eissenberg and Balster2000).
Alcohol reduces social inhibition, discomfort in social situations, and social anxiety; increases talkativeness; and increases the tendency to talk about private affairs (Baum-Baicker Reference Baum-Baicker1985; Booth & Hasking Reference Booth and Hasking2009; Carrigan et al. Reference Carrigan, Ham, Thomas and Randall2008; Peele & Brodsky Reference Peele and Brodsky2000). These “beneficial effects” are brought about by the multiple pharmacological targets of alcohol in the brain (Harris et al. Reference Harris, Trudell and Mihic2008; McBride et al. Reference McBride, Le and Noronha2002; Spanagel Reference Spanagel2009; Tupala & Tiihonen Reference Tupala and Tiihonen2004). Most important for these effects is probably the interaction of alcohol with GABAA-receptors in the brain. γ-Aminobutyric acid (GABA) is the most abundant inhibitory transmitter in the brain (Feldman et al. Reference Feldman, Meyer and Quenzer1997). Alcohol at low to medium doses enhances GABAergic activity, leading to reduced anxiety levels and a behavioral disinhibition. An indirect neurochemical effect of alcohol is to increase dopamine (DA) levels in the nucleus accumbens (NAc; Di Chiara & Imperato Reference Di Chiara and Imperato1988). This neurochemical effect was shown to reduce the brain's reward threshold (Koob et al. Reference Koob, Sanna and Bloom1998) and, thus, may enhance the incentive value of social interaction (Ikemoto & Panksepp Reference Ikemoto and Panksepp1999). No claim is made here that each and every effect of alcohol on the nervous system is beneficial for social interaction. For example, alcohol affects pharmacological targets in subcortical brain regions, shown to be involved in social bonding or social recognition (Ross & Young Reference Ross and Young2009). Social cognition, in contrast, involves also cortical networks, including the medial prefrontal cortex and anterior cingulate cortex (Burnett et al. Reference Burnett, Sebastian, Cohen and Blakemore2010). Although the disinhibitory effects of alcohol mediated at subcortical level may facilitate social behavior, alcohol effects at the cortical level may at the same time have no effect or even impair social cognition (Uekermann & Daum Reference Uekermann and Daum2008). We suggest that it is the sum of alcohol effects on social behavior that requires investigation.
Psychostimulant drugs, such as cocaine, amphetamine, methylphenidate, methamphetamine, and methylenedioxymethamphetamine (ecstasy, MDMA), are also used in a social context with enhanced self-exposure, such as at parties or in club settings (Britt & McCance-Katz Reference Britt and McCance-Katz2005; White et al. Reference White, Becker-Blease and Grace-Bishop2006). In addition to being more generally aroused and having increased attention, people become more talkative, disinhibited, and self-confident after consuming these drugs. In addition, psychostimulants suppress fatigue, which also enables prolonged social interaction (Fischman & Schuster Reference Fischman and Schuster1980). An increase in aggression after psychostimulant consumption (Emley & Hutchinson Reference Emley and Hutchinson1983) may result in dominating social gatherings and the “competition” for partners, further enhancing the beneficial effects for the individual (King et al. Reference King, Johnson and Van Vugt2009). Psychostimulant drugs increase extracellular activity of DA, 5-HT, and noradrenaline (NA) in the CNS by their interaction with the monoamine transporters (Ritz & Kuhar Reference Ritz and Kuhar1989; Ritz et al. Reference Ritz, Cone and Kuhar1990). They block or reverse monoamine transport (Green et al. Reference Green, Mechan, Elliott, O'Shea and Colado2003; Johanson & Fischman Reference Johanson and Fischman1989; Müller et al. Reference Müller, Carey, Huston and De Souza Silva2007a; Pum et al. Reference Pum, Carey, Huston and Müller2007; Seiden et al. Reference Seiden, Sabol and Ricaurte1993). Although high NA levels may account for the suppression of fatigue (Aston-Jones et al. Reference Aston-Jones, Rajkowski and Cohen1999), 5-HT may mediate the anxiolytic (Ho et al. Reference Ho, Pawlak, Guo and Schwarting2004; Müller et al. Reference Müller, Carey, Wilkisz, Schwenzner, Jocham, Huston and De Souza Silva2008; Schwarting et al. Reference Schwarting, Thiel, Müller and Huston1998) and aggression-enhancing effects of these drugs (Licata et al. Reference Licata, Taylor, Berman and Cranston1993; Quadros et al. Reference Quadros, Takahashi, Miczek, Müller and Jacobs2010).
Also, various dissociative anesthetic drugs, such as phencyclidine (PCP), ketamine, and γ-hydroxybutyrate (GHB), can at low doses stimulate social interactions. They can induce a feeling of empathy, reduce anxiety, and increase relaxation. At the same dose range, they are locomotor stimulants. These drugs are noncompetitive glutamate N-methyl-D-aspartate (NMDA)-receptor antagonists (Jentsch & Roth Reference Jentsch and Roth1999). Glutamate is the most abundant excitatory transmitter in the brain (Feldman et al. Reference Feldman, Meyer and Quenzer1997). Blocking NMDA receptors is considered the predominant mechanism for the observed behavioral effects (Britt & McCance-Katz Reference Britt and McCance-Katz2005; Weir Reference Weir2000; Wolff & Winstock Reference Wolff and Winstock2006).
A number of psychoactive drugs can be used to change an individual's mental state in ways to facilitate social interactions. A state in which conditioned and unconditioned anxiety and behavioral suppression are attenuated can be achieved by enhancing GABAergic inhibition and reducing glutamatergic excitation. Alternatively, a state in which the energization of social interaction is required can be achieved by enhancing monoaminergic modulatory transmission. Exaggerated drug use for this instrumentalization goal, however, may result in more pronounced euphoria or high effects, which may facilitate the transition to habitual drug use and addiction (e.g., Chen & Anthony Reference Chen and Anthony2004; Wagner & Anthony Reference Wagner and Anthony2002). An acute overdose reverses the sought effects and may even result in a schizophrenia-like psychotic state for psychostimulants or hallucinations and delirium for alcohol (e.g., Rich & Singer Reference Rich and Singer1991; Siegel Reference Siegel1978).
4.2.2. Facilitated sexual behavior
Closely related to an instrumentalization of psychotropic drug effects for social interaction is their use to facilitate the possibility of sexual behavior (Cooper Reference Cooper2006; Patrick & Maggs Reference Patrick and Maggs2009; Taylor et al. Reference Taylor, Fulop and Green1999). Sexual behavior may still be considered the sine qua non of natural selection. However, many of the same rules that control social interactions in society also restrict occasions for sexual behavior. A “scheduled” and time-dependent (e.g., Saturday night; Patrick & Maggs Reference Patrick and Maggs2009) transition from professional to private microenvironments may therefore significantly enhance the chances of finding a partner or allow already formed couples to escape daily routines. It may, therefore, not be a surprise that drugs that can be instrumentalized to improve social interactions also serve well for sexual behavior.
An important variable determining reproductive success and social behavior in humans is personality (Alvergne et al. Reference Alvergne, Jokela and Lummaa2010). Certain personality traits, such as introversion, may be disadvantageous in some settings but advantageous in others. Because it is argued that psychoactive drugs change mental states, one might view drug instrumentalization also as a self-induced, time-restricted personality change. For example, extroversion may change the likelihood of sexual behavior. The ability of a controlled personality trait change in a certain context may, therefore, help to improve sexual behaviors by overcoming the disadvantages of certain personality traits (e.g., Booth & Hasking Reference Booth and Hasking2009).
In support of popular belief, there is strong evidence for an association among alcohol drinking, drunkenness, and the likelihood for sexual intercourse, in particular in adolescents and young adults (e.g., Lavikainen et al. Reference Lavikainen, Lintonen and Kosunen2009; Patrick & Maggs Reference Patrick and Maggs2009; Sen Reference Sen2002; Wells et al. Reference Wells, Kelly, Golub, Grov and Parsons2010). In addition to the pharmacological effects of alcohol, the expected (conditioned) disinhibitory effects mediate the higher chances of sexual intercourse (Cooper Reference Cooper2006; Crowe & George Reference Crowe and George1989; Patrick & Maggs Reference Patrick and Maggs2009) and may predict future alcohol use (Mooney Reference Mooney1995). Cooper (Reference Cooper2006) suggested that those expectations may even be “instrumental in setting up situations that may lead to alcohol-related disinhibition of sex.” Behavioral disinhibition may also result from diminished cognitive abilities and a narrowed range of perception focusing in a “myopic” way on highly salient stimuli that can drive sexual arousal (Steele & Josephs Reference Steele and Josephs1990). We do not know if situational alcohol consumption in any way improves contemporary “reproductive success,” but this would be an interesting opportunity to distinguish perceived from actual success in the realm of sexual interaction.
As before, psychostimulant drugs may have mixed effects, serving to improve chances for sexual behavior, but may later interfere with physical performance during sexual intercourse in males (Maier Reference Maier1926; Waldorf et al. Reference Waldorf, Reinarman and Murphy1991). In particular, the elevated DA levels in the mesolimbic system may contribute to a mental state that makes the individual respond more effectively to sexual cues, making a potential partner appear more “attractive” (Ikemoto & Panksepp Reference Ikemoto and Panksepp1999; Koob et al. Reference Koob, Sanna and Bloom1998).
One often reported function of drug use is to “enhance sex.” Drugs frequently reported to be used for this purpose are alcohol, cannabis, amphetamines, ecstasy, and cocaine (Boys & Marsden Reference Boys and Marsden2003; Boys et al. Reference Boys, Marsden, Griffiths, Fountain, Stillwell and Strang1999; Reference Boys, Marsden and Strang2001; Maier Reference Maier1926). Although mating behavior can be conceptualized as a flexible approach behavior, sexual intercourse, in contrast, is a consummatory behavior, which is controlled by other neuronal mechanisms (Ikemoto & Panksepp Reference Ikemoto and Panksepp1999). The verbal reports on enhanced pleasure taken from sexual intercourse after psychoactive drug consumption may, therefore, be based on mechanisms that enhance incentive salience.
Altogether, psychoactive drugs facilitate sexual behavior, even enhancing pleasure during sexual intercourse. The mental state they induce is for several drug classes similar to that serving social interaction. Hence, it may be assumed that neuronal mechanisms of this drug instrumentalization are largely overlapping with those facilitating social behavior.
4.2.3. Improved cognitive performance and counteracting fatigue
Highly developed societies put a high cognitive demand on individuals in education and work microenvironments (Arria & Wish Reference Arria and Wish2006). Long working hours lead to fatigue and to decline in cognitive performance. Having the means to “artificially” prolong full cognitive capacity may consequently appear to be beneficial for the individual by increasing external resources for reproduction of self or kin (e.g., money). Although little is known about whether any drug can actually increase cognitive performance in a healthy person with full mental capacity, there is considerable evidence suggesting that mild impairments resulting from exhaustion, fatigue, or mood swings can be compensated with psychoactive drugs (Boys & Marsden Reference Boys and Marsden2003; Lende et al. Reference Lende, Leonard, Sterk and Elifson2007). In this case, no new mental state is desirable, only maintenance of a baseline state over prolonged cognitive effort. Many psychoactive drugs, both legal and illicit in Western societies, improve cognitive performance in this case.
Caffeine, a major psychoactive ingredient of coffee, tea, chocolate, and soft drinks, is a legal drug frequently used to keep people awake. During waking periods, the brain levels of the neurotransmitter adenosine steadily increase and trigger fatigue and sleepiness (Hong et al. Reference Hong, Huang, Qu, Eguchi, Urade and Hayaishi2005; Huston et al. Reference Huston, Haas, Boix, Pfister, Decking, Schrader and Schwarting1996; Porkka-Heiskanen et al. Reference Porkka-Heiskanen, Strecker, Thakkar, Bjorkum, Greene and McCarley1997). As an antagonist at the adenosine A1 and A2A receptors, caffeine effectively blocks adenosine action in the brain (Cauli & Morelli Reference Cauli and Morelli2005). It is thought this action of caffeine is responsible for preventing fatigue and reducing the decline in cognitive performance after prolonged activity.
Another widely used legal drug is nicotine, the active compound in tobacco (Le Foll & Goldberg Reference Le Foll and Goldberg2006). Nicotine is an agonist at the nicotinic ACh-receptor (Markou Reference Markou2008). Nicotinic ACh-receptors in the brain are essentially involved in mediating the action of the neurotransmitter ACh to promote attention and to facilitate learning and memory (Blokland Reference Blokland1995; Sarter et al. Reference Sarter, Hasselmo, Bruno and Givens2005). Nicotine improves attention (Hahn et al. Reference Hahn, Shoaib and Stolerman2002; Hahn & Stolerman Reference Hahn and Stolerman2002) and cognitive performance in animals (Decker et al. Reference Decker, Brioni, Bannon and Arneric1995) and in nonsmoking humans (Rezvani & Levin Reference Rezvani and Levin2001). In human smokers, there is a decline in cognitive abilities after smoking cessation that can be reversed by nicotine administration (Mansvelder et al. Reference Mansvelder, van Aerde, Couey and Brussaard2006). Nicotine was also found to ameliorate cognitive deficits in patients with Alzheimer's disease. It can reduce the cognitive deficits induced by neuroleptic drug treatment in schizophrenic patients (Rezvani & Levin Reference Rezvani and Levin2001). The stimulation of nicotinic ACh-receptors by nicotine increases not only ACh, but also NA activity (Mitchell Reference Mitchell1993; Wonnacott Reference Wonnacott1997), which might contribute to the attention promoting effects of nicotine. Nicotinic ACh-receptors also modulate the activity of the mesolimbic DA system (Markou Reference Markou2008; McBride et al. Reference McBride, Murphy and Ikemoto1999; Wonnacott Reference Wonnacott1997), which could be one mechanism for how nicotine might increase the reinforcing value of non-drug reinforcers (Harrison et al. Reference Harrison, Gasparini and Markou2002; Kenny & Markou Reference Kenny and Markou2006) and hence support goal-directed behavior.
Psychostimulant drugs have been widely used to increase cognitive performance over long periods of time, in particular to maintain arousal and attention. Truck drivers in the United States and Australia use amphetamine and other psychostimulants to stay attentive during long driving hours (Davey et al. Reference Davey, Richards and Freeman2007; Grinspoon & Hedblom Reference Grinspoon, Hedblom and Harris2005). Students use prescription stimulants, such as methylphenidate, demethylphenidate, amphetamine, and methamphetamine, non-medically to promote concentration, to stay awake, to increase alertness, and to help with studying (Arria & Wish Reference Arria and Wish2006; Lende et al. Reference Lende, Leonard, Sterk and Elifson2007; McCabe et al. Reference McCabe, Knight, Teter and Wechsler2005; Sussman et al. Reference Sussman, Pentz, Spruijt-Metz and Miller2006; Teter et al. Reference Teter, Falone, Cranford, Boyd and McCabe2010; White et al. Reference White, Becker-Blease and Grace-Bishop2006). Psychostimulants were shown to effectively increase arousal and attention in humans for long periods of time at doses that induce only a minor and short-lasting “high” and no signs of dysphoria thereafter (Higgins et al. Reference Higgins, Bickel, Hughes, Lynn, Capeless and Fenwick1990; Stillman et al. Reference Stillman, Jones, Moore, Walker and Welm1993). In line with increased attention, improved learning and memory was shown after small doses of cocaine in occasional users (Higgins et al. Reference Higgins, Bickel, Hughes, Lynn, Capeless and Fenwick1990). Attention deficits induced by sleep-deprivation can be ameliorated by a low to medium dose of cocaine (Fischman & Schuster Reference Fischman and Schuster1980). Phasic and tonic NA activity in the brain is well known to control cognitive performance in tasks with a high attention load and potential distraction (Usher et al. Reference Usher, Cohen, Servan-Schreiber, Rajkowski and Aston-Jones1999). Cocaine and amphetamines interact with NA transporters in the brain and effectively block NA reuptake at the synapse (Ritz & Kuhar Reference Ritz and Kuhar1989; Ritz et al. Reference Ritz, Cone and Kuhar1990). This increases extracellular NA levels and causes a hyperactivation of NA receptors (Green et al. Reference Green, Mechan, Elliott, O'Shea and Colado2003; Johanson & Fischman Reference Johanson and Fischman1989; Seiden et al. Reference Seiden, Sabol and Ricaurte1993), which may account for beneficial effects of small doses of psychostimulants on cognition.
Good evidence supports the view that several psychoactive drugs are instrumentalized to enhance cognitive performance by counteracting exhaustion and fatigue. Although little enhancement can be achieved in healthy and “fresh” individuals, the decline in function during fatigue, or in several mental disorders, can be effectively overcome, if temporarily, by these drugs. Likely mechanisms of action are the blockade of adenosine A1 and A2A receptors, activation of nicotinic ACh-receptors, or the enhancement of NA activity in the brain. Exaggerated use of these drugs may acutely result in hyperarousal, restlessness, and a decline in cognitive abilities (e.g., Quednow et al. Reference Quednow, Jessen, Kuhn, Maier, Daum and Wagner2006; Reference Quednow, Kuhn, Hoppe, Westheide, Maier, Daum and Wagner2007). Long-term regular use of these drugs can induce tolerance for the cognitive effects and might lead to an escalation of consumption and to drug addiction.
4.2.4. Facilitated recovery from and coping with psychological stress
Modern societies not only request constant high cognitive and physical performance, but they also allow decreasingly little time for the individual to recover from periods of intense or high work load (Anders Reference Anders1956). This leaves the individual with the pressure of finding a fast recovery and an effective way to cope with the related psychological stress. The goal is then to change the mental state from “tired and stressed” to “fresh and relaxed” in a short period of time. Ideally, after recovery, resources are replenished and stress is under control. Using drugs to accelerate recovery and to enhance coping with stress in a “spare but limited time” microenvironment may, therefore, increase the success of many behaviors in other microenvironments (Amendt Reference Amendt2003; Baum-Baicker Reference Baum-Baicker1985; Peele & Brodsky Reference Peele and Brodsky2000; Segal Reference Segal1985).
A number of pharmacologically different drugs are instrumentalized to facilitate recovery from and coping with psychological stress. Humans as well as animals self-administer alcohol (Cooper et al. Reference Cooper, Russell and George1988; Reference Cooper, Russell, Skinner, Frone and Mudar1992; Kuntsche et al. Reference Kuntsche, Knibbe, Gmel and Engels2005), cannabis (Bonn-Miller et al. Reference Bonn-Miller, Zvolensky and Bernstein2007; Zvolensky et al. Reference Zvolensky, Vujanovic, Bernstein, Bonn-Miller, Marshall and Leyro2007), cocaine (Lende Reference Lende2005; Waldorf et al. Reference Waldorf, Reinarman and Murphy1991), methamphetamine (Lende et al. Reference Lende, Leonard, Sterk and Elifson2007), barbiturates, benzodiazepines, and other sedative anxiolytic drugs (Boyd et al. Reference Boyd, Teter, West, Morales and McCabe2009) to cope with stress (Boys & Marsden Reference Boys and Marsden2003; Boys et al. Reference Boys, Marsden, Griffiths, Fountain, Stillwell and Strang1999; Reference Boys, Marsden and Strang2001; Bradizza et al. Reference Bradizza, Reifman and Barnes1999; De las Cuevas et al. Reference De las Cuevas, Sanz and De la Fuente2003; Griffiths et al. Reference Griffiths, Lamb, Sannerud, Ator and Brady1991; Heberlein et al. Reference Heberlein, Bleich, Kornhuber and Hillemacher2009; Lader Reference Lader1994; Perkins Reference Perkins1999; Segal Reference Segal1985). In the last decade, evidence for a survival promoting effect of moderate alcohol consumption in humans accumulated. Moderate alcohol consumption, which can be maintained with a high degree of stability, was associated with better health, more close friendships, and more family support than was total abstinence (Mondaini et al. Reference Mondaini, Cai, Gontero, Gavazzi, Lombardi, Boddi and Bartoletti2009; Peele & Brodsky Reference Peele and Brodsky2000; Rodgers et al. Reference Rodgers, Korten, Jorm, Christensen, Henderson and Jacomb2000;Skogen et al. Reference Skogen, Harvey, Henderson, Stordal and Mykletun2009; Taylor et al. Reference Taylor, Rehm and Gmel2005; but see also: Sareen et al. Reference Sareen, McWilliams, Cox and Stein2004). Moderate drinkers were also found to face less depression than abstainers in the presence of stress (Lipton Reference Lipton1994). Chronic moderate, but not high, alcohol consumption can reduce the risk of somatic diagnoses, as well as mental disorders such as anxiety and depression (Peele & Brodsky Reference Peele and Brodsky2000; Skogen et al. Reference Skogen, Harvey, Henderson, Stordal and Mykletun2009).
Alcohol inhibits excitatory glutamatergic transmission and enhances inhibitory GABAergic activity at the GABAA-receptor (Spanagel Reference Spanagel2009). Barbiturates and benzodiazepines also modulate the GABAA-receptor (Ito et al. Reference Ito, Suzuki, Wellman and Ho1996), though at other binding sites than alcohol, and allosterically enhance responses to the inhibitory transmitter GABA (Allison & Pratt Reference Allison and Pratt2003). Enhanced GABAergic activity is believed to reduce anxiety and the impact of conditioned aversive stimuli. This is one way in which these drugs may attenuate the processing of stress-related stimuli at a subconscious level. For conscious processing of stress-related stimuli, neocortical circuits, which also contain a high number of GABAA-receptors, are more likely to be involved (Feldman et al. Reference Feldman, Meyer and Quenzer1997). By their interaction with neocortical GABAA-receptors, sedative drugs can dampen cognitive activity and memory of aversive events (Curran Reference Curran1991).
The most widespread illicit psychoactive drug instrumentalized to ameliorate pressure and to reduce stress is cannabis (Boys et al. Reference Boys, Marsden, Griffiths, Fountain, Stillwell and Strang1999; Reference Boys, Marsden and Strang2001). The main psychoactive compound of cannabis is Δ9-tetrahydrocannabinol (THC; Iversen Reference Iversen2000). THC is an exogenous ligand at the brain's cannabinoid 1 (CB1)-receptors, which among others control the emotional impact of external stimuli and thoughts (Mechoulam et al. Reference Mechoulam, Fride and Di Marzo1998). CB1-receptor activation was shown to control the extinction of aversive memories (Marsicano et al. Reference Marsicano, Wotjak, Azad, Bisogno, Rammes, Cascio, Hermann, Tang, Hofmann, Zieglgansberger, Di Marzo and Lutz2002), which might contribute to the stress ameliorating effects of THC.
Interestingly, social stress was also found to increase the self-administration of nonsedating drugs, such as cocaine in animals and humans. The drug-induced increase in arousal (Haney et al. Reference Haney, Maccari, Le, Simon and Piazza1995) might involve another coping mechanism that resembles more a “fight” than a “flight” response. Psychostimulant drugs increase aggression levels and physical strength and can suppress fatigue (Green et al. Reference Green, Mechan, Elliott, O'Shea and Colado2003; Johanson & Fischman Reference Johanson and Fischman1989; Seiden et al. Reference Seiden, Sabol and Ricaurte1993), which are useful effects for an active stress coping mechanism. However, animal studies showed that an increase in cocaine self-administration in order to cope with social stress was only observed in animals with low spontaneous activity (Kabbaj et al. Reference Kabbaj, Norton, Kollack-Walker, Watson, Robinson and Akil2001). Several findings suggest that the ways in which psychoactive drugs are instrumentalized for coping with stress may largely depend on the individual's personality traits and coping strategies.
A number of different classes of drugs can be used to facilitate recovery from and coping with stress. They can be consumed to self-induce a mental state in which conditioned and unconditioned anxiety and mental preoccupation with them are attenuated. This is predominantly achieved by enhancing GABAergic inhibitory activity or by activating cannabinoid receptors. However, an acute overdose of sedating drugs may have fatal effects (Charlson et al. Reference Charlson, Degenhardt, McLaren, Hall and Lynskey2009). For some individuals, an aroused and attentive state of mind might serve to actively cope with stress, when behavioral action is required. This is served by enhanced monoaminergic activation. Chronic exaggerated drug use for this instrumentalization goal may result in restlessness, a hyperanxious state during withdrawal, and compulsive drug use to overcome this state.
4.2.5. Self-medication for mental problems
Psychiatric or mental disorders are characterized by the prolonged persistence of a mental state that is perceived as aversive (American Psychiatric Association 1994). They can be considered as a temporary, recurrent, or continuous breakdown of the homeostatic mechanisms in the neuronal systems that determine mental states. Behavioral functions and reproduction rates are significantly impaired in these disorders (Uher Reference Uher2009).
Certain psychiatric disorders seem to correlate with an increased consumption of psychoactive drugs with a particular neurochemical profile. Although psychoactive drugs do not persistently restore homeostasis in psychiatric patients, they may cause a temporary change to a less aversive mental state. In that, they may provide at least a temporary relief from suffering and an enhanced “functioning” in everyday life (e.g., Lende et al. Reference Lende, Leonard, Sterk and Elifson2007). The self-medication might improve the adaptation to the adverse condition (Nesse & Berridge Reference Nesse and Berridge1997). This might also apply to mental states that are perceived as aversive (e.g., being in a depressed mood), but do not fulfill the diagnostic criteria of a psychiatric disease (Boys & Marsden Reference Boys and Marsden2003; Boys et al. Reference Boys, Marsden, Griffiths, Fountain, Stillwell and Strang1999; Reference Boys, Marsden and Strang2001).
It is well known that people suffering from negative affect use psychoactive drugs to self-medicate and regain some sense of control over their mental state (Boys et al. Reference Boys, Marsden, Griffiths, Fountain, Stillwell and Strang1999; Reference Boys, Marsden and Strang2001; Glynn et al. Reference Glynn, LoCastro, Hermos and Bosse1983; Khantzian Reference Khantzian1985; Reference Khantzian1997; Markou et al. Reference Markou, Kosten and Koob1998; Sher et al. Reference Sher, Grekin and Williams2005). Considerable evidence supports the view that alcohol is consumed to provide relief from negative affect (Peele & Brodsky Reference Peele and Brodsky2000), although it is still unclear whether this is a causal or associative relationship (Room Reference Room2000). The effectiveness of alcohol depends on several factors such as genetic predisposition, expectancies, and environmental factors (Sher et al. Reference Sher, Grekin and Williams2005).
Increased consumption of nicotine and cannabis has been demonstrated in schizophrenic individuals (Hughes et al. Reference Hughes, Hatsukami, Mitchell and Dahlgren1986). It is believed that these drugs may exacerbate positive symptoms such as hallucinations (Perry & Perry Reference Perry and Perry1995). However, the aversive negative symptoms, such as the flattening of affect, and possibly cognitive impairments, might improve with cannabis (Potvin et al. Reference Potvin, Stip and Roy2003). Nicotine might improve cognitive deficits in schizophrenic individuals with prescribed neuroleptics (Rezvani & Levin Reference Rezvani and Levin2001). At present, it can only be speculated that the nicotinic ACh-receptor activation, which enhances cognitive performance under certain circumstances in healthy subjects, might also account for the benefits in cognitively impaired schizophrenics.
THC was shown to alleviate anxious states and to reduce pain in chronic neurological disorders such as multiple sclerosis (Williamson & Evans Reference Williamson and Evans2000). There is a high rate of substance abuse in patients suffering from posttraumatic stress disorder (PTSD) to self-medicate the PTSD symptoms (Jacobsen et al. Reference Jacobsen, Southwick and Kosten2001). Benzodiazepines are used to self-medicate other forms of anxiety disorders and sleep-disturbances off prescription (Heberlein et al. Reference Heberlein, Bleich, Kornhuber and Hillemacher2009). Opiate drugs were reported to be used by people who suffer from physical pain (Boyd et al. Reference Boyd, McCabe, Cranford and Young2006; McCabe et al. Reference McCabe, Cranford, Boyd and Teter2007b; Zacny & Lichtor Reference Zacny and Lichtor2008). People with tendencies for rage and strong violence reported opiate use because they felt that the drug effects help to control the outbursts (Khantzian Reference Khantzian1985; Reference Khantzian1997).
This instrumentalization goal might also explain why non–clinically diagnosed people use psychostimulant drugs to deal with the distress caused by their depression, dysphoria, hypomania, hyperactivity, and attention-deficit problems (Khantzian Reference Khantzian1985; Reference Khantzian1997; Lende et al. Reference Lende, Leonard, Sterk and Elifson2007; Teter et al. Reference Teter, Falone, Cranford, Boyd and McCabe2010). It was suggested that some biomarkers of depression resemble those of drug withdrawal, such as a chronic down-regulation in the activity of the DA- and 5-HT-systems (Maisonneuve et al. Reference Maisonneuve, Ho and Kreek1995; Wyatt et al. Reference Wyatt, Karoum, Suddath and Fawcett1988). Drug use could possibly restore homeostasis and ameliorate depressive symptoms (Markou et al. Reference Markou, Kosten and Koob1998). Nevertheless, attempted self-medication may increase the risk of disease progression in the long run (McLellan et al. Reference McLellan, Woody and O'Brien1979; Weiss et al. Reference Weiss, Mirin, Michael and Sollogub1986).
Sullivan and Hagen (Reference Sullivan and Hagen2002) suggested that at least part of the motivation to use psychoactive drugs for self-medication might be a neurotransmitter deficit in the brain, leading to psychiatric diseases. Indeed, a reduction in DA- and 5-HT transmission can dramatically reduce behavioral activity and incentive motivation (Carey et al. Reference Carey, DePalma, Damianopoulos, Hopkins, Shanahan, Müller and Huston2004; Reference Carey, Huston and Müller2008). Several psychoactive plant compounds resemble structural motifs of those key neurotransmitters that are known to be involved in psychiatric diseases. Sullivan and Hagen (Reference Sullivan and Hagen2002) argued that the drug might compensate for the deficit. This might be true for some drugs. For example, people suffering from depression use cocaine as a preferred drug to ameliorate a negative affective state (Khantzian Reference Khantzian1985; Reference Khantzian1997). Depression is associated with disturbed 5-HT activity (Carr & Lucki Reference Carr, Lucki, Müller and Jacobs2010). Acute administration of cocaine, as well as synthetic antidepressant drugs, increases 5-HT levels (Müller et al. Reference Müller, Thönnessen, Barros, Tomaz, Carey and Huston2004; Reference Müller, Carey, Huston and De Souza Silva2007a; Reference Müller, De Souza Silva and Huston2007b). For the long-term antidepressant effects, however, chronic drug taking is required to increase basal 5-HT levels and 5-HT throughput (Carr & Lucki Reference Carr, Lucki, Müller and Jacobs2010).
An altered mental state and inadequate behavioral responses characterize psychiatric disorders. Several psychotropic drugs were found to be useful by individuals to temporarily ameliorate at least part of their disease-related mental or/and cognitive disturbances. Because disease etiology for major psychiatric disorders is still little understood, it appears difficult to identify those pharmacological actions of the drugs that might serve this instrumentalization goal. Exaggerated drug use for this goal, however, was often found to potentiate disease symptoms in the long term and might add a comorbid addiction to the original disorder (Robbins & Everitt Reference Robbins and Everitt1999).
4.2.6. Sensory curiosity – Expanded perception horizon
Novel stimuli and novel environments carry the potential of new reward contingencies that would allow for the establishment of new behaviors that can lead to a higher overall “reward income” for an individual. The greater the number of distinct behaviors leading to rewards that are established, the more independent an individual will become from changes in the environment when single stimulus-behavior-reward associations lose their contingency. As such, the non–drug related search for novelty and new environments is a driving force to expose an individual to stimuli and environments where new stimulus-reward contingencies exist and can be learned (Kelley et al. Reference Kelley, Cador, Stinus, Boulton, Baker and Greenshaw1990; Thiel et al. Reference Thiel, Müller, Huston and Schwarting1999).
At least in humans, insight may not only be gained by de novo experience of the external world, but also by restructuring of knowledge already gained. As such, a qualitatively or quantitatively altered cognitive performance (Lende et al. Reference Lende, Leonard, Sterk and Elifson2007; Stillman et al. Reference Stillman, Jones, Moore, Walker and Welm1993) may also count as an example of novelty. Although the first is associated with learning and the formation of new representations (McGaugh Reference McGaugh2000; Kandel Reference Kandel2001), the later may involve the coincident activation of previously unrelated representations that are then interlinked. Novelty and new sensations can be considered as primary reinforcers in humans and animals (Weil Reference Weil1998; Zuckerman Reference Zuckerman1990). They were shown to increase DA activity in the mesolimbic system of the brain in a similar way to other primary reinforcers (Feenstra et al. Reference Feenstra, Botterblom and Vanuum1995; Martel & Fantino Reference Martel and Fantino1996). The active enhancement of this exposure may contribute to reward learning as a major behavioral adaptation that enhances an organism's survival chances and reproduction.
Psychoactive drugs, by definition, change mental states. This constitutes a novelty effect on the first consumption episodes for each drug. It is unique to particular substances and is reflected in a drug's discriminative stimulus properties (Overton Reference Overton1968; Stolerman Reference Stolerman1992). It is believed that the distinct pharmacological profile of each drug results in unique discriminative stimulus properties. After repeated exposure, the discriminative stimulus properties still exist, but they are not novel anymore, and something other than the novelty effects are needed to motivate continuation of drug use. If no other motives or instrumentalization goals arise, the “experimental” consumption of the drug may cease. This is supported by drug consumption surveys that collectively show considerably higher rates for trying a particular drug once in a lifetime versus regular consumption, measured as, for example, monthly use (SAMHSA 2005; EMCDDA 2009).
From consumer reports, it can be inferred that some psychoactive drugs may not provide a completely new sensory stimulus or environment but rather change an organism's mental state such that present stimuli or already established memories thereof are perceived and dealt with in a new fashion, including self-perception (Weil Reference Weil1998). However, the psychotropic drug–induced changes in the perception of the external and internal world do not expose the individual to new reward contingencies and may finally offer little improvement in “reward income.” It may, therefore, drive psychotropic drug consumption only for a relatively short period of time – that is, until an individual has learned that the drug-induced changes in stimulus perception are not providing new reward-contingencies. This view is in line with self-administration studies in animals. These studies showed that animals do not regularly self-administer those drugs that humans consume primarily for their sensory perception changing properties, such as hallucinogens (Nichols Reference Nichols2004).
Hallucinogenic drugs, a particular group of psychoactive drugs, are used to change sensation and perception of the external world and to increase self-understanding and self-discovery (Boys & Marsden Reference Boys and Marsden2003; Boys et al. Reference Boys, Marsden, Griffiths, Fountain, Stillwell and Strang1999; Reference Boys, Marsden and Strang2001; Cato Reference Cato1992; Nichols Reference Nichols2004). These drugs include natural compounds, like mescaline and psilocybin, as well as semisynthetic drugs, like lysergic acid diethylamide (LSD). They induce a mental state characterized by perceptual hypersensitivity, illusions, and hallucinations. The experience of time and space and the perception of the self are changed. Highly dependent on expectations and setting, hallucinogens can produce a loss of ego boundaries with an elevated mood but may also cause psychotic ego dissolution with fear, paranoid ideation, and a split ego (Geyer & Vollenweider Reference Geyer and Vollenweider2008; Nichols Reference Nichols2004). In this state, environmental stimuli can be perceived in a new way that is reported to be an enrichment of one's perceptual world.
LSD and other hallucinogenic drugs are 5-HT2-receptor agonists, activating predominantly 5-HT2A and 5-HT2C receptors. There is now wide agreement that the effects on the 5-HT2A receptor are crucial for the hallucinogenic action (Gonzalez-Maeso & Sealfon Reference Gonzalez-Maeso and Sealfon2009; Halberstadt & Nichols Reference Halberstadt, Nichols, Müller and Jacobs2010). They also reduce 5-HT turnover and 5-HT neuronal firing in the raphe nuclei. This suggests a further enhancement of the contrast in activation between 5-HT2A/2C and all other 5-HT receptors. 5-HT2A receptors are found in a high density in the neocortex (Mengod et al. Reference Mengod, Cortes, Teresa Vilaro, Hoyer, Müller and Jacobs2010) where they fine-tune principal- and inter-neurons (Sheldon & Aghajanian Reference Sheldon and Aghajanian1990; Aghajanian & Marek Reference Aghajanian and Marek1997). 5-HT and 5-HT2 receptors play an important role in sensory stimulus processing (Jacobs & Fornal Reference Jacobs, Fornal, Müller and Jacobs2010; Pum et al. Reference Pum, Huston, De Souza Silva and Müller2008; Quednow et al. Reference Quednow, Kuhn, Mossner, Schwab, Schuhmacher, Maier and Wagner2008; Reference Quednow, Schmechtig, Ettinger, Petrovsky, Collier, Vollenweider, Wagner and Kumari2009). The artificial disturbance of this fine-tuning leads to an altered mental state that is relatively selective for the processing of the physical properties of the stimulus without enhancing its incentive salience. However, an interaction of subcortical 5-HT2 receptors, e.g., with the mesolimbic DA system may also affect the emotional properties of a stimulus resulting in, e.g., “horror trips.” Although 5-HT2A and 5-HT2C receptors control DA activity at the level of mesocorticolimbic DA systems (Adell et al. Reference Adell, Bortolozzi, Diaz-Mataix, Santana, Celada, Artigas, Müller and Jacobs2010; McMahon et al. Reference McMahon, Filip and Cunningham2001; Müller & Carey Reference Müller and Carey2006), the effects of hallucinogenic drugs are not considered to induce euphoria or have a major addictive potential (Nichols Reference Nichols2004).
A similar instrumentalization might also apply to the entactogenic drug, MDMA (Boys et al. Reference Boys, Marsden, Griffiths, Fountain, Stillwell and Strang1999; Reference Boys, Marsden and Strang2001), which has a hallucinogenic profile and induces a unique feeling of “divine oneness” with the world. This particular effect might be mediated by an interaction with the peptide transmitter oxytocin, which facilitates social bonding and the feeling of attachment (Halberstadt & Nichols Reference Halberstadt, Nichols, Müller and Jacobs2010). MDMA increases 5-HT activity in the brain (Sprouse et al. Reference Sprouse, Bradberry, Roth and Aghajanian1990), which also hyperactivates 5-HT2A receptors. This effect was shown to be involved in the perceptual changes and emotional excitation following MDMA administration, but did not appear to contribute to the positive effects on mood (Liechti et al. Reference Liechti, Saur, Gamma, Hell and Vollenweider2000). In contrast to LSD, MDMA has a significant effect on DA and NA activity, which might motivate MDMA's use beyond the sensory curiosity.
Phencyclidine, ketamine, and gammahydroxybutyrate (GHB) are drugs used in the club and rave scenes. They are dissociative anesthetics used in the clinic. At high dose, they can have profound hallucinogenic effects. They are unique in that they induce a dissociative state, characterized by sensory deprivation, dreamlike visions, and feelings of the “self” separated from the body. Even near-death experiences were reported. In contrast to serotonergic hallucinogens, they are antagonists at the glutamate NMDA receptor. It is assumed that blocking this receptor, together with an interaction with opiate receptors and monoamine transporters, leads to a functional dissociation of thalamo-cortical and limbic stimulus processing. This was suggested to be the mechanism for the dissociation of the subjective perception from actual environment (Britt & McCance-Katz Reference Britt and McCance-Katz2005; Weir Reference Weir2000; Wolff & Winstock Reference Wolff and Winstock2006).
Also, cannabis was reported to be consumed to expand self- and environmental perception (Bonn-Miller et al. Reference Bonn-Miller, Zvolensky and Bernstein2007; Zvolensky et al. Reference Zvolensky, Vujanovic, Bernstein, Bonn-Miller, Marshall and Leyro2007). In particular, the perception of time is much slower after cannabis consumption (Iversen Reference Iversen2000). It has been speculated that activation of the CB1 receptors in the association cortices of the brain and the presynaptic inhibition of DA, NA, 5-HT, and glutamate release may mediate the changes in self- and environmental perception (Felder & Glass Reference Felder and Glass1998; Porter & Felder Reference Porter and Felder2001).
Several drugs can be used to change one's mental state such that environmental stimuli and the self are perceived in a new fashion, without significant effects on euphoria or on the incentive properties of the stimuli. This can be achieved by drugs that either directly or indirectly activate 5-HT2A receptors or by blocking NMDA receptors. Exaggerated drug use for this instrumentalization goal may result in dangerous activities and schizophrenia-like psychoses.
4.2.7. Euphoria, hedonia, and high
“Euphoria,” “happiness,” “hedonia” – all these concepts describe a more-or-less intense feeling of well-being (the terms are used here synonymously). The pursuit of euphoria, or happiness – as either a series of short-lasting feelings or as a long-lasting mental state – is probably the greatest desire in human life (Marcuse Reference Marcuse1984; Tatarkiewicz Reference Tatarkiewicz1976). Human brains work toward linking this subjective feeling with either the receipt of a primary or secondary reward or with the change in reward contingencies – when a formerly meaningless stimulus now predicts reward availability or a formerly useless behavior now yields a reward. Although the biological function of the subjective perception of euphoria is far from clear, it appears that the amount of euphoria we perceive is related to human well-being. It was argued that mood enhancement alone is a psychological benefit gained from psychoactive drug use (Lende & Smith Reference Lende and Smith2002; Peele & Brodsky Reference Peele and Brodsky2000). An enhanced mood by itself – one without an impact on physical health and behavior – however, would rather constitute a mock benefit. In this respect, the view of drugs “hijacking” the reward system was developed (Nesse & Berridge Reference Nesse and Berridge1997), which is mostly supported by psychoactive drugs with a strong euphoria component. Alternatively, an enhanced mood can be seen as one example of a mental state change, which might allow more efficient performance of many other goal-directed behaviors and, hence, enhance chances for survival and reproduction.
Psychoactive drugs such as heroin, morphine, cocaine, amphetamine, methamphetamine, methylphenidate, and MDMA can induce a potent feeling of euphoria and an emotional “high” (e.g. Javaid et al. Reference Javaid, Fischman, Schuster, Dekirmenjian and Davis1978; Resnick et al. Reference Resnick, Kestenbaum and Schwartz1977) and are used for this reason by nonaddicts (Boyd et al. Reference Boyd, McCabe, Cranford and Young2006; McCabe et al. Reference McCabe, West and Wechsler2007a; Teter et al. Reference Teter, Falone, Cranford, Boyd and McCabe2010; Zacny & Lichtor Reference Zacny and Lichtor2008). A certain degree of euphoria can also be induced by other drugs of abuse, such as alcohol, cannabis, LSD, benziodiapines, and nicotine (e.g., Boys & Marsden Reference Boys and Marsden2003; Boys et al. Reference Boys, Marsden, Griffiths, Fountain, Stillwell and Strang1999; Reference Boys, Marsden and Strang2001; Sher et al. Reference Sher, Grekin and Williams2005). However, the latter are usually not consumed primarily for this reason. The neuronal correlate for the profound euphoria-inducing effects of psychoactive drugs was long believed to be an increase of the extracellular DA activity in the NAc. This view developed from research on the brain's reward circuitry (Olds & Milner Reference Olds and Milner1954; Wise Reference Wise1980; Reference Wise, Legg and Both1994; Reference Wise2002) supported by the hugely influential finding that drugs of abuse increase DA levels in the NAc in vivo, whereas nonabused drugs do not (Di Chiara Reference Di Chiara1995; Di Chiara & Imperato Reference Di Chiara and Imperato1988).
It is now well understood how pharmacologically different classes of drugs converge in their acute neurophysiologic effects of increasing mesolimbic DA activity (Ameri Reference Ameri1999; Di Chiara & North Reference Di Chiara and North1992; Koob Reference Koob1992; McBride et al. Reference McBride, Murphy and Ikemoto1999). Of special importance for the role of DA in these effects appeared to be the D2 receptor (Volkow et al. Reference Volkow, Wang, Fischman, Foltin, Fowler, Abumrad, Vitkun, Logan, Gatley, Pappas, Hitzemann and Shea1997), which becomes hyperactivated during acute drug exposure. In humans, drug-induced euphoria is usually more intense than naturally occurring euphoria. There subsequently appeared to be several conceptual problems with the DA hypothesis, however (Salamone Reference Salamone1996), which led to the view that DA may not code for the euphoria, but rather signal a reward-related prediction error (Hollerman & Schultz Reference Hollerman and Schultz1998; Schultz Reference Schultz2000). This is not only the case for appetitive (i.e., pleasant) stimuli, but also for aversive stimuli (Brischoux et al. Reference Brischoux, Chakraborty, Brierley and Ungless2009; Matsumoto & Hikosaka Reference Matsumoto and Hikosaka2009; Young et al. Reference Young, Joseph and Gray1993). Although these findings may have preserved an outstanding role for DA in reinforcement learning and addiction (Ikemoto & Panksepp Reference Ikemoto and Panksepp1999; Robbins & Everitt Reference Robbins and Everitt1996), they further moved reinforcement learning away from the subjective perception of euphoria.
Robinson & Berridge (Reference Robinson and Berridge1993) suggested that DA in the NAc may be the mechanism to attribute incentive salience (i.e., the “wanting”) to cues associated with either natural or pharmacological reinforcers. However, euphoria, which is most closely conceived as the “liking” of a stimulus, should be independent from its incentive salience and may be mediated by endogenous opioid- and GABAergic mechanisms (Berridge Reference Berridge1996; Berridge & Robinson Reference Berridge and Robinson2003). In their incentive-sensitization theory, Berridge and Robinson (Reference Robinson and Berridge2003) suggested that during repeated drug consumption, a sensitization of neural systems, which attribute incentive salience, occurs. This sensitization increases the “wanting” of a drug, which results in compulsive drug seeking and taking. Thereby, the problems with the original DA hypothesis gave way to an opening for a role of other neuronal adaptations beyond NAc DA (Bardo Reference Bardo1998). Currently, an involvement of many more transmitter systems and various postsynaptic mechanisms in the euphoria-inducing and reinforcing effects of psychoactive drugs is acknowledged (Everitt & Wolf Reference Everitt and Wolf2002; Heilig & Koob Reference Heilig and Koob2007; Kalivas & Volkow Reference Kalivas and Volkow2005; Koob Reference Koob1999; Müller et al. Reference Müller, Pum, Schumann, Huston, Müller and Jacobs2010; Nestler & Aghajanian Reference Nestler and Aghajanian1997; Williams & Adinoff Reference Williams and Adinoff2007).
Euphoria is probably the easiest to accept as an instrumentalization goal for psychoactive drugs. Nevertheless, euphoria is not the most predominant and sought-after effect during most psychoactive drug taking occasions in nonaddicts (for an evolutionary discussion, see: Hagen et al. Reference Hagen, Sullivan, Schmidt, Morris, Kempter and Hammerstein2009; Sullivan et al. Reference Sullivan, Hagen and Hammerstein2008). For those drugs, which are classically associated with euphoria effects, euphoria requires a considerably higher dose than the use of the drug effects for other instrumentalization goals. Nevertheless, the mental state of a mild euphoria can be useful for many other instrumentalization goals as well; for example, for social or sexual behavior. Although an involvement of NAc DA in behavioral adaptations, in particular for the learning of stimulus-behavior-outcome associations, is beyond doubt now, it is still unclear which mechanisms code for the actual subjective feeling of euphoria. Chronic over-instrumentalization of euphoria-inducing drugs may result in tolerance to the euphoria effects and in an escalation of intake. Acute withdrawal effects are characterized by dysphoria and a depression-like mental state. In nonaddicted users, this can also facilitate drug use in order to overcome the aversive withdrawal states. Entering the “vicious circle” of an escalating consumption and withdrawal is considered to be a gateway to addiction (Heilig & Koob Reference Heilig and Koob2007; Koob & Le Moal Reference Koob and Le Moal2008).
4.2.8. Improved physical appearance and attractiveness
Modern societies impose idealized concepts on males and females, respectively, which include expectations for cognitive and social performance, but also “ideal” norms for physical appearance. Given the natural variation among humans and therefore variance around idealized norms, people feel the pressure to perform behaviors that adapt their physical appearance to these norms. There are certain effects of psychoactive drugs that can be used to facilitate these behaviors and enhance their outcome. A currently predominant case in Western societies may be the pressure toward a lean appearance in females and toward a well-defined muscular appearance in males.
A popular belief is that smoking tobacco reduces body weight. Data indicate that smokers weigh less than non-smokers. However, smokers do not eat less or consume fewer calories than nonsmokers. Several lines of evidence suggest that nicotine causes a less efficient storage of calories, most likely by its interaction with the gut (Wack & Rodin Reference Wack and Rodin1982). Nicotine can also reduce the weight gain usually following smoking cessation (Perkins Reference Perkins1992). It was shown that the activation of nicotinic ACh-receptors in the lateral hypothalamus is the most likely central mechanism by which nicotine interacts with hunger and feeding behavior toward specific food (Jo et al. Reference Jo, Talmage and Role2002).
The use of cocaine and amphetamine and its derivatives for their hunger-suppressing effects has been reported as well (Boys & Marsden Reference Boys and Marsden2003; Boys et al. Reference Boys, Marsden, Griffiths, Fountain, Stillwell and Strang1999; Reference Boys, Marsden and Strang2001; Garattini et al. Reference Garattini, Borroni, Mennini, Samanin, Garattini and Samanin1978). The anorectic effects of amphetamine-derivatives are believed to be mediated mainly by their noradrenergic, rather than by their serotonergic or dopaminergic, effects. In particular, α1 receptor stimulation in the hypothalamus was claimed to be responsible for the reduction in food intake (Borsini et al. Reference Borsini, Bendotti, Carli, Poggesi and Samanin1979; Samanin & Garattini Reference Samanin and Garattini1993).
In males, a well-defined, muscular appearance is considered an ideal. Naturally, this may signal health and potency of the male to a female and increase chances of mating. Physical appearance may be enhanced by sport, exercise, or body-building. The predominant classes of drugs whose effects can be instrumentalized to facilitate exercise-dependent muscular appearance are androgenic-anabolic steroids such as testosterone or nandrolone. Their use to improve physique is especially popular among teenagers (Goldstein Reference Goldstein1990). Anabolic-androgenic steroids increase muscle growth by a peripheral mechanism (Kochakian Reference Kochakian1990). However, they also have direct psychoactive effects, such as an increase in self-esteem (Wood Reference Wood2004; Reference Wood2008). The self-perception of superior physical appearance may also feed back and increase self-confidence and, hence, affect other behaviors such as social approach or sexual behavior (Wood Reference Wood2004). As such, there may be an indirect mechanism of how psychoactive drugs can be instrumentalized to first change physical appearance and as a secondary effect to change mental state and potentially all subsequent behaviors (Brower Reference Brower2002). The slow-acting effects of anabolic-androgenic steroids on mental states are likely to be mediated by their slow effects on the opiate system. Chronic treatment in animals increased levels of the endogenous opioid, β-endorphin, in the limbic system and changed opiate receptor densities. This may be the base for an altered self- and social perception. The modulatory effects on the 5-HT system may account for altered levels of aggression (Kanayama et al. Reference Kanayama, Brower, Wood, Hudson and Pope2009; Quadros et al. Reference Quadros, Takahashi, Miczek, Müller and Jacobs2010).
Several drugs are used to facilitate or inhibit behavior directed toward a change in physical appearance. Although weight reduction appears to be a predominant strategy in females, an increase in muscular appearance is an important goal in males. Both are served by different drug classes. Weight reduction can be achieved by either nicotinic-receptor stimulation or by NA activation. The facilitation of muscle growth relies on a peripheral mechanism involving androgen receptors. This effect is supported by androgen receptor–induced changes in mental states that can facilitate exercise behavior.
Altogether, the functional analysis of nonaddictive psychoactive drug consumption suggests that psychoactive drugs are consumed for their effects on mental states. Based on their ability to adapt food consumption for non-nutritional purposes, humans are able to learn that mental states can be changed by drugs in order to facilitate other, non–drug related behaviors. Specific “instrumentalization goals” are suggested. Available evidence on the neuropharmacological effects of the used psychoactive drugs can in detail support a focused use of a great number of single drug effects for instrumentalization. We suggest that drug instrumentalization may enhance efficacy of specific fitness-relevant behaviors in modern environments. It is assumed that in order to effectively instrumentalize psychoactive drugs, the establishment of and retrieval from a drug memory is required. How this could be done is discussed in section 5.
5. The psychological mechanisms of drug instrumentalization
5.1. Drug memories
A fundamental assumption of the psychoactive drug instrumentalization concept is a memory for all drug-related issues and a systematic retrieval of these memories. In a discussion of addiction-related behaviors, Nancy Mello introduced the idea of a “memory of addiction” (Mello Reference Mello, Kissin and Begleiter1972). Norman White (Reference White1996), in summarizing later evidence, suggested that the reinforcing effects of addictive drugs may in part be brought about by their interaction with the brain's multiple memory systems. He proposed three general types of memory that are independently influenced by psychoactive drugs. These systems would be involved in conditioned incentive learning, declarative learning, and habit or stimulus-response learning (White Reference White1996). An important role of habit learning for drug addiction was previously recognized in particular for drug self-administration behavior (White Reference White1989; Reference White1996). This view received important support by more recent studies demonstrating not only anatomical preconditions in the brain (Haber et al. Reference Haber, Fudge and McFarland2000; Porrino et al. Reference Porrino, Lyons, Smith, Daunais and Nader2004), but also its functional relevance for a transfer of information between stimulus-outcome learning and stimulus-response learning systems (Belin & Everitt Reference Belin and Everitt2008).
Another classification of “addiction memories” suggested at least three different memory types in relation to drug consumption: a memory of a drug effect, a memory of drug use, and a memory of addiction (Boening Reference Boening2001; Heyne et al. Reference Heyne, May, Goll and Wolffgramm2000). Jim Orford (Reference Orford2001) suggested in his “excessive appetite model of addiction” an essential involvement of Pavlovian and incentive learning mechanisms. A combination of operant reward would together with cue-induced conditioned responses drive drug consumption within a social context (Orford Reference Orford2001). A more recent review distinguished brain structures involved in declarative and procedural memory and discussed how these contribute to addiction-related behaviors. Thereby, drugs are assumed to work as unconditioned reinforcers that support emotional learning, hence, encompassing Pavlovian, as well as instrumental, conditioning (Robbins et al. Reference Robbins, Ersche and Everitt2008). Overall, these concepts of drug- or addiction memory have in common the explicit focus on drug addiction, ignoring the fact that in order to establish addiction, a preceding period of nonaddicted drug use must occur. During this period, most (if not all) of the drug-related memories are established, and retrieval of these memories drives ongoing drug consumption.
Some have suggested that drug addiction is based on the vulnerabilities of natural memory systems identified for non-drug-related information (Niaura et al. Reference Niaura, Goldstein and Abrams1991; Redish et al. Reference Redish, Jensen and Johnson2008). We suggest here that nonaddictive psychoactive drug instrumentalization also involves a decision-making process that relies on an interaction of all drug-related memory systems. Parallel to non-drug-related memory systems (Milner et al. Reference Milner, Squire and Kandel1998; Squire et al. Reference Squire, Knowlton and Musen1993), two major drug-memory categories are distinguished: a declarative drug memory and a nondeclarative drug memory (see Fig. 1).

Figure 1. Drug memory systems based on normal memories that psychoactive drugs are likely to influence. A time line suggests the temporal order in which different types of drug memories are established. Drug instrumentalization relies mostly on episodic, semantic, and instrumentally conditioned drug memories. Escalating and compulsory drug use may further intensify these memories. Drug addiction is, in addition, characterized by a growing influence of drug habit and drug priming memories, as well as by classically conditioned drug memories.
The declarative drug memory contains information that is consciously accessible and can be reported verbally in humans. The declarative drug memory should comprise a semantic memory for drug facts and a memory for drug episodes. The semantic memory for a drug contains all impersonal facts, rules, and concepts involving drugs – for example, their names, where they come from, recommended doses, what others report about its effects, and the social rules of their consumption. The establishment of this type of drug memory usually starts before a person is engaged in the first episode of consumption by learning from others about the drug (Leigh & Stacy Reference Leigh and Stacy2004; Miller et al. Reference Miller, Smith and Goldman1990; West Reference West2006). An early semantic drug memory thus shapes the first expectations of drug effects, which is then constantly adapted after actual consumption started (Kidorf et al. Reference Kidorf, Sherman, Johnson and Bigelow1995). It has been suggested that the expectation of drug effects (Del Boca et al. Reference Del Boca, Darkes, Goldman and Smith2002; Leigh Reference Leigh1989) should be conceptualized as a retrieval process from different types of memories (Goldman et al. Reference Goldman, Brown, Christiansen and Smith1991). The expectation of drug effects have been shown to dramatically shape the physiological effects of the drug, as well as its subjective perception (Volkow et al. Reference Volkow, Wang, Ma, Fowler, Zhu, Maynard, Telang, Vaska, Ding, Wong and Swanson2003; Reference Volkow, Wang, Ma, Fowler, Wong, Jayne, Telang and Swanson2006) and, hence, influence the establishment of the episodic drug memory. We hypothesize that a person starts to establish a semantic drug memory as a precondition of psychotropic drug instrumentalization by learning from, for example, media or peer groups, long before the first encounter with the drug.
The episodic drug memory comprises the memories of personally experienced episodes with the drug. It is an autobiographical memory on the “what,” “where,” and “when” of the personal drug encounters (Dere et al. Reference Dere, Easton, Nadel and Huston2008). This may include memories of subjectively experienced acute drug effects – for example, the mental states the drug induced. The episodic drug memory system can also contain memories of what was done during a particular drug-induced mental state and even what effects it had in terms of the environmental feedback (Boening Reference Boening2001).
Although the experience of euphoria is often the sought-after mental state, it is not the only one on the timescale of a drug episode. A single drug episode may better be considered as a sequence of several distinct mental states. Experimental studies in animals and humans have shown that it takes a few experiences of the drug effects to reliably distinguish them from placebo. Once established, however, they can be retrieved from memory and provide the base for other behavioral choices (Overton Reference Overton1968; Stolerman Reference Stolerman1992). As such, this type of memory appears to be crucial for establishing drug instrumentalization (Eissenberg & Balster Reference Eissenberg and Balster2000; Miller Reference Miller2001).
The nondeclarative drug memory, in contrast, is not consciously accessible and can be inferred only from behavioral changes. The nondeclarative drug memories contain engrams of the classically conditioned drug memory, instrumentally conditioned drug memory, habit memory, procedural drug memories, and drug-priming memories (see also Orford Reference Orford2001; Robbins et al. Reference Robbins, Ersche and Everitt2008).
Classically conditioned drug memories may contain all drug effects that refer to the process of Pavlovian conditioning (Bouton & Moody Reference Bouton and Moody2004). These may include, for example, the sensitization of the acute drug effects (Kalivas et al. Reference Kalivas, Sorg and Hooks1993; Vanderschuren & Kalivas Reference Vanderschuren and Kalivas2000), drug tolerance, conditioned locomotor activity, conditioned emotional and physiological responses (Foltin & Haney Reference Foltin and Haney2000), and conditioned withdrawal effects (Goldberg Reference Goldberg1975; O'Brien et al. Reference O'Brien, Childress, Ehrman and Robbins1998; Siegel Reference Siegel1988).
Instrumentally conditioned drug memories comprise engrams established by instrumental conditioning. Major behaviors induced by these engrams are drug seeking and drug self-administration (Richardson & Roberts Reference Richardson and Roberts1996; Spealman & Goldberg Reference Spealman and Goldberg1978). These memories also include drug cues that can serve as secondary reinforcers, as shown in conditioned place preference (Bardo & Bevins Reference Bardo and Bevins2000; Childs & de Wit Reference Childs and de Wit2009; Tzschentke Reference Tzschentke2007), or which can reinstate drug seeking and drug self-administration (de Wit & Stewart Reference de Wit and Stewart1981; Shaham et al. Reference Shaham, Shalev, Lu, de Wit and Stewart2003; Wikler Reference Wikler1973). They may also include memories established by social learning, for example, by observation (Bandura Reference Bandura1977). A motivation to use a drug may induce a cognitive process dealing with the outcome expectancies of the drug consumption (Marlatt & George Reference Marlatt and George1984). Behaviors started by this process may then be positively or negatively reinforced by the drug effects (Koob & Le Moal Reference Koob and Le Moal1997; West Reference West2006).
Drug habit memories refer to instrumental behavior that is no longer goal-directed but stimulus-controlled –a behavioral response that is triggered by a cue – but independent from its behavioral consequences (Robbins & Everitt Reference Robbins and Everitt1996). This type of memory plays an important role in the transition from controlled to compulsive drug use and addiction (Belin & Everitt Reference Belin and Everitt2008; Porrino et al. Reference Porrino, Lyons, Smith, Daunais and Nader2004) but may already play a big role in stimulus-driven drug instrumentalization in non-addicted drug users.
Procedural drug memories comprise all memories for skills involved in handling a drug. This may range from its production (e.g., cooking up heroin; building a joint) to the actual method of self-administration (e.g., snorting cocaine; setting a needle for an IV heroin injection).
Drug priming memories refer to those engrams whose activation by a small amount of the drug, which would not induce major subjective and behavioral effects in drug naïve individuals, may in an experienced user induce drug-related behavior (e.g., reinstate drug seeking, conditioned place preference, or self-administration) and subjective effects.
5.2. A two-stage model of drug instrumentalization learning
Based on interplay of different types of drug memories, we propose a two-stage model for drug instrumentalization learning. Because the crucial function of psychoactive drug-instrumentalization is to enhance efficacy of previously learned behaviors, a precondition for this model is an already established behavior that is reflected at the level of the brain, for example in stimulus-response or stimulus-action-outcome associations. In a drug-free state (Fig. 2A) a stimulus (S x ) activates in a particular environmental context, a particular action and an action-outcome representation in the brain. Both facilitate a behavioral response (R x ). The behavior R x yields an action outcome, which is perceived and processed. A comparator function weighs the expected outcome against the actually perceived outcome. The difference in expectation of outcome (ΔEo) serves as a teaching signal to strengthen or weaken the associations of the S x with an action representation and with an action outcome expectation. In constant environments with well-established S-R and stimulus-action-outcome associations, ΔEo will approach zero, indicating an optimal adjustment of action expectation to action outcome. For reasons of simplicity, it will at this stage not be differentiated between approach and consummatory behavior (Ikemoto & Panksepp Reference Ikemoto and Panksepp1999; Robinson & Berridge Reference Robinson and Berridge1993). Both could be considered in this model in a sequential order of approach and consumption.
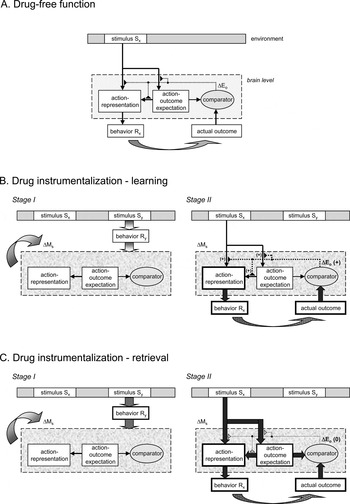
Figure 2. A two-stage model of drug instrumentalization based on the interplay of different drug memories. Drug instrumentalization is based on previously learned instrumental behavior reflected at the level of the brain in stimulus-response and stimulus-action-outcome associations.
During drug instrumentalization – learning (Fig. 2B), the organism learns how to change its mental state in a way that allows a more effective performance of an already established behavior. In Stage I, a specific stimulus (Sy) triggers a psychoactive drug-taking response (R y ). S y summarizes a number of possible and well-described scenarios that lead to initial drug taking, such as peer group pressure, as well as the information on the drug itself as part of the semantic drug memory. The consequence (action outcome) of Ry is an altered mental state of the organism (ΔMs), which is perceived by the organism. It depends in its nature on the pharmacological properties of the drug. The learning of the S y -R y -ΔMs association requires procedural-, instrumental conditioned-, as well as episodic drug memories. Although R y may initially be under control of the action outcome (ΔMs) and semantic memories, this control can shift with excessive repetition. R y then becomes S y -controlled, which involves drug habit memories. During Stage II, the already established instrumental response based on the S x -R x association is performed, but under the new mental state (ΔMs). If ΔMs proves to facilitate the ability of S x to activate the associated action-representation or the way in which the action-representation induces behavioral activity, the R x will be performed more efficiently. Hence, the action outcome is enhanced when the R x is performed under ΔMs. The comparator detects a positive difference between the action-outcome expectation, which was generated during a nondrugged mental state, and the actual outcome achieved under ΔMs. This difference (ΔEo) serves as a teaching signal that not only reinforces the association of S x with an action representation and its action-outcome expectation in Stage II, but also strengthens the association of S y with R y and its outcome ΔMs.
During drug instrumentalization – retrieval (Fig. 2C), an organism is using the learned information of how a self-generated, “on purpose” mental state change can facilitate a subsequent behavior and maximize its outcome. For that to occur, the presence of both stimuli, S y and S x , is required. In Stage I, S y triggers R y , which leads to ΔMs with the expectation to perform the instrumental response R x more efficiently. Once ΔMs is achieved, S x can now more efficiently activate the action and action-outcome representations and induce R x . The enhanced performance of R x under ΔMs is generating a better action-outcome than under a drug-free mental state. If environmental circumstances and ΔMs-enhanced action-outcome remain constant (i.e., no tolerance or sensitization develops), there will be no negative teaching signal ΔEo generated, and the associations of S y -R y -ΔMs and S x -R x are maintained.
6. Ontogeny of drug instrumentalization
6.1. Drug instrumentalization establishment during a lifetime
Although one can read about, and establish a factual knowledge on, the effects of drugs, more is learned during experimental consumption when a number of drug memories become established (Maloff et al. Reference Maloff, Becker, Fonaroff, Rodin, Zinberg and Harding1981). This usually starts during late childhood and early adolescence (Sher et al. Reference Sher, Grekin and Williams2005), when undifferentiated consumption develops into a highly specific pattern of consumption (Kuntsche et al. Reference Kuntsche, Knibbe, Gmel and Engels2006; Spear Reference Spear2000). Experimental consumption, in contrast to instrumentalization and compulsive consumption, refers to a consummatory behavior during which the consequences are initially mostly unknown to the individual. Although there are expectancies of the drug effects in drug naïve consumers (Brown Reference Brown1985; Brown et al. Reference Brown, Goldman, Inn and Anderson1980; Gustafson Reference Gustafson1991; Miller et al. Reference Miller, Smith and Goldman1990; Peele & Brodsky Reference Peele and Brodsky2000), the individual response profile after first consumption is virtually unpredictable (e.g., Jones Reference Jones1971; Waskow et al. Reference Waskow, Olsson, Salzman and Katz1970). During experimental consumption, the effects of a drug are explored at usually different doses and settings (Maloff et al. Reference Maloff, Becker, Fonaroff, Rodin, Zinberg and Harding1981; Patrick & Maggs Reference Patrick and Maggs2008). At the same time, there is also experimentation with how the drug's effects on mental states can be “used” in relation to different settings (Harding & Zinberg Reference Harding, Zinberg and Du Toit1977; Simons et al. Reference Simons, Correia and Carey2000; Zinberg Reference Zinberg1984). The perceived usefulness was found to predict future use of the drug (Boys & Marsden Reference Boys and Marsden2003; Boys et al. Reference Boys, Marsden, Griffiths, Fountain, Stillwell and Strang1999; Leigh & Stacy Reference Leigh and Stacy2004).
The introduction to the drug, appropriate settings, and possible instrumentalizations are usually performed by older and more experienced members of the peer group (e.g., Eissenberg & Balster Reference Eissenberg and Balster2000; Friedman et al. Reference Friedman, Lichtenstein and Biglan1985). However, given interindividual differences in drug pharmacokinetics and -dynamics, in personality, and in life circumstances, each person customizes his or her drug use. In fact, the individual learns about which mental states the drug can induce at different doses and how this new mental state can be used, as well as how to control consumption (Bruehl et al. Reference Bruehl, Lende, Schwartz, Sterk and Elifson2006). For an “optimal” drug instrumentalization that yields the greatest benefits, a well-controlled consumption may be established in relation to the following parameters: goals for instrumentalization, appropriate type of drug, appropriate dose of this drug, and setting for consumption. A systematic drug use can thus become an integral part of a person's life within a socially acceptable range of behaviors (Davies Reference Davies1997; Heath Reference Heath2000; Waldorf et al. Reference Waldorf, Reinarman and Murphy1991; Zinberg et al. Reference Zinberg, Harding, Stelmack and Marblestone1978).
6.2. Benefits versus adverse effects of psychoactive drug use
Although we argue for evolutionary benefits of non-addictive drug use here, we also must emphasize that the instrumentalization of psychoactive drugs comes at a price, which ultimately qualifies it as a risky behavior (Donovan & Richard Reference Donovan and Richard1985; Hill & Chow Reference Hill and Chow2002). Severe damage to the brain and body peripheral organs have been documented, for example, from alcohol (Harper Reference Harper2007; Parsons Reference Parsons1998; Ward et al. Reference Ward, Lallemand and de2009), MDMA (Gouzoulis-Mayfrank & Daumann Reference Gouzoulis-Mayfrank and Daumann2009; Seiden & Sabol Reference Seiden and Sabol1996); nicotine (Ray et al. Reference Ray, Schnoll and Lerman2009), androgenic-anabolic steroids (Wood Reference Wood2004), psychostimulants (Pascual-Leone et al. Reference Pascual-Leone, Dhuna and Anderson1991; Volkow et al. Reference Volkow, Hitzemann, Wang, Fowler, Wolf, Dewey and Handlesman1992), and cannabis (Solowij Reference Solowij1998). Many psychoactive drugs enhance preexisting psychopathologies in vulnerable individuals (e.g., Andreasson et al. Reference Andreasson, Allebeck, Engstrom and Rydberg1987; Reference Andreasson, Allebeck and Rydberg1989; Negrete Reference Negrete, Nahas and Latour1993).
We argue here that in the majority of nonaddicted psychoactive drug users, there are beneficial effects on self-maintenance or reproduction rate – or both – from drug use in the way that established instrumentalization may in particular at a younger age outweigh the drug-induced decline in health later in life (Crawford Reference Crawford2000; Lende Reference Lende, Wenda Trevathan, Smith and McKenna2007; Peele & Brodsky Reference Peele and Brodsky2000). As such, the behavior of psychotropic drug instrumentalization may have a heritable component (Schumann Reference Schumann2007) that is maintained in an antagonistic pleiotropy (Williams Reference Williams1957). This view is supported by life history research, which shows that risky alcohol consumption peaks at an age of 18–19 (males) and 16–17 (females) and declines thereafter (Hill & Chow Reference Hill and Chow2002; Jessor Reference Jessor1987; Johnstone et al. Reference Johnstone, Leino, Ager, Ferrer and Fillmore1996). The peak occurs at a stage of high reproductive efforts. In particular, mating efforts (locating mate, courtship) are high (Chisholm Reference Chisholm1993). Prior to marriage and continuing thereafter, risky alcohol consumption goes down (Leonard & Das Eiden Reference Leonard and Das Eiden1999; Miller-Tutzauer et al. Reference Miller-Tutzauer, Leonard and Windle1991) and many pregnant women give up drinking (Nilsen et al. Reference Nilsen, Holmqvist, Hultgren, Bendtsen and Cedergren2008). At that stage, parenting efforts usually increase (Chisholm Reference Chisholm1993), which are not supported but rather diminished by psychoactive drug effects. We suggest that because individual resource allocations change during life history, so do instrumentalization goals and, ultimately, non-addictive psychoactive drug consumption (Heyman Reference Heyman1996; Hill & Chow Reference Hill and Chow2002).
The major downside of drug instrumentalization, which as yet has prevented the scientific community from acknowledging any adaptational function or effect of drug taking behavior, is the risk of developing drug addiction (Nutt et al. Reference Nutt, King, Saulsbury and Blakemore2007). People who use and instrumentalize drugs are at a higher risk to develop drug addiction as a psychiatric disorder (American Psychiatric Association 1994) than are drug abstainers (Kendler et al. Reference Kendler, Prescott, Myers and Neale2003a). A striking feature of the definition of addiction is the compulsive seeking and consumption of the drug, which is no longer a controlled, goal-directed instrumentalization. Drug addiction is usually associated with an escalating consumption of large amounts of the drug. This significantly increases the impact of any toxic drug effects and directly leads every year to drug-related fatalities (e.g., EMCDDA 2009).
In this case, the adverse drug effects by far outweigh the possible use of any instrumentalization. Natural goals are devalued in the course of addiction development (Redish et al. Reference Redish, Jensen and Johnson2008). It is highly unlikely that this form of drug consumption might have any adaptational function at all. It has been argued that even drug addiction may be an adaptation to cope with “integration failure” (Alexander Reference Alexander1987; Reference Alexander1990). This view highlights the important role of adaptation problems at the personal level for the continuation of psychoactive drug consumption. The first steps of later-stage addicts are indeed to establish non-addictive drug consumption during which they usually instrumentalize the drug effects in the above-described way. Eventually, the capacity of a drug for instrumentalization is exceeded, and no further improvement of non–drug related behavior can be achieved. Then, psychoactive drug use may not help to solve an individual adaptation problem, but, by its toxic side effects, impairs the organism. Altogether, the instrumentalization of psychotropic drugs may have adaptational effects only in non-addicts, being no longer beneficial when the individual loses control and develops drug addiction.
7. Possible implications for drug policy
Epidemiological, ethological, social, psychological, genetic, and psychiatric data on drug addiction are shaping a number of scientific theories, which often have a different focus (West Reference West2006). A major criterion for the quality of a theory is not only how well it integrates empirical evidence and gray literature into a logical framework, but also the quality of its predictions. In terms of theories about drug use and addiction, these criteria might be rephrased as: (1) how well does the framework explain psychoactive drug use in drug addicts and in non-addicts, and (2) how effective are the predictions derived from the framework to avoid adverse effects of drug taking behavior, such as addiction, and treat these effects once they occur. The first criterion is targeted by the presented framework from an evolutionary perspective, suggesting an adaptive function of non-addictive drug use, but stressing reduced chances of survival and reproduction in drug addiction. What can be derived to serve the second criterion when dealing with drug instrumentalization? The proposed concept of non-addictive drug instrumentalization might refine the way in which to deal with psychoactive drugs. As an extension to previous prevention programs that were limited in their success (Brown & Kreft Reference Brown and Kreft1998), these approaches might be tailored to the different stages of the potential and actual drug user's development and a “successful” drug instrumentalization:
-
A. For drug-naïve individuals, who are usually in the adolescent to early adult age, information should be systematically provided not only on the adverse effects of addiction and accidental overdosing, but also on how drugs are instrumentalized. Although this might not prevent the initial experimentation with available drugs, it might change attitudes when it comes to the establishment of regular intake patterns. The goal should not be to prevent drug use in general, but to foster control over it by the individual from early stages of use with a better awareness of the full range of the behavioral mechanisms involved.
-
B. For people who have already integrated drugs in their routines, it is important to emphasize the need to stay in control of drug use. In particular, during periods of transition in life when new demands occur, there is an increased danger of developing new forms of drug instrumentalization. One of these periods is adolescence (NatCent/NFER 2006). Humans appear to be especially sensitive to drug effects during adolescence (Spear Reference Spear2000). In this phase of life, many new demands occur that are prone to drug instrumentalization, such as sexual maturation, peer group pressure, socialization with the opposite sex, and increased cognitive demands at school and work. This is also the first time in life when several drugs become accessible and experimental consumption starts. But this is not the only transition period with an increase in demands. Later in life, when, for example, professional development settles at a high level and performance needs to be maintained at that level, stress load almost certainly increases. Periods of transition should be a major focus when prevention strategies for drug instrumentalization are considered. We argue that psychotropic drug use can have beneficial effects for an individual in modern environments. Nevertheless, one should attempt to limit the extent to which psychotropic drugs are instrumentalized. This can be done by a systematic analysis of the personal instrumentalization pattern, asking, for example, which drugs are instrumentalized, how often, and for which goals. Possibly, educational programs should aim to train young people to self-analyze their drug instrumentalization and seek help when coming to the conclusion that certain goals in life cannot be achieved without extensive psychotropic drug use.
The occurrence of drug instrumentalization can be seen as an indicator that particular goals are not (or are less easily) achieved by “natural means.” Once these goals are identified, particular training may be sought to focus on them and to reduce the extent the drug is used for each goal. Analysis and training could be provided at a personal level or, if an instrumentalization pattern prevails in a particular group, also at family, school, or community levels. People might be trained from early age to learn how to control mental states without pharmacological means (i.e., by own mental resource management, stress-recovery management, relaxation training, controlled disinhibition and approach in social settings).
-
C. For people who have integrated regular drug use in their life's routines and who are at risk of a transition to drug addiction, over-instrumentalization of drugs and, hence, a dependence on the drug to achieve major goals in life, needs to be prevented. Also at this stage, a careful analysis of personal instrumentalization patterns and how they developed over time is required. An initial control for that should be part of a medical routine screen with the intention of preventing over-instrumentalization at an early age. It might be a useful refinement in medical interviews not only to ask for the amount of a drug consumed, but also to identify instrumentalization patterns and the degree the drug is required for achieving major personal goals in life. A more careful investigation might also include a drug-instrumentalization biography and a detailed assessment of the types and intensity of drug memories already established (Orford Reference Orford2008; Redish et al. Reference Redish, Jensen and Johnson2008).
Based on that, alternative ways to achieve these goals may need to be identified and intensely trained. With the advent of a more individualized addiction treatment strategy (Conrod et al. Reference Conrod, Pihl, Stewart and Dongier2000; Reference Conrod, Stewart, Comeau and Maclean2006), factors like personality traits and genetic predictors may be useful identifying individuals who are prone to having problems achieving critical goals in life and who might be at greater risk of losing control over drug instrumentalization (Goldman et al. Reference Goldman, Oroszi and Ducci2005; Kreek et al. Reference Kreek, Nielsen, Butelman and Laforge2005; Spanagel et al. Reference Spanagel, Pendyala, Abarca, Zghoul, Sanchis-Segura, Magnone, Lascorz, Depner, Holzberg, Soyka, Schreiber, Matsuda, Lathrop, Schumann and Albrecht2005; Woicik et al. Reference Woicik, Stewart, Pihl and Conrod2009). However, environmental factors and their interactions with genetic factors might also serve as biomarkers for an increased risk of drug instrumentalization and over-instrumentalization (Blomeyer et al. Reference Blomeyer, Treutlein, Esser, Schmidt, Schumann and Laucht2008; Kendler et al. Reference Kendler, Jacobson, Prescott and Neale2003b; Sher et al. Reference Sher, Grekin and Williams2005).
8. Testing drug instrumentalization empirically
Whether drug instrumentalization can serve as a framework to explain large-scale, non-addictive psychoactive drug consumption in animals and humans needs to be tested empirically. Assumptions, as well as predictions, would require testing. At the level of the assumptions, testing the different types of drug memories and their establishment in the life-course is suggested. This may be done by modifying respective paradigms from non-drug memory assessment. To test predictions in humans, it would be crucial to identify instrumentalization profiles for individual drug users and evaluate the extent of drug-specific instrumentalization in the population of users. Because the full extent of the mechanisms of drug instrumentalization (e.g., non-declarative memory-based behaviors) is not consciously accessible to the user, simple self-reports may not be sufficient. Structured interviews or specifically designed questionnaires, which probe for the individual's relationship with the drugs consumed, with all potential instrumentalization goals, may be more adequate.
At the mechanistic level, animal models of drug instrumentalization may be useful for testing the origin of the behavior and its neurophysiological prerequisites. In contrast to present animal models of drug self-administration (Olmstead Reference Olmstead2006; Sanchis-Segura & Spanagel Reference Sanchis-Segura and Spanagel2006), animals should be given the chance to instrumentalize the drug self-administration behavior to enhance their performance in non–drug related behaviors. It may also be interesting to investigate in ethological studies of drug consumption in animals, whether related behaviors can be identified whose performance is enhanced in a functional way by the pharmacological effects of the psychotropic drug (e.g., Wiens et al. Reference Wiens, Zitzmann, Lachance, Yegles, Pragst, Wurst, von Holst, Guan and Spanagel2008).
9. Summary
Non-addictive psychoactive drug use appears to be much more common than drug addiction in humans around the globe. Although drug addiction as a psychiatric disease results in severe adverse effects on individuals and societies, non-addictive drug use is chosen for its positive effects. We have argued that non-addictive drug use may have a number of beneficial effects on behaviors relevant for survival and reproduction, which may explain the persistence of drug use in human societies. The basic mechanisms establishing non-addictive psychoactive drug use may have arisen in ancient environments, coming to full expression under more recent environmental changes. The key psychological argument is that drugs are used because their psychoactive effects can be instrumentalized. Drug instrumentalization is defined here as a learned behavior to change one's own mental state by consuming a psychoactive drug. Subsequently, this altered mental state allows the more effective pursuit of central survival- and reproduction-relevant goals. A better understanding of the mechanisms of psychotropic drug use in non-addicts might serve to better prevent the transition to drug addiction in the future.
ACKNOWLEDGMENTS
We thank Dr. Cathy Fernandes, Miss Alanna Easton, and the anonymous reviewers for their constructive comments on the manuscript. We also acknowledge the helpful editorial advice from Dr. Barbara Finlay. This work was supported by the UK Department of Health NIHR Biomedical Centre for Mental Health and the MRC-SGDP Centre at the Institute of Psychiatry, King's College London (UK) and funds of the University of Erlangen-Nuremberg (Germany).





Target article
Drug instrumentalization and evolution: Going even further
Related commentaries (1)
Drugs as instruments: A new framework for non-addictive psychoactive drug use