Introduction
There are numerous minerals with the ![]() ${M}_{\rm 2}^{\rm I} $M IIM IVS4 composition that are derived from the sphalerite structure by ordered substitution of Zn. These are divided into the stannite group (space group I
${M}_{\rm 2}^{\rm I} $M IIM IVS4 composition that are derived from the sphalerite structure by ordered substitution of Zn. These are divided into the stannite group (space group I ![]() $\bar{4}$2m) with briartite, Cu2(Fe,Zn)GeS4 (Wintenberger Reference Wintenberger1979, synthetic), černýite, Cu2CdSnS4 (Szymanski et al., Reference Szymanski1978), stannite, Cu2FeSnS4 (Hall et al., Reference Hall, Szymanski and Stewart1978) and velikite, Cu2HgSnS4 (Kaplunik et al., Reference Kaplunnik, Pobedimskaya and Belov1977), and the kësterite subgroup (space group I
$\bar{4}$2m) with briartite, Cu2(Fe,Zn)GeS4 (Wintenberger Reference Wintenberger1979, synthetic), černýite, Cu2CdSnS4 (Szymanski et al., Reference Szymanski1978), stannite, Cu2FeSnS4 (Hall et al., Reference Hall, Szymanski and Stewart1978) and velikite, Cu2HgSnS4 (Kaplunik et al., Reference Kaplunnik, Pobedimskaya and Belov1977), and the kësterite subgroup (space group I ![]() $\bar{4}$) with ferrokësterite, Cu2FeSnS4 (Kissin and Owens, Reference Kissin and Owens1989), hocartite, Ag2FeSnS4 (Caye et al., Reference Caye, Laurent, Picot, Pierrot and Levy1968, structure not solved), kësterite, Cu2ZnSnS4 (Hall et al., Reference Hall, Szymanski and Stewart1978), pirquitasite, Ag2ZnSnS4 (Schumer et al., Reference Schumer, Downs, Domanik, Andrade and Origlieri2013) and zincobriartite, Cu2(Zn,Fe)(Ge,Ga)S4 (McDonald et al., Reference McDonald, Stanley, Ross and Nestola2016). The two subgroups have two distinct but closely related patterns of ordered substitution of the Zn atoms in sphalerite. In the stannite-subgroup minerals, (001) layers of Zn atoms are substituted by layers of Cu atoms which alternate with layers in which Zn atoms are substituted by ordered (M II+M IV) atoms, whereas, in the kësterite subgroup, (001) layers of Zn atoms are substituted by layers of ordered (M I+M II) atoms which alternate with layers in which Zn atoms are substituted by ordered (M I+M IV) atoms. Iron is the only M II case for which both types of structures have been found as minerals (stannite/ferrokësterite). There are closely related minerals
$\bar{4}$) with ferrokësterite, Cu2FeSnS4 (Kissin and Owens, Reference Kissin and Owens1989), hocartite, Ag2FeSnS4 (Caye et al., Reference Caye, Laurent, Picot, Pierrot and Levy1968, structure not solved), kësterite, Cu2ZnSnS4 (Hall et al., Reference Hall, Szymanski and Stewart1978), pirquitasite, Ag2ZnSnS4 (Schumer et al., Reference Schumer, Downs, Domanik, Andrade and Origlieri2013) and zincobriartite, Cu2(Zn,Fe)(Ge,Ga)S4 (McDonald et al., Reference McDonald, Stanley, Ross and Nestola2016). The two subgroups have two distinct but closely related patterns of ordered substitution of the Zn atoms in sphalerite. In the stannite-subgroup minerals, (001) layers of Zn atoms are substituted by layers of Cu atoms which alternate with layers in which Zn atoms are substituted by ordered (M II+M IV) atoms, whereas, in the kësterite subgroup, (001) layers of Zn atoms are substituted by layers of ordered (M I+M II) atoms which alternate with layers in which Zn atoms are substituted by ordered (M I+M IV) atoms. Iron is the only M II case for which both types of structures have been found as minerals (stannite/ferrokësterite). There are closely related minerals ![]() ${M}_{\rm 2}^{\rm I} $M IM VS4, which include luzonite Cu2CuAsS4 (Marumo et al., Reference Marumo and Nowacki1967), famatinite Cu2CuSbS4 (Garin and Parthé, Reference Garin and Parthé1972, synthetic), and keutschite Cu2AgAsS4 (Topa et al., Reference Topa, Fredrickson and Stanley2014). Some uncertainty remains with respect to the structures of other
${M}_{\rm 2}^{\rm I} $M IM VS4, which include luzonite Cu2CuAsS4 (Marumo et al., Reference Marumo and Nowacki1967), famatinite Cu2CuSbS4 (Garin and Parthé, Reference Garin and Parthé1972, synthetic), and keutschite Cu2AgAsS4 (Topa et al., Reference Topa, Fredrickson and Stanley2014). Some uncertainty remains with respect to the structures of other ![]() ${M}_{\rm 2}^{\rm I} $M IIM IVS4 minerals, as the structure of barquillite Cu2CdGeS4 has not been determined using single-crystal methods (Murciego et al., Reference Murciego, Pascua, Babkine, Dusausoy, Medenbach and Bernhardt1999) for the natural occurrence, and synthetic Cu2CdGeS4 has a wurtzite/enargite derived structure related to agmantinite (Parthé et al., Reference Parthé, Yvon and Deitch1969). Uncertainty also remains with respect to the structures of kuramite and petrukite. Synthetic compounds with
${M}_{\rm 2}^{\rm I} $M IIM IVS4 minerals, as the structure of barquillite Cu2CdGeS4 has not been determined using single-crystal methods (Murciego et al., Reference Murciego, Pascua, Babkine, Dusausoy, Medenbach and Bernhardt1999) for the natural occurrence, and synthetic Cu2CdGeS4 has a wurtzite/enargite derived structure related to agmantinite (Parthé et al., Reference Parthé, Yvon and Deitch1969). Uncertainty also remains with respect to the structures of kuramite and petrukite. Synthetic compounds with ![]() ${M}_{\rm 2}^{\rm I} $M IIM IVS4 composition derived from the wurtzite ZnS structure are known, including Cu2MnGeS4 and Cu2MnSiS4 (Bernert and Pfitzner, Reference Bernert and Pfitzner2005), but no such cases have been reported previously for minerals. The only mineral of
${M}_{\rm 2}^{\rm I} $M IIM IVS4 composition derived from the wurtzite ZnS structure are known, including Cu2MnGeS4 and Cu2MnSiS4 (Bernert and Pfitzner, Reference Bernert and Pfitzner2005), but no such cases have been reported previously for minerals. The only mineral of ![]() ${M}_{\rm 2}^{\rm I} $M IM VS4 type derived via ordered substitution of Zn in wurtzite is enargite, Cu2CuAsS4 (Karanovic et al., Reference Karanovic, Cvetkovic, Balić-Žunić and Makovicky2002), a dimorph of luzonite.
${M}_{\rm 2}^{\rm I} $M IM VS4 type derived via ordered substitution of Zn in wurtzite is enargite, Cu2CuAsS4 (Karanovic et al., Reference Karanovic, Cvetkovic, Balić-Žunić and Makovicky2002), a dimorph of luzonite.
In this paper we report occurrence, physical properties and crystal structure of agmantinite (ideally, Ag2MnSnS4), which is the first mineral with ![]() ${M}_{\rm 2}^{\rm I} $M IIM IVS4 composition derived from the wurtzite ZnS structure. It was found in one sample from the alabandite zone of the Uchucchacua polymetallic deposit, Oyon district, Lima Department, Peru. The mineral was named for its composition and the new mineral and mineral name have been approved by the International Mineralogical Association Commission on New Minerals, Nomenclature and Classification (IMA2014-083, Keutsch et al., Reference Keutsch, Topa, Takagi Fredrickson, Makovicky and Paar2015). Holotype material is deposited in the reference collection of the Naturhistorisches Museum Wien, Wien, Austria, specimen number N 9736.
${M}_{\rm 2}^{\rm I} $M IIM IVS4 composition derived from the wurtzite ZnS structure. It was found in one sample from the alabandite zone of the Uchucchacua polymetallic deposit, Oyon district, Lima Department, Peru. The mineral was named for its composition and the new mineral and mineral name have been approved by the International Mineralogical Association Commission on New Minerals, Nomenclature and Classification (IMA2014-083, Keutsch et al., Reference Keutsch, Topa, Takagi Fredrickson, Makovicky and Paar2015). Holotype material is deposited in the reference collection of the Naturhistorisches Museum Wien, Wien, Austria, specimen number N 9736.
Occurrence and physical properties
The sample with agmantinite was found during a study of the mineralogy of the Uchucchacua polymetallic deposit that focuses on a zone with high Ag and Mn content. This zone is characterised by the occurrence of alabandite and silver minerals. Uchucchacua is the type locality for six Mn-bearing sulfides/sulfosalts: uchucchacuaite, AgPb3MnSb5S12 (Moëlo et al., Reference Moëlo, Oudin, Picot and Caye1984); benavidesite, Pb4MnSb6S14 (Oudin et al., Reference Oudin, Picot, Pillard, Moëlo, Burke and Zakrzewski1982); manganoquadratite, AgMnAsS3 (Bonazzi et al., Reference Bonazzi, Keutsch and Bindi2012); menchettiite, AgPb2.40Mn1.60Sb3As2S12 (Bindi et al., Reference Bindi, Keutsch and Bonazzi2012); oyonite, Ag3Mn2Pb4Sb7As4S24 (Bindi et al., Reference Bindi, Biagioni and Keutsch2018); agmantinite, Ag2MnSnS4 (this work); and including keutschite, Cu2AgAsS4 (Topa et al., Reference Topa, Fredrickson and Stanley2014) and spryite, Ag8(As3+, As5+)S6 (Bindi et al., Reference Bindi, Keutsch, Morana and Zaccarini2017). It is the type locality of seven silver minerals, as well as being the type locality of hyršlite, Pb8As10Sb6S32 (Keutsch et al., Reference Keutsch, Topa and Makovicky2017). Geological and metallogenetic data concerning the Uchucchacua deposit district have been reported by Oudin et al. (Reference Oudin, Picot, Pillard, Moëlo, Burke and Zakrzewski1982).
The sample with agmantinite consists mainly of calcite and quartz with associated minor manganoquadratite, alabandite, proustite, probable kutnohorite, sphalerite and Pb–Sb–As–S minerals. Agmantinite occurs as extremely rare translucent, orange–red, flattened, free-standing crystals rarely reaching 100 μm on small quartz and calcite crystals with small manganoquadratite crystals as the only other minerals in direct association (Fig. 1). It is the least common mineral discovered in the course of the study of the alabandite zone in Uchucchacua, and despite exhaustive searches only one sample with two groups of small crystals has been discovered.

Fig. 1. Photograph of part of the type specimen showing agmantinite crystals on quartz and manganoan calcite. The typical orange–red colour and habit can be seen in the two main crystals, which are viewed from two different directions. The size of the largest crystal is 100 μm. Naturhistorisches Museum Wien, specimen number N 9736.
Agmantinite is orange–red in colour and has a red streak. The mineral is translucent with adamantine lustre. It is brittle. No fracture or cleavage was observed in the material available. Due to the small crystal size the density could not be measured. Based on the empirical formula the calculated density is 4.574 g/cm3. The microhardness could not be determined. On the basis of chemically similar compounds the Mohs hardness is estimated to be in the range of 2 to 2½.
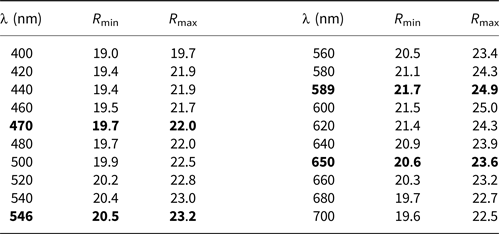
In plane-polarised light, agmantinite is greyish white with no discernible pleochroism and weak bireflectance. With crossed polars, the mineral is weakly anisotropic (air) in reddish brown to greenish rotation tints. Agmantinite has red internal reflections. Reflectance measurements were made within the visible spectrum (400–700 nm) at intervals of 20 nm using a Leitz MPV-SP microscope-spectrophotometer. A WTiC reflectance standard (Zeiss 314) was used as the reference. The measurements were performed on an untwined grain with a ×50 objective, the effective numerical apertures of which were confined to 0.28, and the diameters of the measured discs were 10 μm. The results are presented in Table 1. The spectra of agmantinite are unique and cannot be compared to members of the stannite and kësterite subgroups.
Table 1. Reflectance data for agmantinite

The reference wavelengths required by the Commission on Ore Mineralogy (COM) are given in bold.
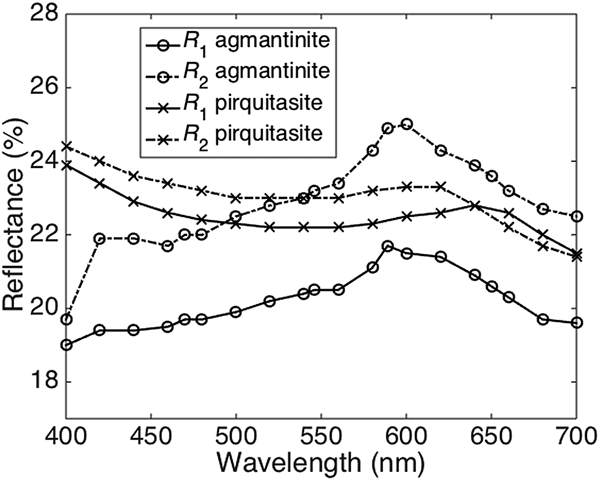
The reflectance spectra obtained for agmantinite and compared to those of pirquitasite, Ag2ZnSnS4 (Johan and Picot, Reference Johan and Picot1982) is shown in Fig. 2. There is an obvious increase of R of agmantinite up to a maximum at 600 nm whereas R of pirquitasite decreases. In the region after R = 600 nm the behaviour of both minerals is similar (reflectance decreases). At 600 nm the bireflectance of agmantinite is twice as large as for pirquitasite.
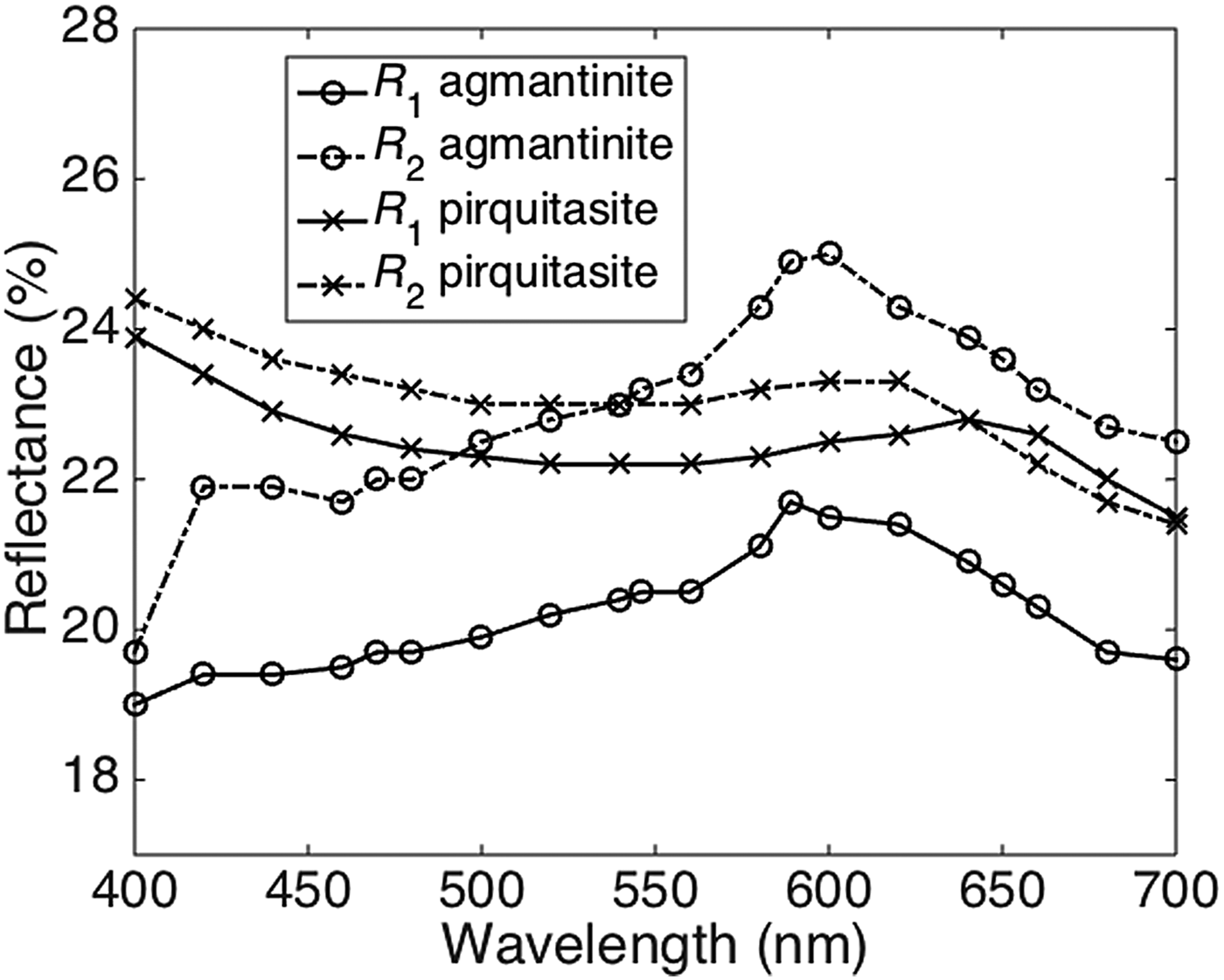
Fig. 2. Reflectance spectra (air) for agmantinite and pirquitasite (Johan and Picot Reference Johan and Picot1982).
X-ray crystallography and crystal-structure determination
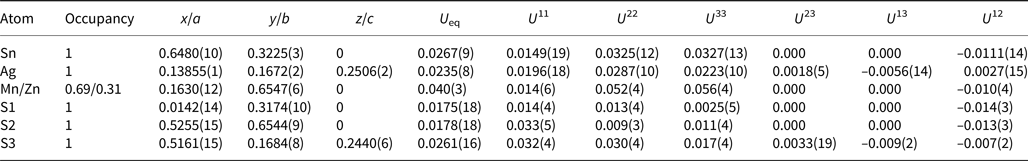
A crystal fragment of agmantinite with irregular shape and 0.05 mm × 0.04 mm × 0.03 mm in size was selected from the rock sample and examined by means of a Bruker AXS three-circle diffractometer equipped with a CCD area detector using graphite-monochromatised MoKα radiation. The SMART (Bruker AXS, 1998a) system of programs was used for unit-cell determination and data collection, SAINT+ (Bruker AXS, 1998b) for the reduction of the intensity data, and XPREP (Bruker AXS, 1997) for space-group determination and empirical absorption correction based on pseudo ψ-scans. The non-centrosymmetric space group P21nm proposed by the XPREP program was chosen. The structure of agmantinite was solved by direct methods (Sheldrick, Reference Sheldrick1997a), which revealed most of the atom positions. In subsequent cycles of the refinement (Sheldrick, Reference Sheldrick1997b), remaining atom positions were deduced from difference-Fourier syntheses by selecting from among the strongest maxima at appropriate distances. The structure of agmantinite contains one Ag site, one Sn site, one mixed Mn(Zn) site and three S sites. Atom coordinates and anisotropic displacement parameters are given in Table 2, bond lengths and bond angles in Table 3. The amount of Zn in the mixed site (occupancy value) is higher than in the empirical chemical formula, indicating a possible variation of the Mn/Zn ratio from grain to grain. Crystal data and a summary of parameters describing data collection and refinement for agmantinite are given in Table 4.
Table 2. Final atom coordinates and U eq values (Å2) and anisotropic displacement parameters (Å2).
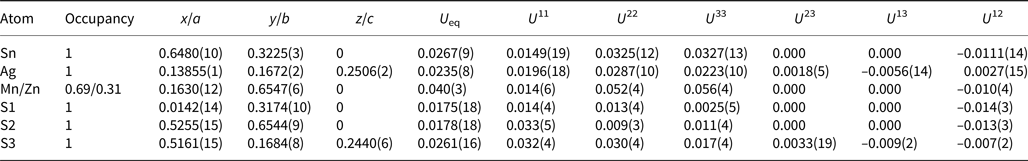
Table 3. Selected bond lengths (Å) and angles (°) in agmantinite.

Table 4. Details of data collection and refinement.
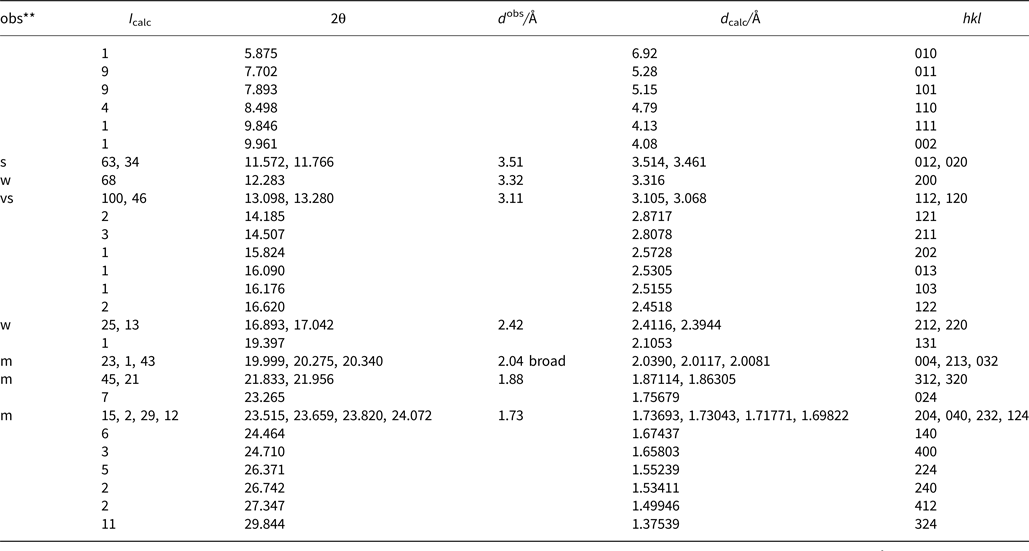
Powder X-ray diffraction data were derived from measurements of the same single-crystal specimen on an Oxford Diffraction Xcalibur E diffractometer using graphite-monochromatised MoKα radiation (λ = 0.7107 Å) at ambient temperature. Observed intensities were added for different 2θ values and d spacings were calculated from these. The data obtained had broad peaks (full width at half maximum ~0.08) but showed good consistency with the intensities and d spacings calculated using Jade9 (v9.5.0, MDI Materials Data, California, USA, 2012) software on the basis of the structural model (see below); only calculated reflections with I > 1 are reported (if not observed). Observed intensities were estimated visually; vs = very strong; s = strong; m = medium; w = weak. Data (in Å for MoKα) are listed in Table 5.
Table 5. Measured and calculated powder X-ray diffraction data*.
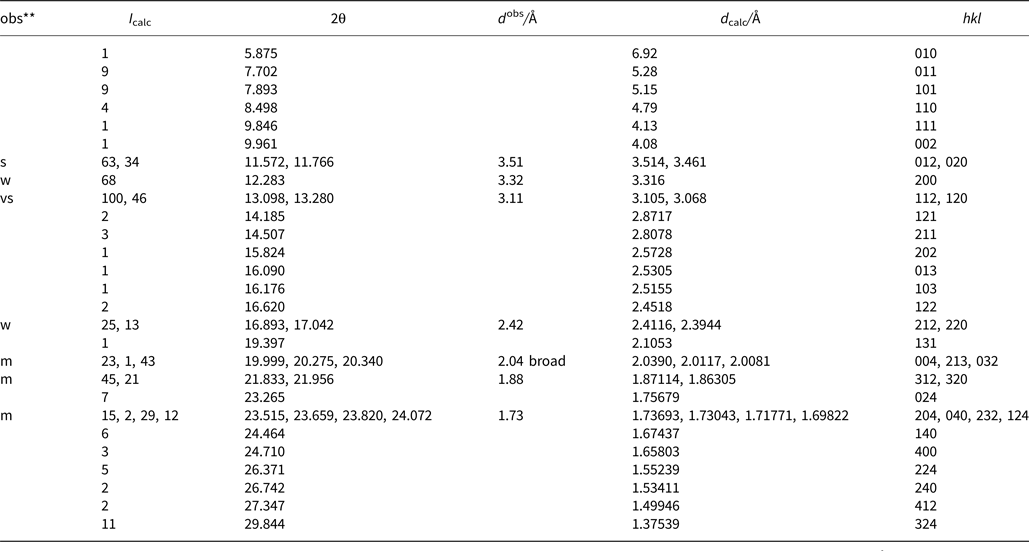
* The theoretical pattern was calculated with PowderCell 2.3 software (Kraus and Nolze, Reference Kraus and Nolze1999) in Debye-Scherrer configuration with MoKα radiation (λ = 0.7107 Å), a fixed slit, and no anomalous dispersion. Cell parameters, space group, atom positions, site-occupancy factors and isotropic displacement factors from the crystal-structure determination were used.
** s = very strong; m = medium; w = weak
Chemical composition
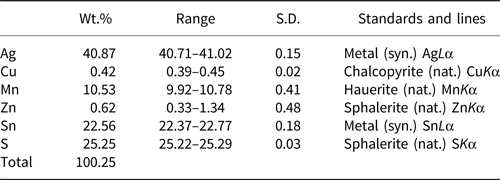
The chemical composition of an agmantinite grain (Fig. 3) was determined using wavelength dispersive analysis (WDS) by a JEOL Hyperprobe JXA-8530F field-emission electron microprobe, installed at Natural History Museum, Vienna, Austria (25 kV, 20 nA, beam size = 2 μm); standards used (element): pure metal (Ag); chalcopyrite (Cu); hauerite (Mn); sphalerite (Zn); pure metal (Sn); and sphalerite (S). No other elements with atomic number >11 were detected. The chemical compositions (four analyses on one grain) are reported in Table 6. The chemical formula, (Ag1.94Cu0.03)Σ1.97(Mn0.98Zn0.05)Σ1.03Sn0.97S4.03, was calculated on the basis of 8 atoms. The ideal formula is Ag2MnSnS4.

Fig. 3. Secondary electron image of an agmantinite grain and its optical image under crossed polars. Scale bar = 10 μm.
Table 6. Analytical data for agmantinite.

S.D. = standard deviation.
Description of the structure and discussion
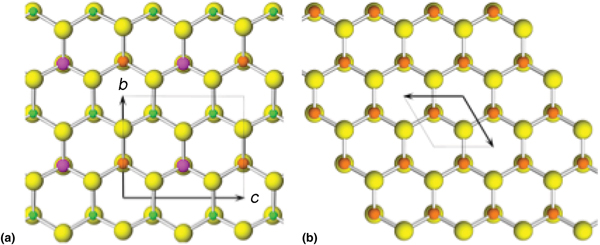
The crystal structure of agmantinite (Fig. 4) is derived from that of wurtzite by ordered substitution of Zn. This substitution pattern is analogous to that for deriving stannite from sphalerite. In stannite (001) layers of sphalerite Zn atoms are substituted by layers of Cu atoms alternating with layers in which Zn atoms are substituted by ordered (Fe + Sn) atoms [in contrast to kësterite, in which (001) layers of Zn atoms are substituted by layers of ordered (Cu + Zn/Fe) which alternate with layers of Zn atoms that are substituted by ordered (Cu + Sn)]. Analogously to stannite, in agmantinite, layers of Zn atoms are substituted by layers of Ag atoms in alternation with layers in which Zn atoms are substituted by ordered (Sn + Mn/Zn) atoms, but starting with the wurtzite ZnS structure rather than the sphalerite one. These substitutions proceed on alternating (001) cation levels. The substitution results in an orthorhombic unit cell and an increase of unit-cell dimensions compared to the corresponding dimensions in wurtzite. The corresponding dimensions in wurtzite are a = 6.188, b = 6.542 and c = 7.554 Å, whereas those in agmantinite are a = 6.632, b = 6.922 and c = 8.156 Å. Although not obvious from Fig. 4, Ag tetrahedra are situated on (001) planes at about ¼ and ¾ c; Ag–S distances are 2.50 Å along the a direction whereas they appear to adjust to the differences in atomic radii of Mn2+ and Sn4+ in the (100) plane: 2.44 Å towards S bound to Sn in the adjacent [100] Sn–Mn populated columns (in the (001) plane, Sn–S bonds are equal to 2 × 2.42 Å and 2.44 Å) and 2.50 Å towards Mn in these columns (in this plane Mn–S bonds are equal to 2 × 2.61 Å and 2.54 Å). The remaining Ag–S bond, subparallel to the b direction, towards S in the adjacent Ag populated [100] column, is 2.46 Å, a compromise between similarly oriented 2.54 Å and 2.44 Å bonds of Mn and Sn with S, respectively. Along a, Sn4+ preserves a regular tetrahedral coordination, with the Sn–S distance equal to 2.43 Å, whereas Mn has a short distance of 2.40 Å and Ag the longest one, 2.50 Å; they display small distortions of their tetrahedral coordination, but with the opposite sense. Although ![]() ${M}_{\rm 2}^{\rm I} $M IIM IVS4 compounds are common minerals, the structure of agmantinite is the first example of a
${M}_{\rm 2}^{\rm I} $M IIM IVS4 compounds are common minerals, the structure of agmantinite is the first example of a ![]() ${M}_{\rm 2}^{\rm I} $M IIM IVS4 type mineral that is derived from the wurtzite structure and not sphalerite. However, synthetic
${M}_{\rm 2}^{\rm I} $M IIM IVS4 type mineral that is derived from the wurtzite structure and not sphalerite. However, synthetic ![]() ${M}_{\rm 2}^{\rm I} $M IIM IVS4 phases derived from the wurtzite structure have been described, including those of Cu2MnGeS4 and Cu2MnSiS4 (Bernet and Pfitzner, 2005), and enargite, Cu2CuAsS4, of
${M}_{\rm 2}^{\rm I} $M IIM IVS4 phases derived from the wurtzite structure have been described, including those of Cu2MnGeS4 and Cu2MnSiS4 (Bernet and Pfitzner, 2005), and enargite, Cu2CuAsS4, of ![]() ${M}_{\rm 2}^{\rm I} $M IM VS4 type is derived from the wurtzite structure.
${M}_{\rm 2}^{\rm I} $M IM VS4 type is derived from the wurtzite structure.
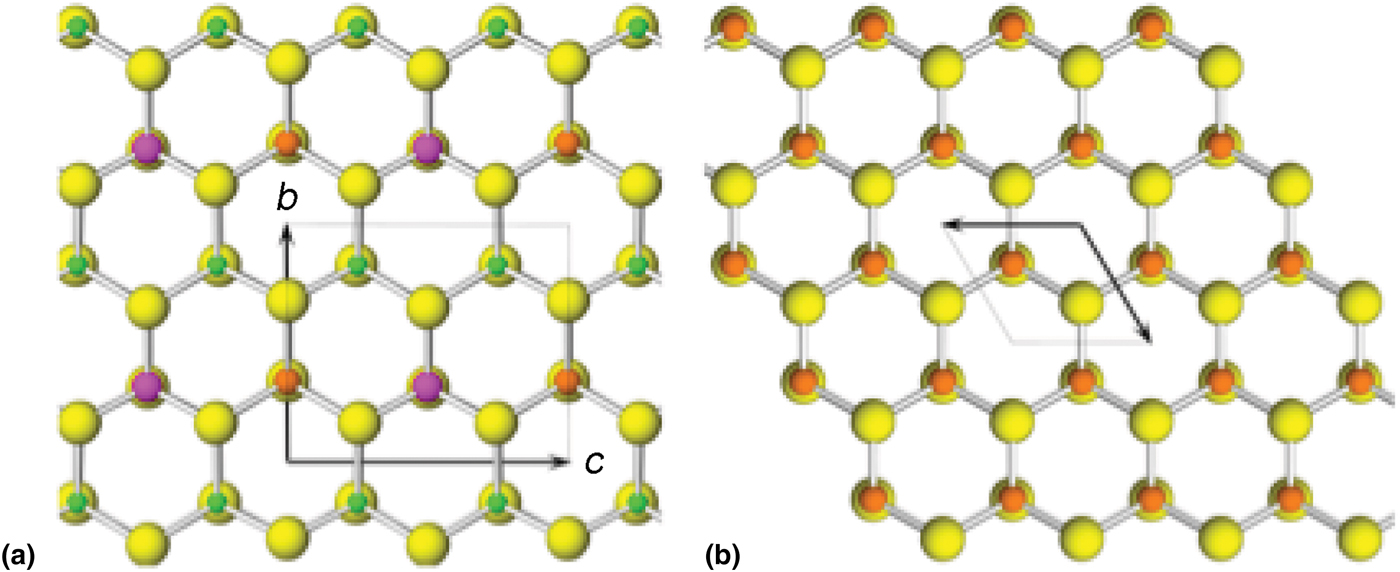
Fig. 4. The crystal structure of agmantinite (a) viewed along [100]; yellow: S sites, green: Ag sites, magenta: Sn sites, brown: Mn(Zn) sites and (b) the crystal structure of wurtzite viewed along [001]; yellow: S sites, brown: Zn sites.
The mineral adds to the list of Ag- and Mn-bearing minerals described from the Uchucchacua deposit. In addition, during study of the alabandite zone other minerals chemically related to agmantinite, such as stannite, kësterite and keutschite were found. The Uchucchacua deposit is unusual for the occurrence of Ag–Mn bearing minerals (uchucchacuaite, manganoquadratite, menchettiite, oyonite and agmantinite) and Mn-bearing sulfosalts (benavidesite, in addition to those listed above). The only Mn-bearing sulfosalts so far not encountered during the study within the alabandite zone at Uchucchacua are clerite and samsonite. Investigations of the exotic mineralogy of the ‘alabandite zone’ are ongoing and will possibly result in the description of new and chemically and structurally exciting species.
Acknowledgements
The authors thank Daniel Fredrickson (University of Wisconsin-Madison, USA) for helpful discussions and Christian Rewitzer (Furth im Wald, Germany) for taking the photograph of agmantinite.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2018.139