Introduction
Until 2008, the family Bornaviridae consisted of one virus – Borna disease virus (BDV), known primarily as a cause of neurological disease in horses and ruminants in a geographically restricted area of Central Europe. The 2008 identification of multiple genotypes of a novel avian bornavirus (ABV) opened the door to investigations on the role of these viruses in causing disease in captive and free-ranging birds. The purpose of this article is to review the associations between members of the family Bornaviridae and birds, especially North American waterfowl.
Borna disease virus
History
The town of Borna lies in the eastern part of Germany near Leipzig, in the state of Saxony. The region has long been associated with epidemics of a unique neurologic disease of horses, especially a devastating epidemic among cavalry horses between 1894 and 1896 (Richt et al., Reference Richt, Grabner and Herzog2000). Affected animals show a diverse array of changes in behavior including ataxia, head tilt, muscle fasciculation, hind-limb paresis, localized hypo- or hyper-aesthesia, disturbances in chewing and swallowing, and aggression. A similar disease occurs in sheep. The pathology of the disease consists of a virally induced progressive, non-suppurative encephalomyelitis characterized by lymphocytic infiltrates affecting the gray matter (Lipkin and Briese, Reference Lipkin, Briese, Knipe and Howley2006). This disease is restricted to a fairly small geographic area encompassing central and southern Germany and neighboring countries, and was named Borna disease after its region of origin. Serologic studies have shown that while BDV infection is widespread in horses and sheep within the affected region, only a small fraction of these infected animals actually develop clinical disease. Once disease develops, however, Borna disease mortality may reach 100% in horses and 50% in sheep.
Characteristics of the virus
BDV is an enveloped, non-segmented negative strand RNA virus with a genome size of approximately 8.9 kb. The bornaviral genome encodes six proteins: nucleocapsid (N), X protein (X), phosphoprotein (P), matrix (M), envelope glycoprotein (G) and the RNA-dependent RNA polymerase (L). Its overall genomic organization is similar to that of other viruses in the order Mononegavirales including the paramyxoviruses and rhabdoviruses. However, BDV is the only non-segmented, negative strand RNA virus to replicate within the nuclei of infected cells and hence was placed into its own family, the Bornaviridae. Bornaviruses also have a unique genome replication strategy that involves trimming of 5′-terminal nucleotides (Schneider et al., Reference Schneider, Schwemmle and Staeheli2005).
Virions appear to be enveloped particles of 80–100 nm, with 50–60 nm electron dense cores, but are difficult to visualize (Lipkin et al., Reference Lipkin, Briese and Hornig2011). In tissue culture, BDV is highly cell-associated, non-cytopathic, and found in only small amounts in cell culture supernatants (Schneider, Schwemmle and Staeheli, Reference Schneider, Schwemmle and Staeheli2005; Tomonaga et al., Reference Tomonaga, Carbone and Carbone2002). Many mammalian cell types are permissive for BDV replication. Cells in which BDV can be cultured include, but are not limited to human oligodendrocyte (OL), African green monkey kidney (Vero), Madin–Darby canine kidney (MDCK), rat glial cells (C6,) human embryonic kidney cells (HEK) and guinea pig 1505 cells (Herzog and Rott, Reference Herzog and Rott1980; Staeheli et al., Reference Staeheli, Sauder, Hausmann, Ehrensperger and Schwemmle2000; Schneider, Schwemmle and Staeheli, Reference Schneider, Schwemmle and Staeheli2005).
BDV spreads predominantly by direct cell-to-cell contact. Indirect immunofluorescence assays using antibodies against the viral N or P proteins reveal a characteristic speckled pattern in the nucleus. These intranuclear inclusions (karyosphaeridia) are known as Joest–Degen bodies and form as a result of the close association between BDV proteins and cellular chromatin (Matsumoto et al., Reference Matsumoto, Hayashi, Omori, Honda, Daito, Horie, Ikuta, Fujino, Nakamura, Schneider, Chase, Yoshimori, Schwemmle and Tomonaga2012). This association probably ensures that viral genomes are effectively delivered to daughter cells during mitosis.
Borna disease pathology/immunopathology
BDV can infect a variety of mammals and birds, including rodents, non-human primates, chickens and ostriches (Carbone, Reference Carbone2001; Tomonaga and Carbone, Reference Tomonaga, Carbone and Carbone2002). In many cases, animals become persistently infected but fail to develop disease. This is not surprising as, in tissue culture, the virus appears to have minimal adverse effects on cell function or survival. A small proportion of infected animals develop a progressive neurologic disease. Experimentally, disease development varies with host species and age (reviewed in Lipkin et al., Reference Lipkin, Briese and Hornig2011). For example, 4–5-week old Lewis rats respond to intracranial infection with transient behavioral abnormalities and develop meningoencephalitis and retinitis characterized by perivascular mononuclear cell infiltrations (Narayan et al., Reference Narayan, Herzog, Frese, Scheefers and Rott1983). In contrast, newborn rats can be chronically infected and develop subtle behavioral abnormalities but the infection is non-fatal (Lipkin et al., Reference Lipkin, Briese and Hornig2011). The differences in these scenarios appear to result from differences in host immune function. Specifically, brain damage results from the activities of host T cells (Rott et al., Reference Rott, Herzog, Richt and Stitz1988). The presence of CD8+ T cells in the brain parallels the onset of neurologic dysfunction. Conversely, suppression of T cell responses reduces the severity of experimental BDV infections (Narayan et al., Reference Narayan, Herzog, Frese, Scheefers and Rott1983). BDV spreads throughout the host largely through neural networks (Carbone et al., Reference Carbone, Duchala, Griffin, Kincaid and Narayan1987; Ackermann et al., Reference Ackermann, Guelzow, Staeheli, Schneider and Heimrich2010) and any route of infection that allows virus access to the nervous system will eventually lead to CNS infection and disease in susceptible adult rats (Lipkin and Briese, Reference Lipkin, Briese, Knipe and Howley2006).
Borna disease epidemiology
Serologic studies based on ELISA assays suggest that up to 12% of horses in central and southern Germany may have been exposed to BDV, implying that inapparent infections are not uncommon (Lipkin and Briese, Reference Lipkin, Briese, Knipe and Howley2006). The status of Borna disease in hoofstock, outside its core range, is unclear but it appears to be present in Israel, Japan and other Asian countries (Richt et al., Reference Richt, Pfeuffer, Christ, Frese, Bechter and Herzog1997). Studies within the core range have long suggested that the virus is maintained within a natural reservoir, and rodents have been the prime suspects. BDV has been found in the bicolored white-toothed shrew (Crocidura leucodon) in Central Europe (Hilbe et al., Reference Hilbe, Herrsche, Kolodziejek, Nowotny, Zlinszky and Ehrensperger2006), but the shrew cannot be held wholly responsible for the transmission of Borna disease, as it is not found everywhere that disease occurs. In Finland, seropositive bank voles (Myodes glareolus) were identified by Kinnunen et al. (Reference Kinnunen, Billich, Ek-Kommonen, Henttonen, Kallio, Niemimaa, Palva, Staeheli, Vaheri and Vapalahti2007). Domestic mammals are believed to acquire infection as a result of eating or inhaling on pastures contaminated with shrew urine. While other reservoirs have not been formally demonstrated, there is evidence that birds may play a role in maintaining and disseminating this disease.
BDV and birds
A few reports have noted the presence of BDV in birds, either as a cause of clinical disease or as inapparent infections playing a part in transmission to more susceptible species. A review by Rott and Becht (Reference Rott, Becht, Koprowski and Lipkin1995) reported that chickens are ‘frequently susceptible’ to BDV and cited previous studies, including those of Zwick et al. (Reference Zwick, Seifried and Witte1927). Experimental infections of chickens were also described by Ludwig et al. (Reference Ludwig, Becht and Groh1973). In that study, 1-day old chicks were inoculated intracerebrally with brain homogenates from rabbits with Borna disease; at 5–8 weeks post-inoculation, 9 of 13 chicks developed paralysis of legs and wings. After sacrifice the affected birds were found to have intranuclear Joest-Degen inclusion-bodies in brain sections and anti-BDV antibodies were demonstrated by immune-diffusion tests using sera from recovered chickens (Ludwig et al., Reference Ludwig, Becht and Groh1973).
BDV was identified as the cause of an outbreak of neurologic disease in ostriches (Struthio camelus) in Israel (Malkinson et al., Reference Malkinson, Weisman, Ashash, Bode and Ludwig1993a, Reference Malkinson, Weisman, Perl, Ashash, Koprowski and Lipkinb). Between 1989 and 1992, 7–26% of all hatched chicks on affected farms died from paresis (Asash et al., Reference Asash, Malkinson, Meir, Perl and Weisman1996). In one group of ostriches BDV antigen was detected, using an ELISA, in 7 of 13 brain homogenates from paralyzed birds, whereas it was found in only 1 of 10 brains from healthy birds. Brain extracts from paralyzed ostriches, given orally or intramuscularly to 5-week-old ostrich chicks, reproduced the clinical signs and microscopic lesions of naturally infected birds. Whether this disease outbreak was in fact caused by an avian bornavirus is unknown; the described pathologic lesions were not at all similar to those seen in parrots or waterfowl. Unfortunately these tissues are not available for additional testing.
Beginning in the late 1980s, a neurologic disease characterized by stiffness leading to paralysis and death was observed in cats in Central Europe and Scandinavia (Lundgren, Reference Lundgren1992). BDV was subsequently isolated from some of these cats and challenge of pathogen-free cats with BDV induced a neurologic disease resembling the natural disease. The source of this cat infection was unclear but Berg and his colleagues in Sweden (Berg et al., Reference Berg, Johansson, Montell and Berg2001) speculated that the cats might have acquired this infection from wild birds. They investigated this possibility by using a nested RT-PCR assay to test for the presence of BDV in bird droppings and in fact detected BDV sequences in the droppings of a mallard (Anas platyrhyncos) and a jackdaw (Corvus monedula) from an urban pond in Uppsala, Sweden. The partial genome sequences of these Swedish bird isolates were clearly BDV, sharing 95.9–99% nucleotide sequence identity with BDV sequences from mammals. The jackdaw and mallard sequences were not identical to one another, sharing 98–98.5% nucleotide sequence identity (Berg et al., Reference Berg, Johansson, Montell and Berg2001).
The publications cited above predate the discovery of ABV and involve little or no genetic characterization of the putative bornaviruses. The role of avian species in the epidemiology of BDV thus remains unclear, and there is as yet no evidence for the presence of any other members of the family Bornaviridae in wild birds in Europe.
Additional evidence also suggests that links may exist between BDV and an avian vector. For example, Borna disease recurs in specific areas or on individual farms during spring and summer months (April, May and June) at several year intervals (Dürrwald, Reference Dürrwald1993; Richt et al., Reference Richt, Grabner and Herzog2000). The prevalence of disease drops significantly in the autumn and winter months. BD tends to occur at low altitudes and there may be an association with river valleys – sites where waterfowl occur. Staeheli et al. (Reference Staeheli, Sauder, Hausmann, Ehrensperger and Schwemmle2000) pointed out the remarkable stability of the endemic area in central Europe over many years, despite the widespread movement of animals into and out of the area. Clusters of BDV appear to have different origins, which tends to exclude the spread of a single virus from a single point of origin. This is somewhat surprising given the widespread movement of livestock, especially horses, between these areas, but is consistent with the apparent lack of direct spread among farm animals (Staeheli et al., Reference Staeheli, Sauder, Hausmann, Ehrensperger and Schwemmle2000). More recently, Kolodziejek and her colleagues have also demonstrated, by genetic analysis, that within the Borna disease endemic area there is clear clustering of different genetic strains of the virus (Kolodziejek et al., Reference Kolodziejek, Durrwald, Herzog, Ehrensperger, Lussy and Nowotny2005). Thus specific genetic sequences are associated with certain German, Austrian and Swiss regions, which have no obvious geographic barriers between them.
Studies such as those described above support multiple introductions of genetically stable bornaviruses into livestock. Small mammal reservoirs of BDV have been identified, but there is no evidence of widespread BDV infection in the Borna disease endemic area or elsewhere.
An avian vector of BDV was also suggested by studies on the epidemiology of the outbreak of Borna disease in ostriches in Israel. Teplitsky et al. (Reference Teplitsky, Pitlik, Richt, Herzog, Meir, Marcus, Sulkes, Weisman and Malkinson2003) showed that horses in the region were seropositive for BDV as determined by an ELISA. Interestingly, the positive samples came either from the coast or the Jordan valley rather than from the highlands between these two regions. The authors pointed out that the coast and valley are major flyways for migrating birds and went on to suggest the possibility that birds may serve as vectors of BDV in Israel.
Avian bornaviruses
Work thus far suggests that ABV behave much like BDV in culture. Infections are non-cytopathic and there is no evidence that a significant amount of virus is released (Rinder et al., Reference Rinder, Ackermann, Kempf, Kaspers, Korbel and Staeheli2009; Staeheli et al., Reference Staeheli, Rinder and Kaspers2010). In ABV-infected cells, viral antigen is found in the nucleus and shows the same speckled immunofluorescence pattern as BDV (Rinder et al., Reference Rinder, Ackermann, Kempf, Kaspers, Korbel and Staeheli2009; Gray et al., Reference Rinder, Ackermann, Kempf, Kaspers, Korbel and Staeheli2010). Not surprisingly, bird cells are more permissive for ABV replication than are mammalian cells. Cells used successfully for ABV culture include primary duck embryo fibroblasts (DEF), the quail fibroblast cell line CEC32, the quail skeletal cell line (QM7) and a chicken hepatoma cell line (Rinder, et al. Reference Rinder, Ackermann, Kempf, Kaspers, Korbel and Staeheli2009; Gray et al., Reference Gray, Hoppes, Suchodolski, Mirhosseini, Payne, Villanueva, Shivaprasad, Honkavuori, Lipkin, Briese, Reddy and Tizard2010; Villanueva et al., Reference Villanueva, Gray, Mirhosseini, Payne, Hoppes, Honkavuori, Briese, Turner and Tizard2010). Attempts to grow ABV in mammalian cells (Vero, MDCK or C6 cell lines) have been unsuccessful (Rinder, et al., Reference Rinder, Ackermann, Kempf, Kaspers, Korbel and Staeheli2009). In vivo, ABV appears to be widely disseminated. Viral antigen can be found in a broad spectrum of organs and cell types in diseased birds (Rinder et al., Reference Rinder, Ackermann, Kempf, Kaspers, Korbel and Staeheli2009; Raghav et al., Reference Raghav, Taylor, Delay, Ojkic, Pearl, Kistler, Derisi, Ganem and Smith2010). This is in sharp contrast to BDV, which has a preference for cells of the central and peripheral nervous systems.
ABV genotypes
In the two studies that first identified ABV in parrots, five genotypes (ABV1–5) were recognized on the basis of nucleotide and amino acid sequence identity (Honkavuori et al., Reference Honkavuori, Shivaprasad, Williams, Quan, Hornig, Street, Palacios, Hutchison, Franca, Egholm, Briese and Lipkin2008; Kistler et al., Reference Kistler, Gancz, Clubb, Skewes-Cox, Fischer, Sorber, Chiu, Lublin, Mechani, Farnoushi, Greninger, Wen, Karlene, Ganem and DeRisi2008). The ABV genotypes, all with <70% nucleotide sequence identity with BDV, were sufficiently different as to comprise a new species (Honkavuori et al., Reference Honkavuori, Shivaprasad, Williams, Quan, Hornig, Street, Palacios, Hutchison, Franca, Egholm, Briese and Lipkin2008; Kistler et al., Reference Kistler, Gancz, Clubb, Skewes-Cox, Fischer, Sorber, Chiu, Lublin, Mechani, Farnoushi, Greninger, Wen, Karlene, Ganem and DeRisi2008). Two additional psittacine bornavirus genotypes (ABV 6 and 7) were subsequently identified (Weissenbock et al., Reference Weissenbock, Bakonyi, Sekulin, Ehrensperger, Doneley, Durrwald, Hoop, Erdelyi, Gal, Kolodziejek and Nowotny2009a; Rubbensroth et al., Reference Rubbensroth, Rinder, Kaspers and Staeheli2012). Among these seven genotypes, ABV4 and ABV2 are by far the most common genotypes in captive parrots worldwide (Ogawa et al., Reference Ogawa, Sanada, Kudo, Tuchiya, Kodama and Uetsuka2011; Rubbensroth et al., Reference Rubbensroth, Rinder, Kaspers and Staeheli2012).
Two additional ABV genotypes have recently been identified. One genotype was recovered from a canary (Serinus canaria) and is identified as ABV-canary (Weissenbock et al., Reference Weissenbock, Sekulin, Bakonyi, Högler and Nowotny2009b; Rinder et al., Reference Rinder, Kronthaler, Hufen and Korbel2012). The second non-psittacine ABV genotype was recovered from a wild Canada goose (Branta canadensis) (Delnatte et al. Reference Delnatte, Berkvens, Kummrow, Smith, Campbell, Crawshaw, Ojkic and DeLay2011), and was named ABV-CG. This was the first ABV identified from wild birds. As described below, ABV-CG is common across North America and has been isolated from trumpeter swans (Cygnus buccinator) and mute swans (Cygnus olor) (Delnatte et al., Reference Delnatte, Berkvens, Kummrow, Smith, Campbell, Crawshaw, Ojkic and DeLay2011; Guo et al., Reference Guo, Covaleda, Heatley, Baroch, Tizard and Payne2012). Thus, to date nine ABV genotypes have been identified.
To illustrate the genetic relationships among the bornaviruses, the phylogeny (shown in Fig. 1) was generated using a short fragment of the nucleocapsid (N) gene. The phylogeny shows the psittacine ABVs on multiple branches of the tree. As ABV genotypes 1–7 and ABV-canary were recovered from captive birds it is impossible to meaningfully correlate virus genotype to the region of origin of the host. It also appears that there is no obvious correlation between psittacine species and infecting genotype. For example, ABV4 and ABV2 are found in captive birds of South American, African and Australian species. In contrast, the bornaviruses identified in wild Canada geese, mute swans and trumpeter swans form a tight cluster with over 90% nucleotide sequence identity among them (Payne et al., Reference Payne, Covaleda, Jianhua, Baroch, Ferro, Lupiani, Heatley and Tizard2011a, Reference Payne, Shivaprasad, Mirhosseini, Gray, Hoppes, Weissenbock and Tizardb; Guo et al., Reference Guo, Covaleda, Heatley, Baroch, Tizard and Payne2012). Thus, it appears that, at least in North America, there may be a predominant genotype circulating among free-living waterfowls.
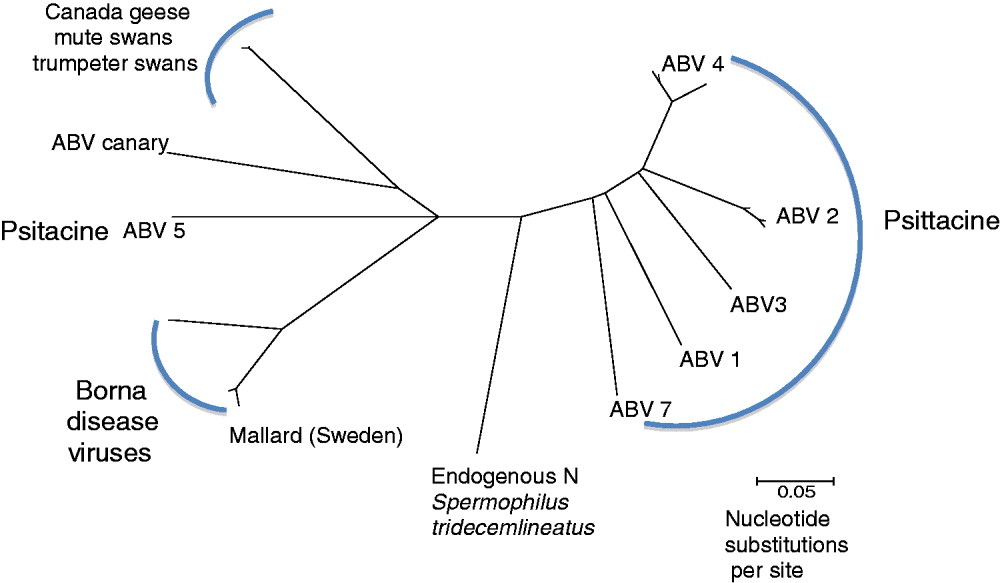
Fig. 1. A phylogeny was generated using partial N-gene sequences of bornaviruses from birds and mammals. A consensus tree was generated using a Neighbor-Joining algorithm, with no outgroup assigned. To generate the consensus tree 1000 bootstrap replicates were generated.
Natural cases of Borna disease are most prevalent in Central Europe, and the BDVs from that region are for the most part closely related, forming their own cluster. BDV was also found in ducks in Sweden, and that finding suggests a possible correlation between genotype and geographic location.
A particularly intriguing question is the nature of the relationship between ABV infection in waterfowl and in parrots. As described earlier, PDD first appeared in captive parrots in the USA in the mid-1970s at a time when many species of birds were being captured in the wild and imported into Europe and the USA. These birds were subjected to quarantine while being tested for Newcastle disease, and facilities were not designed to prevent cross species contact. Based on the lack of recognition of PDD in psittacine birds prior to this date, on the rapid spread of the disease globally, and the lack of reports of PDD (or ABV infection) in wild psittacine populations, it is tempting to hypothesize that macaws were infected by contact with ABV-infected waterfowl while in quarantine. Mixing of species and high stress levels would have enhanced viral transfer and perhaps facilitated development of disease in the new host. The presence of multiple discrete ABV genotypes in psittacine birds suggests multiple introductions, perhaps from different sources. Evaluation of waterfowl (or other wild birds) for ABV genotypes has hardly begun, and has thus far largely been restricted to examining birds within continental North America. There is no information currently available regarding the presence of ABV in wild birds in South America, Asia, Africa or Australasia; thus the potential for discovering other genotypes in other species and geographic locations is high.
A recent observation that further complicates our understanding of the relationships among bornaviruses is the identification of bornavirus sequences integrated into the genomes of a variety of mammals (Belyi et al., Reference Belyi, Levine and Skalka2010; Horie et al., Reference Horie, Honda, Suzuki, Kobayashi, Daito, Oshita, Ikuta, Jern, Gojobori, Coffin and Tomonaga2010; Katzourakis and Gifford, Reference Katzourakis and Gifford2010). It appears that bornavirus genes found their way into primate genomes over 40 million years ago, and that integration events occurred more than once. One North American mammal, the 13-lined ground squirrel (Spermophilus tridecemlineatus) has an intact bornavirus N gene in its genome. The endogenized N gene is more closely related to currently circulating ABV than to the endogenous bornavirus-like elements (EBLN) in human and non-human primates.
Proventricular dilatation disease (PDD)
PDD is a fatal neurologic condition of captive parrots (Hoppes et al., Reference Hoppes, Gray, Payne, Shivaprasad and Tizard2010). The disease was first identified in North America and Europe during the mid-1970s at a time when extensive trading in wild parrots was permitted. Its source or sources are unknown (Graham, Reference Graham1984), but South America was suspected to be the site of origin as the disease was often observed in macaws imported from Bolivia (S. Clubb, personal communication, October, 2012). International trade in captive parrots subsequently resulted in the disease being widely spread geographically. PDD has yet to be identified in a wild parrot population. In 2008, the intensive search for an etiology ended when a novel bornavirus was identified and subsequently shown to be the causative agent of PDD (Honkavuori et al., Reference Honkavuori, Shivaprasad, Williams, Quan, Hornig, Street, Palacios, Hutchison, Franca, Egholm, Briese and Lipkin2008; Kistler et al., Reference Kistler, Gancz, Clubb, Skewes-Cox, Fischer, Sorber, Chiu, Lublin, Mechani, Farnoushi, Greninger, Wen, Karlene, Ganem and DeRisi2008; Gancz et al., Reference Gancz, Kistler, Greninger, Farnoushi, Mechani, Perl, Berkowitz, Perez, Clubb, DeRisi, Ganem and Lublin2009; Gray et al., Reference Gray, Hoppes, Suchodolski, Mirhosseini, Payne, Villanueva, Shivaprasad, Honkavuori, Lipkin, Briese, Reddy and Tizard2010).
PDD is characterized histopathologically by non-suppurative inflammation in the central, peripheral and autonomic nervous systems (Berhane et al., Reference Berhane, Smith, Newman, Taylor, Nagy, Binnington and Hunter2001; Raghav et al., Reference Raghav, Taylor, Delay, Ojkic, Pearl, Kistler, Derisi, Ganem and Smith2010). Clinical signs reflect the wide distribution of lesions, but are generally classified as neurologic or gastrointestinal. They include weakness, ataxia, proprioceptive deficits, seizures and blindness; weight loss, passage of undigested food, regurgitation and delayed crop emptying, respectively. Gastrointestinal malfunction results in the most common gross pathologic lesions – dilation and thinning of the walls of the proventriculus and ventriculus, and in maldigestion, emaciation and death by starvation (Hoppes et al., Reference Hoppes, Gray, Payne, Shivaprasad and Tizard2010). Birds may present with any combination of these signs, and the severity and progress of the disease can vary tremendously. Subclinically affected birds may show very few if any clinical signs over long periods of time (Raghav et al., Reference Raghav, Taylor, Delay, Ojkic, Pearl, Kistler, Derisi, Ganem and Smith2010; Villanueva et al., Reference Villanueva, Gray, Mirhosseini, Payne, Hoppes, Honkavuori, Briese, Turner and Tizard2010).
ABV infects primarily the central and enteric nervous systems of birds. Although the virus is non-cytopathic it can induce inflammation and a selective loss of glial cells and neurons. Based on the known pathogenesis of BDV, it is believed that this cell loss is secondary to T cell cytotoxicity although this has not yet been formally demonstrated for ABV. ABV can be detected throughout the CNS and enteric nervous systems but is also found in the kidneys, adrenals and gonads and in cell types other than neurons (Rinder et al., Reference Rinder, Ackermann, Kempf, Kaspers, Korbel and Staeheli2009; Raghav et al., Reference Raghav, Taylor, Delay, Ojkic, Pearl, Kistler, Derisi, Ganem and Smith2010; Weissenbock et al., Reference Weissenbock, Fragner, Nedorost, Mostegl, Sekulin, Maderner, Bakonyi and Nowotny2010; Payne et al., Reference Payne, Shivaprasad, Mirhosseini, Gray, Hoppes, Weissenbock and Tizard2011b).
Identification of ABV in birds
Psittacines
ABV is readily detected by both RT-PCR and immunohistochemistry (IHC) in necropsy tissues from parrots with clinical and histopathological evidence of PDD (Raghav et al., Reference Raghav, Taylor, Delay, Ojkic, Pearl, Kistler, Derisi, Ganem and Smith2010). It is present in all major organs, especially the central nervous system. In live birds, the identification of virus and the determination of its significance are less straightforward. ABV is often detectable in cloacal swabs and the urofeces of birds affected with PDD and this has been recommended as the primary antemortem method of identifying birds affected with the disease. However, surveys have shown that ABV can be found in droppings from apparently healthy captive psittacines, and shedding of virus from the ABV-positive birds is often intermittent and unpredictable (Raghav et al., Reference Raghav, Taylor, Delay, Ojkic, Pearl, Kistler, Derisi, Ganem and Smith2010; Villanueva et al., Reference Villanueva, Gray, Mirhosseini, Payne, Hoppes, Honkavuori, Briese, Turner and Tizard2010). For example, ABV was detected in droppings from apparently healthy parrots of several species, including multiple cockatiels (Nymphicus hollandicus) purchased from several local aviaries without a history of PDD (Villanueva et al., Reference Villanueva, Gray, Mirhosseini, Payne, Hoppes, Honkavuori, Briese, Turner and Tizard2010). Some clinically unaffected birds shed virus for at least 5 years.
Recently, serologic tests appear to be of limited usefulness in disease diagnosis. Indirect fluorescent antibody testing (Lierz et al., Reference Lierz, Piepenbring, Heffels-Redmann, Herzog, Herden and Enderlein2012) and Western blot assays (Villanueva et al., Reference Villanueva, Gray, Mirhosseini, Payne, Hoppes, Honkavuori, Briese, Turner and Tizard2010) appear to be the most sensitive and specific tests. Both tests appear to be 90–95% sensitive and specific. They cannot however differentiate between diseased birds and healthy carriers.
Passerines
Since the first appearance of PDD in large psittacines, avian pathologists have, from time to time, noted neurologic lesions in other bird species that ‘look like’ PDD (Daoust et al., Reference Daoust, Julian, Yason and Artsob1991; Perpinan et al., Reference Perpinan, Fernandez-Bellon, Lopez and Ramis2007). However, this was impossible to prove without the ability to detect the presence of ABV in the lesions. Since the discovery of ABV, infections have been detected in canaries (S. canaria). Weissenbock et al., (Reference Weissenbock, Sekulin, Bakonyi, Högler and Nowotny2009b) examined a bird that had died after a few days of ‘apathy’. On necropsy the bird had a severely dilated proventriculus. On histopathology the bird had a non-suppurative encephalitis and ganglioneuritis in the proventriculus and ventriculus. Bornaviral antigen was detected in multiple tissues and confirmed by RT-PCR. Sequence analysis demonstrated that this was a unique genotype of ABV. Subsequently, Rinder et al. (Reference Rinder, Kronthaler, Hufen and Korbel2012) described two canaries with neurologic disease associated with the presence of ABV. One bird showed ‘apathy’ and sudden death; the other showed prolonged depression, neurologic disease (head tilting and inability to fly), and visual impairment with chorioretinitis. Necropsy showed a dilated proventriculus with ganglioneuritis and non-suppurative encephalitis. Sequencing identified variants of the previously reported canary genotype. Canaries are archetypical passerines and the ability of ABV to cause disease in this species suggests the possibility that all passerines may be susceptible to this virus.
Geese
Smith and colleagues were the first to identify the presence of ABV in waterfowl, specifically Canada geese and trumpeter swans during a retrospective survey of diseases of wild birds in Southern Ontario (Smith et al., Reference Smith, Berkvens, Kummrow, Crawshaw, Okjic, Campbell and DeLay2010). These samples had been obtained from birds suffering from neurologic disease of unknown origin but with consistent neuropathology. Careful examination of central, peripheral and autonomic nervous tissue revealed non-suppurative inflammatory lesions similar to those seen in psittacine birds with PDD. ABV was identified in 11/12 goose brains and 2/2 swan brains by IHC, using rabbit polyclonal antiserum against ABV N-protein, as well as by RT-PCR testing for N-protein genes. Sequence analysis of the amplified gene products confirmed the presence of ABV in Canada geese and identified it as a unique new genotype (Delnatte et al., Reference Delnatte, Berkvens, Kummrow, Smith, Campbell, Crawshaw, Ojkic and DeLay2011).
Subsequently, Delnatte and her colleagues selected 51 necropsy reports from Canada geese, trumpeter swans and mute swans, euthanized or found dead in southern Ontario, based on the presence of upper gastrointestinal impaction, central nervous system histopathology or a clinical history suggestive of ABV infection (Delnatte et al., Reference Delnatte, Ojkic, DeLay, Campbell, Crawshaw and Smith2012a). IHC and conventional RT-PCR for the N-protein gene and quantitative RT-PCR (qPCR) for the M-gene on fresh and formalin fixed paraffinized tissue again revealed the presence of ABV in birds with widespread non-suppurative encephalomyelitis and ganglioneuritis. As with psittacine PDD, the clinical history and gross necropsy findings in these birds generally reflected the occurrence of nervous system lesions and included neurologic disease, weakness or inability to fly, and signs suggesting possible defects in gastrointestinal motility such as upper gastrointestinal tract impaction. In 1991, Daoust and colleagues described two cases of PDD-like disease in Canada geese from Prince Edward Island (Eastern Canada) (Daoust el al., Reference Daoust, Julian, Yason and Artsob1991), but this was before the identification of ABV. Recently, archived tissues from these birds were found to be positive for ABV based on RT-PCR (Delnatte et al., Reference Delnatte, Ojkic, DeLay, Campbell, Crawshaw and Smith2012a), and one Canada goose from the province of Quebec showing consistent neuropathology has also been shown to be ABV-positive (D.A. Smith, unpublished work). The conclusion of this work was that ABV infection can be associated with significant neuropathology in waterfowl species, and that the resulting disease is very similar to PDD in psittacine birds.
Comparisons of the site of origin of 40 Canada geese affected by ABV-related neurological disease suggested an uneven geographic distribution. Using retrospective necropsy data, the proportion of affected wild geese found dead or euthanized at a large urban zoo was significantly higher than that found elsewhere in the province. Unequal surveillance intensity made estimation of the true prevalence of the disease in these two locations impossible to determine.
Cloacal swabs were subsequently collected from 200 free ranging Canada geese in four different locations, including the zoo site, to better estimate the prevalence of ABV infection in Ontario (Delnatte et al., Reference Delnatte, Ojkic, DeLay, Nagy, Campbell, Crawshaw and Smith2012b). The prevalence of fecal shedding at the zoo site was significantly higher than at the other three collection sites (7/50 versus 0/150), supporting their premise that environmental or ecological factors can affect the prevalence of ABV infection and subsequent disease.
Canada geese are often considered to be nuisance birds when they gather in large permanent flocks in public spaces such as parks and playgrounds. In the USA, these flocks are culled by the Wildlife Services Agency of the US Department of Agriculture. These culled birds are routinely swabbed in the oropharynx and the cloaca to determine the presence of avian influenza viruses. Payne and her colleagues extracted RNA from influenza-negative swabs and subjected them to RT-PCR analysis for ABV M-genes (Payne et al., Reference Payne, Covaleda, Jianhua, Baroch, Ferro, Lupiani, Heatley and Tizard2011a, Reference Payne, Shivaprasad, Mirhosseini, Gray, Hoppes, Weissenbock and Tizardb). In their first survey, 300 samples consisting of 25 samples from each of 12 states across the USA were obtained. Subsequently, an additional 109 swab samples were surveyed from the three western coastal states. Of the 409 goose swab samples tested, 24 from 5 states were positive for ABV M-genes (Fig. 2). Twelve of these products (2.9%) were subsequently sequenced and confirmed to be of the ABV goose strain genotype. Of the states where Canada geese were tested, only Ohio had no positive samples detected. These results also showed that viral prevalence was not uniform across the continent, and that infections could be clustered within flocks. For example, 20–42% of birds within selected flocks in Kansas, New Hampshire and New York were RT-PCR-positive. There were no apparent gender differences in infection prevalence in either this study or the Canadian studies.

Fig. 2. Sampling locations and the number of RT-PCR-positive samples at each site. The smallest circles represent 1–5 positive samples, the medium circles show 5–10 positive samples and the largest circles represent states or provinces from which >10 positive samples were obtained. The numbers do not reflect prevalence at any site. Of the states where Canada geese were tested, only Ohio had no positive samples detected. Likewise, of the states where mute swans were tested, only New York provided no positive samples.
While cloacal/oropharyngeal swabs provided a convenient method of testing wild goose flocks, experience based on psittacine testing suggests that this is a relatively insensitive method of detecting ABV infection (Villanueva et al., Reference Villanueva, Gray, Mirhosseini, Payne, Hoppes, Honkavuori, Briese, Turner and Tizard2010). For this reason 25 freshly frozen Canada goose heads were obtained from sites in New Jersey. Brain tissue from these geese was extracted and tested by RT-PCR. Of these, 11 (44%) were RT-PCR-positive (Payne et al., Reference Payne, Covaleda, Jianhua, Baroch, Ferro, Lupiani, Heatley and Tizard2011a, Reference Payne, Shivaprasad, Mirhosseini, Gray, Hoppes, Weissenbock and Tizardb). Seven of the positive samples were from a single nuisance flock while four were from hunter-killed birds.
RT-PCR testing also has been carried out on the brain materials of hunter-killed geese in Texas and Kansas. These include 115 snow geese (Chen caerulescens), 58 Ross's geese (Chen rossii) and 10 greater white-fronted geese (Anser albifrons). None of the samples from greater white-fronted geese were ABV-positive, but 10% of Ross's geese and 19% of snow geese samples were positive.
Swans
Trumpeter swans were native to Eastern Canada until extirpated as a result of overhunting by the early 20th Century. Beginning in the 1980s, a restoration project has succeeded in reintroducing this bird to its former range. There are now more than 1000 trumpeter swans in Ontario and more than 100 breeding pairs established (Moser, Reference Moser2006; Lumsden, Reference Lumsden2009). Close monitoring of this population and an appreciation of the importance of lead poisoning as a cause of morbidity and mortality, result in the early recognition and capture of clinically abnormal birds, and an enhanced likelihood of the submission for necropsy of birds that die. In the retrospective study performed by Delnatte et al. (Reference Delnatte, Ojkic, DeLay, Campbell, Crawshaw and Smith2012a), ABV-associated neuropathology was present in six of eight trumpeter swans that met the study inclusion criteria intended to select ABV affected birds from a large database. Among the birds presented for necropsy, the proportion of trumpeter swans meeting the inclusion criteria was lower than that for Canada geese but did not differ between the two sites of sample origin. There was no significant difference in virus detection frequency between the two sites of origin, which is not surprising as all swans in Southern Ontario belong to a single mobile population.
In contrast to trumpeter swans, mute swans are an introduced species in North America and are found predominantly in the northeast. They are often considered nuisance birds because of their aggression and their destructive effects on the environment. As a result, some US states permit their capture and euthanasia. Captured birds were therefore available to permit estimation of the prevalence of ABV in this species. Combined oropharyngeal and cloacal swabs were collected from 219 mute swans. Fourteen of these samples gave a product of appropriate size on gel electrophoresis (Guo et al., Reference Guo, Covaleda, Heatley, Baroch, Tizard and Payne2012). Four of these positive samples were selected for sequencing and were confirmed to be ABV M-gene sequences. Phylogenetic analysis of these amplified sequences indicated that the genes clustered in a single group closely related to the M-genes of the previously described goose isolates (Payne et al., Reference Payne, Covaleda, Jianhua, Baroch, Ferro, Lupiani, Heatley and Tizard2011a, Reference Payne, Shivaprasad, Mirhosseini, Gray, Hoppes, Weissenbock and Tizardb).
Brain samples taken from 197 mute swan were tested by RT-PCR by Guo et al. and ABV sequences were detected in 45 (23%) (Guo et al., Reference Guo, Covaleda, Heatley, Baroch, Tizard and Payne2012). Two mute swan isolates were cultured, their M, N and X–P sequences were analyzed, and they were found to closely match the Canada goose genotype. Positive mute swan samples were obtained from Michigan, Rhode Island and New Jersey (Fig. 2). Further analysis of brain samples obtained from selected counties in Michigan suggested that ABV prevalence could be as high as 50% in some populations. The ‘health’ of these mute swans was assessed at the time of capture and five were determined to be ‘sick’. However, at the time, their ill health was believed to be a result of either parasitic worms or of lead poisoning. No necropsies were performed on any of these birds and it is entirely possible that some may have suffered from subclinical nervous system disease. Of the states where mute swans were tested, only New York provided no positive samples (Guo et al., Reference Guo, Covaleda, Heatley, Baroch, Tizard and Payne2012). ABV-associated neuropathology has also been described in one mute swan in the retrospective study by Delnatte et al. (Reference Delnatte, Ojkic, DeLay, Campbell, Crawshaw and Smith2012a).
It is of interest to note the great difference between apparent viral prevalence in mute swan cloacal swabs (6%) and brains (23%). This disparity is similar to that observed in ABV-infected parrots and almost certainly reflects the intermittent nature of viral shedding. The presence of ribonucleases in the feces may also contribute to this disparity. In addition, Delnatte et al. (Reference Delnatte, Ojkic, DeLay, Nagy, Campbell, Crawshaw and Smith2012b) collected cloacal swabs from 67 mute swans and 132 free ranging trumpeter swans, and found six positive mute swan samples (qRT-PCR analysis for ABV M-gene) compared with no positive samples in trumpeter swans. This was despite the fact that these species commingle and that ABV-diseased trumpeter swans had previously been identified in the sampled flock. The reason for this difference is unclear, but a species effect on fecal shedding of ABV is one possibility.
Wild ducks
Given the high prevalence of ABV in Canada geese and mute swans, it was logical to test for the presence of virus in ducks. Thus 212 fresh duck heads were obtained from processors of hunter-killed ducks in Texas and brain samples were extracted (Payne et al., Reference Payne, Guo, Tizard, Hambrick and Gammon2012). These samples reflected the species composition in the areas hunted. One set of samples was harvested near Houston, Texas and consisted of a mixture of the predominant wintering dabbling ducks. Most were northern pintails (Anas acuta) and gadwalls (Anas strepera). Other species tested included northern shovelers (Anas clypeata), mallards (Anas platyrhynchos) and American widgeons (Anas americana). ABV was detected by RT-PCR in 4 of 61 northern pintails (6.5%), 6 of 25 gadwalls (24%), 1 of 10 mallards (10%) and 4 of 12 American widgeons (33%). It was not detected in either of the two wood ducks (Aix sponsa) tested. Diving ducks, represented by redheads (Aythya americana), were obtained from a hunting camp on the central Texas coast, where this species winters in large numbers. ABV was detected in 7 of 84 redheads (8.3%) tested.
Domestic ducks
While growing primary duck fibroblasts (DEF) from commercially available fertile Pekin duck eggs, Payne et al. (Reference Payne, Guo, Tizard, Hambrick and Gammon2012) found that these cells were infected with a strain of ABV whose M-protein gene sequence was different from those of previous laboratory isolates. Eighteen fertile eggs were subsequently purchased from each of four commercial duck hatcheries. Upon receipt, eggs were incubated for 8–14 days before the embryo was removed and processed for DEF production. The cultured DEFs were subsequently tested by RT-PCR for ABV-M protein. Two eggs from two of the four sources were ABV-positive. Sequencing of these PCR products indicated that the viruses belonged to the ABV-CG genotype. Minor differences in sequence from ABV genotypes previously cultured in the laboratory supported the premise that these viruses were not laboratory contaminants. This result also suggests that ABV may be vertically transmitted in domestic ducks. This has implications for transmission, diagnosis and immunopathology. Thus birds infected in-ovo may be immunologically tolerant of ABV. If, as is believed, disease develops as a result of T cell responses to the virus, this tolerance may ensure that ABV infection is inapparent in many birds. Whether in-ovo infection occurs in Canada geese is currently under investigation by Delnatte and colleagues.
Gulls
Brains from several species of gull were tested for the presence of ABV M-gene RNA by RT-PCR (Payne et al., Reference Payne, Guo, Tizard, Hambrick and Gammon2012). Birds had been culled as a result of collision avoidance strategies at airports (New Jersey, New York and New Hampshire) or euthanized after submission to rehabilitation centers (Texas). Three of 26 herring gulls (Larus argentatus), 1/5 ring-billed gulls (L. delawarensis), 3/13 laughing gulls (L. atricilla) and 0/4 great black-backed gulls (L. marinus) were RT-PCR-positive; none of these birds were from Texas.
Raptors
A bald eagle (Haliaeetus leucocephalus) that was unable to fly was submitted to an avian rehabilitator in East Texas in February, 2011 and died soon thereafter. Necropsy showed the presence of acute encephalitis and ABV-CG strain was present in the brain as demonstrated by RT-PCR. Bald eagles are known to predate on waterfowl flocks in Texas, killing and eating sick birds. Thus transmission of ABV from geese to eagles is not unexpected.
Experimental infection with ABV
ABV-induced disease in psittacines
The causal association between experimental ABV infection of psittacine birds and the development of PDD has been demonstrated by multiple investigators. Gancz et al. (Reference Gancz, Kistler, Greninger, Farnoushi, Mechani, Perl, Berkowitz, Perez, Clubb, DeRisi, Ganem and Lublin2009) were the first to demonstrate that PDD could be transmitted to healthy birds by the use of infected-brain tissue. They inoculated cockatiels by multiple routes with a brain homogenate from either an ABV4-positive bird or from a PDD-/ABV-control bird. The birds inoculated with healthy control bird homogenate remained healthy, whereas all three birds inoculated with brain homogenate from ABV-infected birds developed both gross and microscopic lesions typical of PDD. Two of these had also exhibited clinical signs. These investigators went on to demonstrate the presence of ABV, with a sequence nearly identical to that of the challenge strain, in the brains of the challenged birds. High throughput pyrosequencing of the inoculum suggested however, that other viruses may have been present in the inoculums although they were not identified in the challenged birds. While persuasive, these results could not prove that ABV alone was responsible for the development of PDD.
In a formal attempt to prove Koch's postulates, Gray et al. (Reference Gray, Hoppes, Suchodolski, Mirhosseini, Payne, Villanueva, Shivaprasad, Honkavuori, Lipkin, Briese, Reddy and Tizard2010) isolated ABV in cultured DEF. After six passages, these infected cells were injected intramuscularly into two Patagonian conures (Cyanoliseus patagonis). Clinical signs of PDD developed within 66 days post-infection in both challenged birds. The presence of typical PDD was demonstrated on necropsy and histopathology. RT-PCR demonstrated the presence of the inoculated strain in the brains of the challenged birds. A third, uninoculated control bird remained healthy. These conures, although apparently healthy, had previously been shown to be carriers of psittacine herpesvirus and as a result, some uncertainty persisted regarding the etiology of PDD.
Four apparently healthy cockatiels from a flock known to be shedding ABV4 were challenged with a known virulent strain of ABV4 (strain M24) (Payne et al., Reference Payne, Shivaprasad, Mirhosseini, Gray, Hoppes, Weissenbock and Tizard2011b). The challenged birds either died or were euthanized for humane reasons between days 92 and 110. Typical PDD was apparent on necropsy but the histopathologic lesions were reported to be unusually severe. Control birds inoculated with uninfected tissue culture cells remained healthy until euthanized on day 150, and no histopathologic lesions of PDD were found at necropsy.
Recently, Piepenbring et al. (Reference Piepenbring, Enderlein, Herzog, Kaleta, Heffels-Redmann, Ressmeyer, Herden and Lierz2012) inoculated 18 cockatiels by both the intracerebral and intravenous routes with an isolate of ABV4 cultured for six passages in a quail cell line (CEC-32). All challenged birds became persistently infected but the clinical disease patterns that developed varied among individuals. Five birds developed clinical signs of PDD, while on necropsy 7 of the 18 had a dilated proventriculus. All infected birds did, however, show mononuclear cell infiltrates characteristic of PDD in a wide range of organs.
Mirhosseini et al. (Reference Mirhosseini, Gray, Hoppes, Tizard, Shivaprasad and Payne2011) isolated ABV genotype 2 from a cockatiel and used infected DEF to inoculate two adult cockatiels by the oral and intramuscular routes. One bird developed clinical signs on day 33 and the second on day 41. While both challenged birds had slightly enlarged proventriculi, histopathology showed typical PDD lesions in the brain, spinal cord, heart, adrenal gland and intestine. A control, uninoculated cockatiel was apparently healthy when euthanized on day 50 and at necropsy, no gross abnormalities were observed. On histopathologic examination, the liver, pancreas and spleen had mild-to-moderate infiltration of lymphocytes, some of which were forming lymphoid nodules (Mirhosseini et al., Reference Mirhosseini, Gray, Hoppes, Tizard, Shivaprasad and Payne2011). However, all tissues from this bird were negative for ABV by RT-PCR. Lierz and his colleagues have subsequently conducted a similar challenge study with ABV2 and suggested that this genotype is more pathogenic in cockatiels than is genotype 4 (Lierz et al., Reference Lierz, Piepenbring, Heffels-Redmann, Herzog, Herden and Enderlein2012).
The results of all these experiments provide overwhelming support for the proposition that ABV is the sole cause of PDD in psittacines. That is not to say that PDD inevitably develops in ABV-infected parrots. As with both BDV and the Canada goose strains of ABV, it is also clear that many, apparently healthy psittacines may carry ABV for prolonged periods.
ABV infection of ducks
Gray et al. (Reference Gray, Villanueva, Mirhosseini, Hoppes, Payne and Tizard2009) inoculated specific-pathogen-free domestic mallard ducklings with ABV4 (psittacine origin) cultured in DEF. Although virus could be identified in the brains of two infected birds and most seroconverted, clinical disease and pathologic lesions did not result. Delnatte and colleagues inoculated domestic Embden geese intramuscularly with brain homogenates from ABV-infected Canada geese (P. Delnatte, unpublished work). Inoculated birds showed mild non-specific clinical signs, and mild-to-moderate non-suppurative encephalitis was present in 4/13 experimental birds. ABV was identified by qRT-PCR in the brain of one of these birds; further RT-PCR testing on tissue samples, characterization of the fecal shedding of ABV and the development of any serologic response are ongoing.
ABV in North America
Within North America, ABV are associated with two very different patterns of disease. In captive psittacine birds, infection is often confined to particular flocks and is considered to have a high morbidity and mortality although, as described earlier, subclinical or chronically infected birds have been identified and are likely under recognized. In wild birds, particularly waterfowl, infection appears to be present in populations across enormous geographic ranges, sometimes at very high prevalence, and frequently associated with a lack of observable clinical disease.
While the initial identification of ABV in free-ranging waterfowl was surprising, the broad distribution of the virus is not. North American waterfowl often occur in large flocks that can migrate long distances, and mix together and separate into subgroups through the course of a year. The waterfowl are widely distributed and some species, for example, Canada geese and mute swans occur in both migratory and resident populations. Close mixing of waterfowl species occurs frequently, particularly in areas with limited appropriate habitat, enhancing transmission of the virus among, as well as within, species.
Density-dependent transmission of disease in waterfowl has been shown for a number of different avian pathogens and ABV infection is likely similarly influenced. The predominant means of spread of ABV is likely fecal-oral, and waterfowl graze and defecate both on land and in water. Viral infection probably occurs through ingestion, but intranasal, inhalation and even trans-cloacal routes of spread are all possible. Viral survival and spread may be enhanced by the propensity of waterfowl to spend time in moist areas, and by the abundant, large, wet droppings with which they heavily contaminate their environment. Persistent high bird density is seen with some species, such as Canada geese, which remain in geographically restricted areas while raising their young each year, and in locations that are particularly attractive. Based on studies in parrots that show persistent infection and shedding (in our aviary we have detected intermittent ABV shedding by healthy birds for at least 5 years), seemingly healthy waterfowl could transmit virus for long periods of time and migrating birds could transmit ABV over long distances. The presence of virus in the brains and feces of large numbers of apparently healthy birds suggests that subclinical disease, or even more benign forms of viral carriage, may be the normal state. The conditions that result in the development of the impressive pathologic lesions described by Delnatte et al. (Reference Delnatte, Ojkic, DeLay, Campbell, Crawshaw and Smith2012a) are yet to be elucidated. As pointed out above, vertical transmission may also occur and this may play a role in determining the outcome of ABV infections.
Conclusions
Since 2008 our knowledge of the family Bornaviridae has undergone a remarkable transformation. Borna disease virus, the sole member of the family and known as the cause of a geographically restricted disease of mammalian hosts, has been joined by a number of avian bornavirus genotypes. Bornaviruses infecting psittacine birds show genetic diversity and proventricular disease of parrots fits the epidemiologic profile of an emerging disease. Bornaviruses infecting waterfowl, in contrast, show a remarkable lack of genotypic diversity and appear to be stable and endemic in North America, although they are also capable of producing significant pathology and mortality. Indeed, based on current information, wild waterfowl appear to constitute the largest pool of bornaviruses. Given the migratory nature of these birds, it is unlikely that waterfowl bornaviruses are restricted to a single continent. We anticipate that they will eventually be found among Central/South American and Eurasian waterfowl as well. We suggest, therefore, that the Bornaviridae, like avian influenza, are predominantly waterfowl viruses. However, based on their possible ‘jump’ into parrots, their identification in canaries, and the sporadic reports of ABV identification and PDD-like disease in a wide range of avian species, we have likely only started to recognize the broad range of avian species that may be infected, and affected by this virus.
Many questions remain to be answered. For example, our understanding of the pathogenesis of ABV-related disease is limited. Much has been assumed on the basis of rodent studies using BDV. On a deeper level, what is the relationship among the avian bornaviruses, and what is the origin of the psittacine ABV genotypes? EBLN are present in the genome of a variety of mammals, especially African ones, implying a very ancient origin and a much wider distribution of the Bornaviridae in the past. The presence of EBLN in ground squirrels implies that ABV is not new to North America and that a mammalian reservoir may remain undetected in this region. There are no significant EBLN in the genomes of chickens, zebra finch and scarlet macaw, implying that, at least in the past, bornaviruses were predominantly found in mammals. The topic of birds and bornaviruses is one that will continue to occupy researchers for decades to come.




