Introduction
Environmental influences on the mother have a significant effect on the offspring’s phenotypic performance. Maternal effects include the influence of dam milk production, uterine feeding and mothering abilities on her lamb that may be temporary or permanent (Tosh and Kemp, Reference Tosh and Kemp1994; Saatci et al., Reference Saatci, Ap Dewi and Ulutas1999; Maniatis and Pollott, Reference Maniatis and Pollott2002; Gowane et al., Reference Gowane, Chopra, Prakash and Arora2010a; Bangar et al., Reference Bangar, Magotra and Yadav2020; Magotra et al., Reference Magotra, Bangar, Chauhan, Malik and Malik2021). Maternal permanent environmental effects explain the dam effect for each lambing rather than the genetic influence. Additionally, neonatal lamb behaviours are also important indicators for lamb survival and growth (Matheson et al., Reference Matheson, Bünger and Dwyer2012). Lamb growth rate is an expression of the adaptability and economic viability of the animal and can be considered as a selection criterion for superior germplasm. Therefore, a sequential selection procedure should be adopted for the improvement of growth rate in sheep. The investigation of pre-weaning and post-weaning body weight additionally directs the breeders to choose the ideal management practices to achieve the gain at optimum level (Van den Bergh, Reference Van den Bergh1990; Kumar et al., Reference Kumar, Gangaraju, Kumar and Nath2018).The existence of covariance components and genetic variability among different growth traits are a guiding light for formulating appropriate selection strategies for the genetic improvement of small ruminants.
Many researchers (Tosh and Kemp, Reference Tosh and Kemp1994; Saatci et al., Reference Saatci, Ap Dewi and Ulutas1999; Maniatis and Pollott, Reference Maniatis and Pollott2002; Van Wyk et al., Reference Van Wyk, Fair and Cloete2009; Gowane et al., Reference Gowane, Chopra, Prakash and Arora2010a; Bangar et al., Reference Bangar, Magotra and Yadav2020) have indicated that maternal environmental effects make substantial contributions to the offspring’s phenotypic performance. Therefore, incorporation of maternal component in the analytical models will increase the accuracy of parameter estimates, while exclusion may lead to biased estimates (Saatci et al., Reference Saatci, Ap Dewi and Ulutas1999; Prince et al., Reference Prince, Gowane, Chopra and Arora2010; Singh et al., Reference Singh, Pannu, Narula, Chopra, Naharwara and Bhakar2016; Gowane et al., Reference Gowane, Chopra, Prakash and Arora2010a, 2018; Bangar et al., Reference Bangar, Magotra and Yadav2020; Magotra et al., Reference Magotra, Bangar, Chauhan, Malik and Malik2021).
Munjal is a mutton-type non-descript sheep breed of Indian origin. The Munjal is a quite massive sheep with a dark brown face (Figure 1). Wool obtained from this breed is very coarse and hairy. Munjal sheep is economically a very efficient animal due to its early maturity, faster growth rate and shorter lambing interval compared with Magra, Malpura and Muzaffarnagri sheep breeds (Poonia, Reference Poonia2008; Yadav et al., Reference Yadav, Arora, Bhatia and Singh2011). There has been no published study on the estimation of (co)variance components and genetic parameters for additive and maternal effects for growth traits, average daily gain and Kleiber ratio in Munjal sheep.

Figure 1. Photograph of a Munjal sheep.
Therefore, the objective of the present investigation was to estimate the genetic parameters of direct and maternal effects on the growth traits of Munjal sheep by fitting six animal models.
Materials and methods
Data records
The data and pedigree information on Munjal sheep were collected from the Sheep Breeding Farm, Department of Animal Genetics and Breeding, LUVAS, Hisar (India), over the period from 2004 to 2019. This information included pedigree information (animal, sire and dam number), birth information (date of birth and lamb’s sex) and performance records [birth weight (BWT), weaning weight (WT3), 6-months body weight (WT6) and 12-month body weight (WT12)]. Average daily gain [0–3 months (ADG1), 3–6 months (ADG2) and 6–12 months (ADG3)] and Kleiber’s ratio (KR1 = ADG1/WT30.75; KR2 = ADG2/WT60.75 and KR3 = ADG3/WT120.75) were also calculated from primary data included in the study.
The data structure, numbers of sires and dams, least squares means, standard deviation (SD) and coefficient of variation for each trait are summarized in Table 1. Data that were available for analysis included 706 lamb records born from 48 sires and 199 dams for BWT, 678 lamb records born from 48 sires and 198 dams for WT3, 511 lamb records born from 46 sires and 181 dams for WT6, 383 lamb records born from 45 sires and 154 dams for WT12 were included in this study.
Table 1. Data structure for growth traits in Munjal sheep
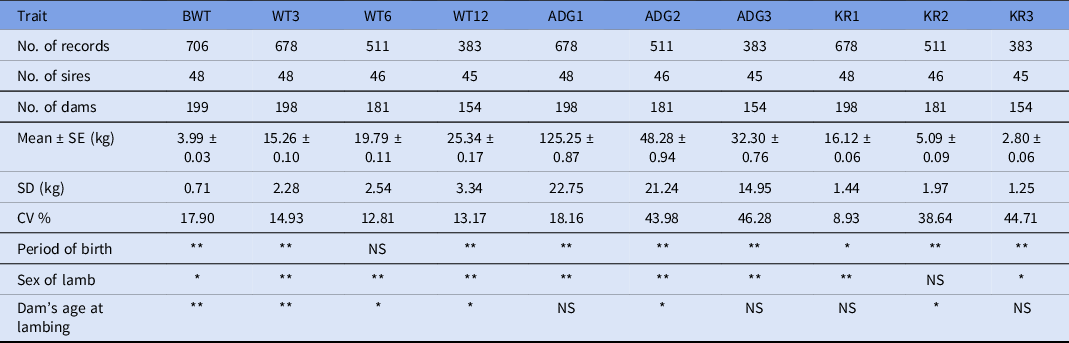
CV, coefficient of variation; SD, standard deviation; SE, standard error. *P < 0.05; **P < 0.01; NS: non-significant.
Statistical analysis
The general linear model that consisted of the fixed effects of period of birth [two groups: (1) 2004–2011; and (2) 2012–2019], sex of the lamb (two groups: male and female) and dam’s age at lambing (three groups: less than 3 years; 3–5 years and more than 5 years) was used to estimate its significance on targeted traits. Then, the following six univariate animal models were used under restricted maximum likelihood method (AI-REML) using WOMBAT software (Meyer, Reference Meyer2006):
where Y is the vector of observations; β, a, m, c and ϵ are vectors of fixed, direct additive genetic, maternal genetic, maternal permanent environmental effects and residual effects, respectively; with respective association matrices X, Za, Zm and Zc; A is the numerator relationship matrix between animals; and σam is the covariance between additive direct and maternal genetic effects. The selection of the most appropriate animal model for a particular trait was done using log-likelihood ratio. Furthermore, genetic, phenotypic and residual correlation among targeted traits was obtained under a bivariate model.
Results
Least squares analysis revealed significant (P < 0.05) association of period of birth and sex of lamb with the traits under study except for BWT6 and KR2 respectively. The least squares mean with standard error for BWT, BWT3, BWT6 and BWT12 in Munjal sheep was 3.99 ± 0.03, 15.26 ± 0.10, 19.79 ± 0.11, and 25.34 ± 0.17 kg, respectively. ADG1, ADG2, ADG3, KR1, KR2 and KR3 were observed as 125.25 ± 0.87 g, 48.28 ± 0.94 g, 32.30 ± 0.76 g, 16.12 ± 0.06, 5.09 ± 0.09 and 2.80 ± 0.06, respectively. Age of dam at lambing showed significant (P < 0.05) association with all the traits except ADG1, ADG3 and their corresponding Kleiber ratio (Table 1). Based on the best model, the estimates of variance components and genetic parameters for various traits under study are presented in Table 2. The respective log-L value obtained after successful convergence for best model is also given for each trait. The model including direct additive genetic and maternal genetic effect (Model 2) without taking covariance between them into account was the most appropriate model for BWT and weaning weight, i.e. WT3. While for the remaining traits, the addition of maternal genetic or environmental effects (Models 2–6) was non-significant. Therefore, Model 1 with direct additive effects only was considered as most appropriate model for these traits.
Table 2. Estimates of variance components and heritability for growth traits in Munjal sheep
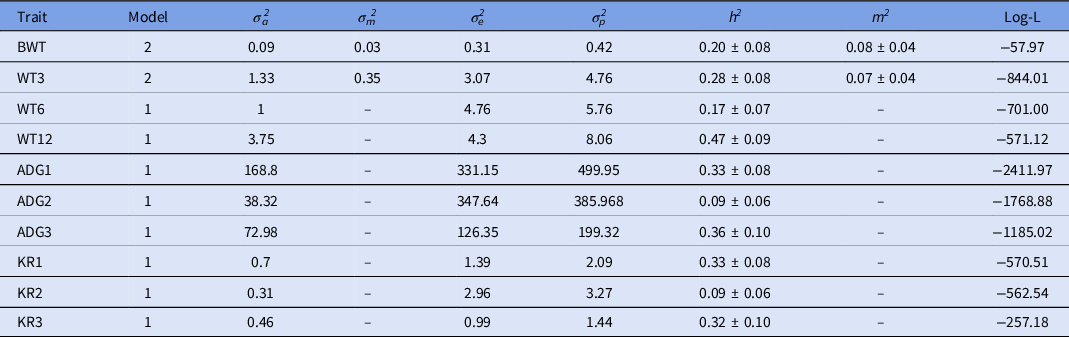
σa 2 , σm 2 , σe 2 and σp 2 are additive genetic, maternal genetic, residual variance and phenotypic variance, respectively; h2 and m2 are direct and maternal heritability respectively; and log-L is log-likelihood.
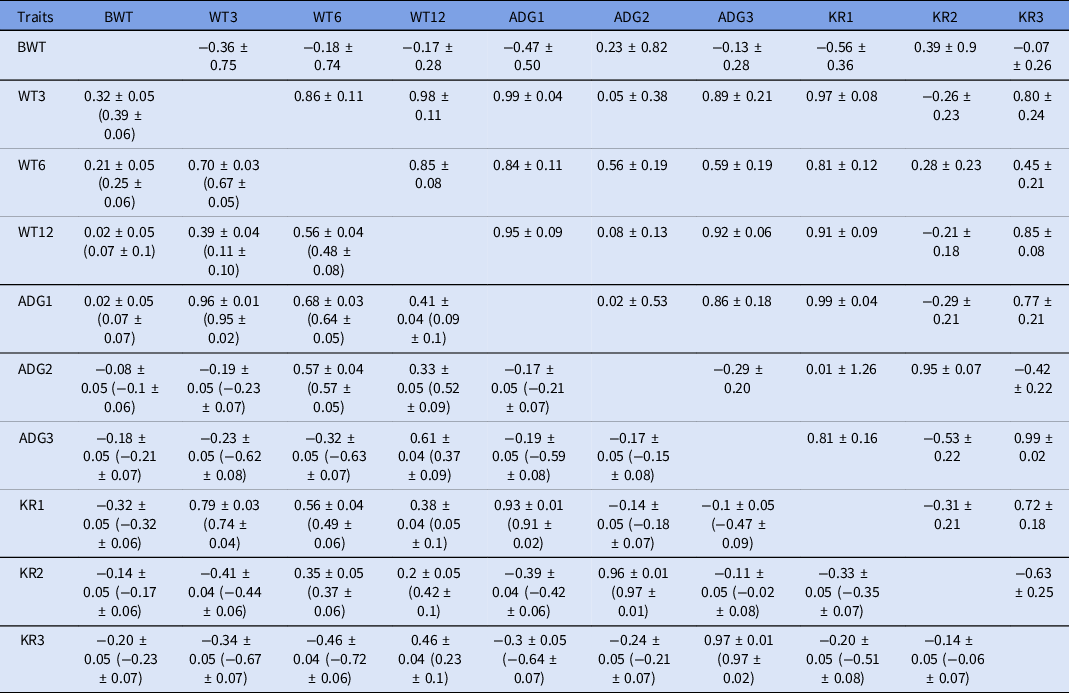
The genetic and phenotypic correlations among various growth traits were estimated under a bivariate model and are given in Table 3. The genetic correlation estimates of BWT were positive, with ADG2 (0.23) and KR1 (0.39) only. While, they were negative and ranged from −0.56 to −0.07 with remaining traits. The genetic correlation of WT3 was high and positive with post-weaning growth traits (0.86–0.98), growth rates (0.05–0.99) and Kleiber ratios (0.80–0.97). Additionally, the phenotypic correlations of WT3 with WT6, WT12, ADG1 and KR1 were moderate to high (0.39–0.96).
Table 3. Genetic (above diagonal), phenotypic (below diagonal) and residual (in parenthesis) correlations among various growth traits in Munjal sheep
Discussion
Our findings implied that the addition of maternal genetic effects to a direct additive model led significantly to change in log-likelihood values and provided a low-to-moderate estimate of direct and maternal heritability to birth weight. Under the best model, Model 2, the estimates of direct and maternal genetic variances were 0.09 and 0.03 respectively, which indicated the significant influence of maternal effects on BWT trait. The inclusion of maternal effects in the model showed reduction in direct additive variance as well as the estimate of direct heritability that was also reported by Kushwaha et al. (Reference Kushwaha, Mandal, Arora, Kumar, Kumar and Notter2009).
The estimate of direct heritability for BWT was 0.20 ± 0.08, which was in agreement with the estimate of Matika et al. (Reference Matika, van Wyk, Erasmus and Baker2003) in Sabi (0.25), Abegaz et al. (Reference Abegaz, van Wyk and Olivier2005) in Horro (0.20), Eskandarinasab et al. (Reference Eskandarinasab, Ghafouri-Kesbi and Abbasi2010) in Afshari (0.23), Gowane et al. (Reference Gowane, Chopra, Prakash and Arora2010a) in Malpura (0.19), Jafaroghli et al. (Reference Jafaroghli, Rashidi, Mokhtari and Shadparvar2010) in Moghani (0.25), Prince et al. (Reference Prince, Gowane, Chopra and Arora2010) in Avikalin (0.28), Prakash et al. (Reference Prakash, Prince, Gowane and Arora2012) in Malpura (0.21) and Singh et al. (Reference Singh, Pannu, Narula, Chopra, Naharwara and Bhakar2016) in Marwari sheep (0.28). Lower estimates than the current study were reported by Bangar et al. (Reference Bangar, Magotra and Yadav2020) in Harnali (0.10), Gowane et al. (Reference Gowane, Chopra, Prince, Paswan and Arora2010b) in Bharat Merino (0.05), Mohammadi et al. (Reference Mohammadi, Rashidi, Mokhtari and Esmailizadeh2010) in Sanjabi (0.14) and Rashidi et al. (Reference Rashidi, Mokhtari, Jahanshahi and Abadi2008) in Kermani sheep (0.04).
The maternal heritability for BWT in this study was 0.08 ± 0.04, which was in accordance with findings of Baneh et al. (Reference Baneh, Hafezian, Rashidi, Gholizadeh and Rahimi2010) in Ghezel sheep (0.04), Mohammadi et al. (Reference Mohammadi, Shahrebabak, Shahrebabak, Bahrami and Dorostkar2013) in Shal (0.12) and Singh et al. (Reference Singh, Pannu, Narula, Chopra, Naharwara and Bhakar2016) in Marwari sheep (0.09). However, it was lower than Duguma et al. (Reference Duguma, Schoeman, Cloete and Jordaan2002) in Tyger-hoek Merino (0.25), Rashidi et al. (Reference Rashidi, Mokhtari, Jahanshahi and Abadi2008) in Karmani sheep (0.24) and Bangar et al. (Reference Bangar, Magotra and Yadav2020) in Harnali sheep (0.16). The strong influence of maternal genetic effect at birth weight indicated the potential of maternal ability for lamb’s initial performance.
Weaning weight (WT3)
The direct and maternal genetic variance for weaning weight (WT3) under the best Model 2 was observed as 1.33 and 0.35, respectively. This estimate was in accordance with estimates reported by Duguma et al. (Reference Duguma, Schoeman, Cloete and Jordaan2002) in Tyger-hoek Merino, Baneh et al. (Reference Baneh, Hafezian, Rashidi, Gholizadeh and Rahimi2010) in Ghezel, Abbasi and Ghafouri-Kesbi (Reference Abbasi and Ghafouri-Kesbi2011) in Makooei, Kamjoo et al. (Reference Kamjoo, Baneh, Yousefi, Mandal and Rahimi2014) in Iran-Black and Lalit et al. (Reference Lalit, Dalal, Dahiya, Patil and Dahiya2016) in Harnali sheep, but was higher than reports of Ozcan et al. (Reference Ozcan, Ekiz, Yilmaz and Ceyhan2005) in Turkish Merino, Mohammadi et al. (Reference Mohammadi, Shahrebabak, Shahrebabak, Bahrami and Dorostkar2013) in Shal and Boujenane et al. (Reference Boujenane, Chikhi, Ibnelbachyr and Mouh2015) in D’man sheep. That the role of maternal effects reduces from birth to weaning and post-weaning was also reported previously by Mandal et al. (Reference Mandal, Neser, Rout, Roy and Notter2006) and Kushwaha et al. (Reference Kushwaha, Mandal, Arora, Kumar, Kumar and Notter2009).
The direct heritability estimates for weaning weight from the best model was 0.28 ± 0.08. This moderate estimate was in accordance with estimates reported by Duguma et al. (Reference Duguma, Schoeman, Cloete and Jordaan2002) in Merino (0.26), Bahreini Behzadi et al. (Reference Bahreini Behzadi, Shahroudi and Van Vleck2007) in Kermani (0.22), Eskandarinasab et al. (Reference Eskandarinasab, Ghafouri-Kesbi and Abbasi2010) in Afshari (0.27), Baneh et al. (Reference Baneh, Hafezian, Rashidi, Gholizadeh and Rahimi2010) in Ghezel (0.29), Prakash et al. (Reference Prakash, Prince, Gowane and Arora2012) in Malpura (0.24) and Singh et al. (Reference Singh, Pannu, Narula, Chopra, Naharwara and Bhakar2016) in Marwari (0.27). Lower estimates than found in the current study were obtained by Gowane et al. (Reference Gowane, Chopra, Prince, Paswan and Arora2010b) in Bharat Merino (0.04) and Jafaroghli et al. (Reference Jafaroghli, Rashidi, Mokhtari and Shadparvar2010) in Moghani sheep (0.17). Higher estimates were reported by El Fadili et al. (Reference El Fadili, Michaux, Detilleux and Leroy2000) in Moroccan Timahdit (0.49), Abbasi and Ghafouri-Kesbi (Reference Abbasi and Ghafouri-Kesbi2011) in Makooei, Kamjoo et al. (Reference Kamjoo, Baneh, Yousefi, Mandal and Rahimi2014) in Iran-Black, Lalit et al. (Reference Lalit, Dalal, Dahiya, Patil and Dahiya2016) and Bangar et al. (Reference Bangar, Magotra and Yadav2020) in Harnali sheep (0.38 and 0.45, respectively). The maternal heritability for WWT in this study was 0.07 ± 0.04 and was within the range of published values by Hanford et al. (Reference Hanford, Van Vleck and Snowder2005) in Rambouillet (0.08), Ekiz et al. (Reference Ekiz, Ozcan, Yilmaz and Ceyhan2004) and Ozcan et al. (Reference Ozcan, Ekiz, Yilmaz and Ceyhan2005) in Turkish Merino (0.03) and Mandal et al. (Reference Mandal, Neser, Rout, Roy and Notter2006) in Muzaffarnagri sheep. Whereas, Bahreini Behzadi et al. (Reference Bahreini Behzadi, Shahroudi and Van Vleck2007) in Kermani sheep (0.19) reported higher estimates. Maternal effects are defined as the maternal genotype or phenotype causal influence on the offspring’s phenotype. Because the mother contributes a specific mRNA or protein to the oocyte, maternal effects are common (Schier, Reference Schier2007). Therefore, maternal influences at the weaning stage must be taken into consideration, along with direct effects in our resource population to make effective selection strategies.
Post-weaning traits
The estimates of direct additive heritability were due to most appropriate model for WT6 0.17 ± 0.07. This finding for WT6 was in agreement with results reported by Kushwaha et al. (Reference Kushwaha, Mandal, Arora, Kumar, Kumar and Notter2009) in Chokla (0.16), Gowane et al. (Reference Gowane, Chopra, Prakash and Arora2010a) in Malpura (0.27), Mohammadi et al. (Reference Mohammadi, Rashidi, Mokhtari and Beigi Nassiri2011) in Zandi sheep (0.13) and Mohammadi et al. (Reference Mohammadi, Shahrebabak, Shahrebabak, Bahrami and Dorostkar2013) in Shal sheep (0.16). These estimates were lower than those accounted by Kamjoo et al. (Reference Kamjoo, Baneh, Yousefi, Mandal and Rahimi2014) in Iran-Black and Singh et al. (Reference Singh, Pannu, Narula, Chopra, Naharwara and Bhakar2016) in Marwari sheep (0.28 and 0.29) and Bangar et al. (Reference Bangar, Magotra and Yadav2020) in Harnali sheep (0.32 and 0.23). As WT6 was the existing selection criteria at the farm, our results showed low levels of additive variation at this stage that may be less effective for improving the performance of lambs. For the high expected genetic gain, one must choose a trait with at least moderate range additive variation, which was the weaning stage under the present study. The selection criteria can be switched depending upon additive variation among the traits. However, optimization of variability and selection criteria over the years is of utmost importance for setting efficient breeding programmes.
For the WT12 trait, surprisingly, we observed moderate level heritability (0.47 ± 0.09) under Model 1 that was contrary to and higher than the reports by Bahreini Behzadi et al. (Reference Bahreini Behzadi, Shahroudi and Van Vleck2007) in Kermani (0.10 and 0.14), Gowane et al. (Reference Gowane, Chopra, Prince, Paswan and Arora2010b) in Bharat Merino (0.00 and 0.09) and Mohammadi et al. (Reference Mohammadi, Rashidi, Mokhtari and Beigi Nassiri2011) in Zandi sheep (0.13). However, estimates on a similar line for this trait have been reported in previous publications such as Ozcan et al. (Reference Ozcan, Ekiz, Yilmaz and Ceyhan2005) in Turkish Merino (0.25), Kushwaha et al. (Reference Kushwaha, Mandal, Arora, Kumar, Kumar and Notter2009) in Chokla (0.23), Kamjoo et al. (Reference Kamjoo, Baneh, Yousefi, Mandal and Rahimi2014) in Iran-Black, Singh et al. (Reference Singh, Pannu, Narula, Chopra, Naharwara and Bhakar2016) in Marwari sheep (0.29) and Bangar et al. (Reference Bangar, Magotra and Yadav2020) in Harnali sheep (0.23). This high heritability estimate in our study might be due to the lesser dataset.
Average daily gain (ADG) and Kleiber ratio (KR)
The estimates of direct heritability for ADG1, ADG2, ADG3, KR1, KR2 and KR3 resulting from the best model were 0.33 ± 0.08, 0.09 ± 0.06, 0.36 ± 0.10, 0.33 ± 0.08, 0.09 ± 0.06 and 0.32 ± 0.10, respectively. The estimates of direct additive heritability due to Model 1 for ADG1, ADG2, KR1 and KR2 were in conformity with those reported by Bangar et al. (Reference Bangar, Magotra and Yadav2020) in Harnali sheep. Lower estimates than in the present study were reported by Eskandarinasab et al. (Reference Eskandarinasab, Ghafouri-Kesbi and Abbasi2010) in Afshari, Ghafouri-Kesbi et al. (Reference Ghafouri-Kesbi, Abbasi, Afraz, Babaei, Baneh and Arpanahi2011) in Zandi, Prakash et al. (Reference Prakash, Prince, Gowane and Arora2012) in Malpura, Kesbi et al. (Reference Kesbi, Eskandarinasab and Hassanabadi2008) in Mehraban, Mandal et al. (Reference Mandal, Karunakaran, Sharma, Baneh and Rout2015) in Muzaffarnagari, Jafari and Razzagzadeh (Reference Jafari and Razzagzadeh2016) in Makuie and Kumar et al. (Reference Kumar, Gangaraju, Kumar and Nath2018) in Nellore sheep. However, higher estimates than in the present study were reported by Illa et al. (Reference Illa, Gollamoori and Nath2019) in Nellore sheep.
As these ADG and KR traits are generated from the growth traits under this study, moderate estimates of additive variation for these traits were similar to those of pre-weaning growth traits. These generated traits can be combined with weaning weight, i.e. the moderately heritable trait under this study to set stringent selection plan accounting growth rates and feed conversion efficiency.
Correlation estimates
For the estimated genetic correlation of BWT with remaining traits, the present findings were in accordance with estimates reported by Kamjoo et al. (Reference Kamjoo, Baneh, Yousefi, Mandal and Rahimi2014) in Iran-Black sheep. However, these were higher than the findings reported by Kesbi et al. (Reference Kesbi, Eskandarinasab and Hassanabadi2008) in Mehraban and Gowane et al. (Reference Gowane, Chopra, Prince, Paswan and Arora2010b) in Bharat Merino sheep. The high and positive genetic correlation of WT3 with post-studied traits was in accordance with reports by Swain et al. (Reference Swain, Gopikrishna, Gaur and Sanyal2004) in Bharat Merino and by Bangar et al. (Reference Bangar, Lawar, Nimbalkar, Shinde and Nimase2018) in Deccani sheep. This indicated a strong linear relationship between weaning weight and other traits and also suggested that the selection for one trait can improve other traits. The genetic correlations between WT6 and other traits were also positive and ranged from 0.28 to 0.85 and were in accordance with reports by Kamjoo et al. (Reference Kamjoo, Baneh, Yousefi, Mandal and Rahimi2014) in Iran-Black and Bangar et al. (Reference Bangar, Lawar, Nimbalkar, Shinde and Nimase2018) in Deccani and Kesbi et al. (Reference Kesbi, Eskandarinasab and Hassanabadi2008) in Mehraban sheep. The genetic correlations of ADG1 were low to highly positive (0.02 to 0.99) with all traits except for BWT (−0.47) and KR2 (−0.24). The positive genetic associations of ADG1 and KR1 with BWT, WT3, WT6, WT9 and WT12 were also reported by Mohammadi et al. (Reference Mohammadi, Shahrebabak, Shahrebabak, Bahrami and Dorostkar2013) in Shal sheep. ADG2 also provided positive genetic correlation with all traits except ADG3 and KR3.
The phenotypic correlations of BWT with other traits were very low and negative for all traits except WT3 (0.32) and WT6 (0.21), which were lower than the report by Singh et al. (Reference Singh, Pannu, Narula, Chopra, Naharwara and Bhakar2016) on Marwari sheep. These low estimates may be due to improper care of newborn lambs in the initial days. The phenotypic correlations of WT3 with WT6, WT12, ADG1 and KR1 were moderate to high (0.39 to 0.96), which was also reported by Mohammadi et al. (Reference Mohammadi, Shahrebabak, Shahrebabak, Bahrami and Dorostkar2013) in Shal sheep. However, WT3 had a negative phenotypic correlation with ADG2, ADG3, KR2 and KR3 due to compensatory growth effects, whereas, all average daily gains, i.e. ADG1, ADG2 and ADG3 were positively correlated with their corresponding KRs, i.e. KR1, KR2 and KR3. Phenotypic correlation was found to be negative among all KRs. Similar findings were reported by Mandal et al. (Reference Mandal, Karunakaran, Sharma, Baneh and Rout2015) in Muzaffarnagari sheep.
In conclusion, the moderate level of additive genetic variability at weaning weight estimated under alternate animal modelling indicated the scope for genetic improvement through early selection at weaning weight. In addition to this, maternal effects were found to be contribute significantly to variation at early growth traits. It is worth to mention here that the genetic relationship of weaning trait was also moderately positive with other traits. Therefore, it is suggested that the optimization of maternal effects along with direct effects should be done under stringent breeding plans to achieve the desirable performance of offspring in their lifetime.
Conflict of interest
The authors report no declarations of interest.
Acknowledgements
The authors are thankful to the Director of Research, LUVAS, Hisar (Haryana), India for providing the necessary facilities to conduct this study.






