INTRODUCTION
The prevalence of depression is particularly high in patients with advanced cancer. A systematic literature review has found the prevalence of major depressive disorder, defined by a diagnostic interview, to range from 5% to 26% (median 15%), whereas the prevalence of clinically significant depressive symptoms, assessed using the Hospital Anxiety and Depression Scale, ranges from 23% to 45% (Hotopf et al., 2002). This indicates that a considerable proportion of palliative care patients suffer from significant depression that could impair their quality of life. Yet, depression remains largely underdiagnosed and undertreated in this population (Wilson et al., 2000).
Beck's cognitive therapy (Beck et al., 1979b), a type of cognitive-behavioral therapy (CBT), is an empirically supported treatment for depression in the general population (DeRubeis & Crits-Christoph, 1998). Cognitive therapy is at least as efficacious as antidepressants in the treatment of major depression (Rush et al., 1977; Murphy et al., 1984; Hollon et al., 1992), even for moderate to severe major depression (DeRubeis et al., 2005). Furthermore, cognitive therapy is associated with a better maintenance of therapeutic gains over time, in comparison to short-term antidepressant therapy (Kovacs et al., 1981; Blackburn et al., 1986; Simons et al., 1986; Evans et al., 1992; Hollon et al., 2005).
A number of studies have assessed the efficacy of psychological interventions incorporating cognitive-behavioral strategies in cancer patients. Most of these studies have found significant positive outcomes, including reduction of depression, anxiety, fatigue, and general psychological distress (e.g., Fawzy et al., 1990; Edgar et al., 1992; Greer et al., 1992; Marchioro et al., 1996; Watson et al., 1996; Moorey et al., 1998; Antoni et al., 2001). However, to our knowledge, only one of these studies has been conducted exclusively in women with metastatic breast cancer (Edelman et al., 1999a). In that study, 124 women with metastatic breast cancer were assigned either to eight weekly sessions of CBT administered in a group format or to standard care (i.e., no CBT). The intervention incorporated a variety of behavioral and cognitive techniques (e.g., relaxation, problem solving, goal setting, communication strategies, cognitive restructuring), and expression of feelings and building of group support were encouraged in the group. Patients who received CBT showed greater improvements in depression, total mood disturbance, and self-esteem relative to patients in the control group at posttreatment. However, there were no group differences at the 3- and 6-month follow-up assessments. One of the explanations put forth by the authors for this lack of sustained gains over time is the impossibility in a group intervention of individualizing the intervention to each patient's specific needs. Studies assessing the efficacy of individual CBT, the most common treatment format used in clinical settings (Goodwin, 2004), were consequently recommended.
A recent pilot study supported the feasibility of short-term (i.e., eight weekly sessions) cognitive therapy administered individually in six women with metastatic cancer (Lévesque et al., 2004). This study, using an experimental case study design, revealed significant improvements in depression symptoms (e.g., anhedonia) and some associated features (i.e., anxiety, fatigue) for each participant, effects that were generally well maintained at the 6-month follow-up evaluation. Although promising, these preliminary findings need to be replicated in a randomized controlled trial.
Another area that warrants further investigation is the impact of psychological interventions on patients' medical status or physiological functioning potentially relevant to cancer progression. Except for the early study published by Spiegel et al. (1989), none of the other studies conducted among women with metastatic breast cancer have found any increase in survival associated with the administration of a psychological intervention (Cunningham et al., 1998; Edelman et al., 1999b; Goodwin et al., 2001). Assessing the effect of a treatment targeting a particular psychological disorder that has been found to be associated with impaired physiological functioning has the potential to shed new light on this matter. Cross-sectional studies conducted in noncancer populations have consistently found a relationship between depression and immunosuppression (Weisse, 1992; Herbert & Cohen, 1993; Cohen & Herbert, 1996; Irwin, 1999). Although still being debated (Garssen & Goodkin, 1999), the psychoneuroimmunology model of cancer postulates that this down-regulating effect of depression on immune functioning could ultimately affect cancer progression (Reiche et al., 2004). Conversely, depression treatment may be associated with improved immunological functioning, which could, in turn, translate into increased cancer survival, a hypothesis that has yet to be investigated.
The main goal of this study was to assess the efficacy of cognitive therapy, administered individually, in reducing depression among women with metastatic cancer. An exploratory goal was to assess the effect of this intervention on immunological functioning.
METHODS
Participants
Study Population
The participants were recruited between May 1999 and June 2003, mainly through systematic screening of depressive symptoms in three cancer clinics (Centre des maladies du sein Deschênes-Fabia of the Hôpital St-Sacrement [HSS] and haematooncology departments of L'Hôtel-Dieu de Québec [HDQ] and L'Hôtel-Dieu de Lévis [HDL]) using the depression subscale of the Hospital Anxiety and Depression Scale (HADS-D; Zigmond & Snaith, 1983). A small number of participants were recruited with the aid of posters and pamphlets as well as through physician referrals.
The study inclusion criteria were as follows: (1) a diagnosis of metastatic breast cancer (stage IV) and (b) a score of 7 or more on the HADS-D or of 15 or more on the Beck Depression Inventory (BDI; Beck et al., 1961). Exclusion criteria were (1) being in a terminal stage of the disease defined as a life expectancy of less than 2 months; (2) meeting DSM-IV criteria (American Psychiatric Association, 1994) for a severe psychiatric disorder other than major depression (e.g., psychotic, substance use disorders); (3) presenting severe suicidal ideations with a risk of acting out, as evaluated by the Scale for Suicide Ideation (SSI; Beck et al., 1979a); (4) having recently (i.e., within the past 2 months) started an antidepressant medication or recently altered the dosage taken of an antidepressant medication; and (5) currently receiving a psychological intervention targeting depression. Patients excluded from the study were referred to the Psycho-oncology Service of HDQ.
Sample Characteristics
Figure 1 shows the rates of recruitment, exclusion, refusal, and dropout throughout the study. Of the 333 women screened for depressive symptoms, 75 (23% of women initially screened) obtained a HADS-D score of 7 or higher at some point. Of those, 45 (14% of women initially screened) were found eligible at the clinical interview and were enrolled in the study and randomized. Demographic and clinical characteristics of the 37 participants included in the analyses are presented in Table 1. All patients were Caucasian. Cardiovascular disease was the most common comordid physical condition (n = 7). Of all demographic and clinical variables examined, the only significant group difference at pretreatment was the time passed since the initial cancer diagnosis, with the cognitive therapy group having a longer duration of cancer (M = 7.61 years) compared to the control group (M = 4.35), t(35) = 2.08, p = .05.
Demographic and medical variables


Flow diagram of participants' progress through the study phases.
Experimental Design
Participants were first stratified according to the cancer clinic where they were recruited (HSS, HDQ, or HDL), and then randomly assigned either to the (1) cognitive therapy (CT) or (2) waiting-list control (WLC) condition (see Fig. 2). The group allocation was contained in individually sealed envelopes, prepared by the principal investigator prior to study initiation using a computer-generated random numbers table. Participants assigned to the WLC group waited for a period corresponding to the duration of the intervention (8 weeks) and were reassessed on the study variables before receiving CT. This second assessment (pretreatment or postwaiting assessment) of control patients was contrasted to the posttreatment evaluation of treated patients to assess the short-term effects of CT. Additional evaluations were conducted 3 and 6 months after the end of their respective treatment to assess the maintenance of treatment effects over time. The study was approved by the ethical review boards of HSS, HDQ, HDL, and l'Université Laval.
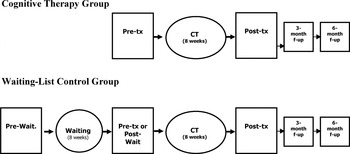
Study design. Pre-tx: pretreatment; post-tx: posttreatment; f-up: follow-up; pre-wait.: prewaiting; post-wait.: postwaiting.
Measures
Psychological Measures
Structured Clinical Interview for DSM-IV (SCID; First et al., 1996). The purpose of this interview is to evaluate the presence of current and past psychiatric disorders according to DSM-IV diagnostic criteria (American Psychiatric Association, 1994).
Scale for Suicide Ideation. The SSI (Beck et al., 1979a) is a semistructured interview evaluating the severity of suicidal ideation. The SSI was translated into French by the first author for use in this study.
Hospital Anxiety and Depression Scale (HADS; Zigmond & Snaith, 1983). This is a 14-item questionnaire divided into two sub-scales: depression (HADS-D: 7 items) and anxiety (HADS-A: 7 items). The HADS contains no somatic items that may be confused with symptoms of the physical illness. The French-Canadian version possesses psychometric qualities equivalent to those of the original English version (Savard et al., 1998).
Beck Depression Inventory (Beck et al., 1961). The BDI includes 21 items evaluating the severity of depressive symptoms, each with four response choices. The French-Canadian version used was developed and empirically validated by Bourque and Beaudette (1982), who found the psychometric properties to be comparable to those of the English version.
Hamilton Depression Rating Scale (HDRS; Hamilton, 1960). This clinical interview contains 17 items assessing the severity of depression symptoms. In this study, the Structured Interview Guide for the Hamilton Depression Rating Scale (Williams, 1988) was used to standardize its administration. This interview was translated into French by the first author.
Insomnia Severity Index (ISI; Morin, 1993). The ISI is a 7-item questionnaire evaluating insomnia severity (e.g., difficulties falling asleep). Each item is rated using a 5-point Likert scale ranging from 0 (not at all) to 4 (very much), for a total score ranging from 0 to 28. The French-Canadian version of the ISI was recently validated in the context of cancer (Savard et al., 2005) and psychometric properties equivalent to those of the original version were found (Bastien et al., 2001; Smith & Trinder, 2001).
Multidimensional Fatigue Inventory (MFI; Smets et al., 1995). The French-Canadian version used, which possesses adequate psychometric properties (Fillion et al., 2003), is a short form of the original MFI. It contains 15 items (on a scale from 1 to 5) divided into four subscales including general and physical fatigue, reduction in activity, reduction in motivation, and mental fatigue. A global score of fatigue is obtained by calculating a mean for all items.
The European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ-C33; Aaronson et al., 1993; Osoba et al., 1997). This questionnaire was developed and validated with cancer patients. Only the global quality of life scale, comprised of three items (i.e., physical condition, overall health, overall quality of life), was used as an outcome measure in this study. Other scales were used as potential confounding variables, including the physical functional scale, two symptom scales (pain and nausea/vomiting), and four single items (dyspnea, appetite loss, constipation, and diarrhea). The scores obtained are transformed to give a score ranging from 0 to 100. The French version was developed by the authors of the original English version.
The EORTC Breast Cancer-Specific Quality of Life Questionnaire Module (QLQ-BR23; Sprangers et al., 1996). This questionnaire was developed to evaluate the physical symptoms frequently associated with breast cancer. One functional scale (sexuality) and one symptom scale (systemic therapy side effects) were used as potential confounders in this study. As for the QLQ-C33, scores are transformed to give a score ranging from 0 to 100. The French-Canadian version was developed by our research team and has not yet been empirically validated.
Health Behaviors Questionnaire (HBQ). The HBQ assesses recent (i.e., past week) health behaviors that may potentially confound the relationship between psychological aspects and immune function (e.g., use of alcohol, tobacco, and exercise level) (Savard et al., 2003).
List of Life Events (LLE). This 19-item questionnaire was adapted from the Inventory of Recent Life Experiences for Cancer Patients (Fillion et al., 2001), which is a measure of cancer-related life events, and the List of Threatening Experiences (Brugha et al., 1985), which is a measure of general stressful life events. The presence of stressful life events was used as a potential confounding variable.
Immunological Measures
At each assessment point, 36 ml of venous blood was collected in four heparinized tubes. The tubes were centrifuged at 1800 r.p.m. for 5 min at room temperature and the buffy coat was collected (3 ml by tube). Analyses were performed by laboratory personnel blind to the patients' randomization.
Lymphocyte subpopulations. White blood cell (WBC) subsets were determined in the whole blood by three-color direct immunofluorescence using a Coulter flow cytometer (Coulter EPICS Elite ESP, Beckman Coulter, Miami, FL). A minimum of 10,000 cells per sample was analyzed. To analyze lymphocyte surface antigens, monoclonal antibodies (Abs) were directly conjugated with either fluorescein isothiocyanate (FITC), phycoerythrin (PE), or peridinin chlorophyll protein (PerCP). Briefly, for each subset analysis, 10 μl Abs (TriTEST) were added to 50 μl of buffy coat and incubated for 20 min. The erythrocytes were then disintegrated using OPTILYSE-C reagent (Immunotech, Miami, FL). Enumeration by flow cytometry included the following cells: T cells (CD3+; CD3 FITC), T helper cells (CD3+CD4+; CD3 FITC/CD4 PE/CD45 PerCP), T suppressor/cytotoxic cells (CD3+CD8+; CD3 FITC/CD8 PE/CD45 PerCP), and natural killer (NK) cells (CD3−/CD16+CD56+; CD3 FITC/CD16+CD56 PE/CD45 PerCP). All Abs and immunofluorescence reagents were purchased from Becton Dickinson (San Jose, CA). The absolute number per unit volume bearing each lymphocyte marker was determined by multiplying data obtained by flow cytometry with the absolute lymphocyte count derived from the complete blood count (CBC) and differential. The CBC was performed within 2 h after the blood was drawn, whereas flow cytometry was conducted the next morning (within 12 to 15 h after the blood was drawn).
NK cell activity. Peripheral blood lymphocytes were separated by density gradient centrifugation on a Ficoll-Hypaque gradient (Amersham Pharmacia Biothech, Piscataway, NJ) the morning following the blood withdrawal (within 12 to 15 h) and were stored (20% dimethyl sulfoxide; 80% fetal bovine serum [FBS] at −80°C) until the assay was performed within a 3-year period after. The NK cytotoxicity was determined by flow cytometry (Robinson, 1993). This method is fast, reliable, and correlates well with the standard 51 CR-release assay while avoiding the use of radioactive material (Kroesen et al., 1992; Chang et al., 1993; Papadopoulos et al., 1994). Peripheral blood lymphocytes were thawed rapidly in a 37°C water bath, washed with RPMI 1640 + 10% FBS, and kept for one night in a humidified 5% CO2 atmosphere at 37°C. The following morning, they were transferred in culture flasks and kept for 30 min in a humidified 5% CO2 atmosphere at 37°C. Effector cells were washed, counted, and adjusted to 5 × 106/ml. Target cells, K562, a human erythroleukemic cell line (American Type Culture Collection, Rockville, MD; CC L243) in log phase were washed, counted, and adjusted to 1 × 105/ml. Subsequently, the effector cells and target cells were mixed at four ratios of effector:target (E:T) cells (50:1, 25:1, 12.5:1, and 6.25:1) and were incubated at 37°C in a humidified 5% CO2 incubator for 10 min to promote conjugate formation. Then, 10 μl of propidium iodide working solution (100 μl/ml) were added into each tube and incubated for 90 min at 37°C in a 5% CO2. Finally, cells were stored in a dark ice bath, and flow cytometric data acquisition was performed using a Coulter flow cytometer (Coulter EPICS Elite ESP, Beckman Coulter). A previous study in which the same procedure was used revealed that cryopreservation of blood samples had no influence on NK cell activity (Savard et al., 2003).
Cytokine secretion. For determination of IL-1β and IFN-γ production, a whole blood assay was performed (Kirchner et al., 1982). For stimulation of IL-1β, aliquots of 50 μl of buffy coat were resuspended in 445 μl of RPMI 1640 medium (Cellgro, Winset Canadian Laboratories, St-Bruno, Canada) and 5 μl of lipopolysaccharide from Escherichia coli (0.1 mg/ml). For stimulation of IFN-γ, aliquots of 50 μl of buffy coat were resuspended in 440 μl of RPMI 1640 medium, and 10 μl phytohemagglutinin (2%) were added. Every sample was stimulated in duplicate the morning following the blood withdrawal (within 12 to 15 h). Then, the samples were incubated for a minimum of 72 h at 37°C with 5% CO2. The supernatant was stored at −80°C until the assay was performed within a 3-year period after the blood withdrawal. A minimum incubation time of 72 h was chosen on the basis of previous kinetic studies indicating that it provides a good estimate for cytokines assessed in this study (De Groote et al., 1992). All cytokine levels were measured by ELISA kits (Biosource International, Camarillo, CA). The sensitivities of the assays were 1 pg/ml for IL-1β and 4 pg/ml for IFN-γ.
Procedure
Screening
To identify women potentially eligible for the study, a systematic screening for depressive symptoms was conducted in each cancer clinic. All women with metastatic breast cancer were invited to provide informed consent to complete at repeated intervals the HADS-D, either at the clinic or by telephone, and to be approached to participate in the present study if considered eligible based on this information. Women potentially eligible were thus contacted by telephone by the research coordinator who provided brief information about the study and invited them to a face-to-face clinical interview to further assess their eligibility for the study.
Clinical Interview
During this interview, patients were first provided with the detailed information about the study, and their written consent to participate was obtained. Subsequently, the patients were asked to complete once again the HADS-D and the BDI to confirm the presence of clinical levels of depressive symptoms. A clinical psychologist then administered the SCID and the SSI to rule out the presence of severe psychiatric disorders and suicidal ideations. This was followed by the administration of the HDRS by an independent evaluator, a resident in psychiatry, who was blind to study objectives and procedures.
Patients found eligible for the study and who accepted the randomization procedure were then randomized. Then, blood samples were drawn by a registered nurse and the Health Behavior Questionnaire was administered. To control for diurnal variations, all blood samples for a particular participant were drawn at the same time (±1 h). Finally, the participant received a battery of self-report scales to be completed at home (i.e., MFI, ISI, QLQ-C33, QLQ BR-23, LLE). The first treatment session was scheduled 2 weeks later for CT patients to allow completion of questionnaires, whereas it was scheduled 10 weeks later for WLC patients.
Treatment
CT was administered individually and involved eight weekly sessions of 60 to 90 min. Strategies that were elaborated for the treatment of depression in the general population (Beck et al., 1979b) were slightly adapted to meet the specific needs of women with metastatic cancer. The ultimate goal was to develop an optimistic but realistic attitude toward their situation, as opposed to a negative (e.g., only thinking of death) or overly positive attitude (e.g., hoping to be cured). Two licensed psychologists with experience in the application of CT (i.e., 5 and 10 years) conducted CT sessions. Regular meetings were held to ensure treatment integrity. Because missed treatment sessions were rescheduled (n = 18), all patients received the entire treatment program, excluding the seven patients who dropped out of the study during the course of CT (see Fig. 1).
CT began with the presentation of a cognitive theory of emotions (Beck et al., 1979b). Then, participants were encouraged to increase their level of daily activities, initially by self-monitoring their activities, then by planning more pleasant and energizing activities every day. This was done sensitively, taking into consideration each participant's physical condition. In some cases, the goal was rather to decrease extenuating activities (e.g., house cleaning) in favor of doing more leisure activities and activities leading to a sense of accomplishment. Participants were then trained to identify their negative thoughts (e.g., “I am going to die alone and in pain”; “Life is no longer worth living since I know I am going to die”; “I am no longer useful to my family; I am a burden”; “My metastases are progressing; it means that I am going to die within the next two months”) and to use cognitive restructuring to modify dysfunctional or irrational cognitions about cancer and other situations in their life (e.g., “I know that the most important people to me will be there when I die and my doctor will ensure that I am sufficiently medicated to control pain”; “No one knows how long I am going to live; I may have enough time to achieve some goals that are important to me”; “It is true that I can't do as much as I used to, but I'm sure they are happy that I am still alive and happy to take care of me”; “My physician told me that there are still a couple of other treatment options that may slow down the progression”). Patients were then encouraged to redefine their life goals. Patients with advanced cancer often believe they can no longer have life goals because they have an incurable condition, an attitude that strongly enhances depression. During treatment, participants were encouraged to identify short-, medium- and even long-term objectives, the rationale being that they are better off to set life goals that they may not have time to achieve than to have no life goals, wait only for death to come, and to feel depressed. Finally, to help prevent a relapse of depression, future high-risk situations (e.g., cancer progression, treatment failure, terminal stage of the disease, hospitalization, autonomy loss) were identified, as well as strategies to cope with them.
Booster Sessions
Three booster sessions of CT were administered to participants every 3 weeks following treatment. The goal of these sessions was to review the difficulties the patient had experienced since the last session and the strategies used (or that could have been used) to cope with them.
Posttreatment and Follow-up Evaluations
At the posttreatment evaluation, as well as 3 and 6 months after the end of treatment, the participants again met the independent evaluator, who was blind to study objectives and procedures and the patients' group allocation, for the administration of the HDRS and to complete the battery of self-report scales (i.e., HADS, BDI, MFI, ISI, QLQ-C33, QLQ BR-23, LLE).
Analyses
Statistical Analyses
Data were carefully inspected to identify missing data and outliers and to assess normality (Tabachnik & Fidell, 2001). No significant outliers were found and no missing data imputation was performed. Descriptive and inferential statistics were conducted using SAS 8.2 statistical software (SAS Institute, 2001). The alpha level was set at 5% (two-tailed) for all inferential tests. The main analyses were based on a split-plot group (two conditions) × time (four assessments; pretreatment, posttreatment, and 3-month and 6-month follow-ups) randomized design, although WLC patients actually completed two pretreatment assessments (prewaiting and pretreatment). Due to the unequal number of time assessments across groups, two subsets of analyses were performed. First, analyses were conducted to determine whether treated patients had greater improvements on all dependent variables at posttreatment compared to patients in the control group following their waiting period. These findings are reported in the Group Comparisons subsections, and because of this objective, significant group × time interactions are emphasized. Second, all data were pooled together (while including the group effect to control for possible cohort effects) to assess, with a larger sample size, the possible benefits associated with the intervention at posttreatment and evaluate whether the therapeutic gains observed at posttreatment were maintained at follow-up assessments. These findings are reported in the Pooled Analyses subsections and significant time effects are emphasized.
Data were analyzed within an intent-to-treat framework. Thus, all patients with at least one observation post-randomization were included in the analyses (see Fig. 1; CT: n = 21; WLC: n = 16). Linear mixed models were used to test group, time, and interaction effects for all continuous dependent variables. A priori contrasts were used to break down these effects. Within-group effect sizes (and their 95% CI) were computed as the raw difference (and its 95% CI) divided by the square root of the mixed model mean square error (Bird, 2002). Satterthwaite F tests were computed because they are typically more robust to nonnormality, unbalanced data, and violations of multisample sphericity (Keselman et al., 2001).
Following the strategy suggested by Frigon and Laurencelle (1993), various covariates were tested to assess their capacity to reduce the error term. To be included in the mixed model analyses as a covariate, a variable had to meet these three criteria: (1) a significant relationship between the covariate and the dependent variables, (2) within-group slope homogeneity between groups, and (3) a significant reduction of error variance for at least 50% of dependent variables. For psychological dependent variables, 10 covariates met these criteria and were therefore included in the mixed models to control for their potential impact on psychological outcomes: lifetime use of hormone therapy, oxazepam (Serax®) use, lifetime use of pamidronate (Aredia®), systemic therapy side effects (QLQ BR-23), appetite loss (QLQ-C33), pain level (QLQ-C33), alcohol use (HBQ), tobacco use (HBQ), activity level (HBQ), and the perceived impact of life events (LLE). For immunological dependent variables other than NK cell activity, five covariates were included in the mixed models: lifetime use of chemotherapy, appetite loss (QLQ-C33), alcohol use (HBQ), tobacco use (HBQ), and the perceived impact of life events (LLE). Finally, for NK cell activity, the seven covariates included in the mixed models were oxazepam (Serax®) use, number of local recurrences, delay since adjuvant radiation therapy, natural product use (HBQ), physical functioning (QLQ-C33), nausea/vomiting (QLQ-C33), and constipation (QLQ-C33) symptoms. Because it was difficult to obtain a sufficient quantity of blood in many patients (and sometimes even to collect blood at all), these analyses were computed on a more limited number of patients. In fact, 39% and 50% of immune data were missing for CT and WLC groups, respectively. Moreover, because there were insufficient cells to perform the NK cell activity assay at ratios 50:1 and 25:1, only two E:T ratios were used, that is, 12.5:1 and 6.25:1.
Clinical Significance
Four criteria of clinical significance of treatment effects were used. First, the proportion of patients having a significant mood improvement as assessed by the patient herself (score of 5 or higher on a scale ranging from 0, not at all, to 7, extremely), the clinician, and a significant other (score of 3 or greater on a scale ranging from 0, not at all, to 4, extremely) was calculated. Second, the proportion of patients with a depression score falling under the clinical cut-off score was used. The cut-off scores used were 7 for the HADS-D (Savard et al., 1999), 15 for the BDI (Bourque & Beaudette, 1982), and 12.6 for the HDRS, the later corresponding to the mean score obtained in six studies assessing the diagnostic accuracy of this instrument (Bagby et al., 2004). Third, the proportion of patients with a reduction in depression scores of at least 50% was assessed. Finally, the proportion of patients obtaining a score greater than 67 on the global quality of life scale of the QLQ-C33 was used. This score corresponds to the median score obtained by a group of 150 women with metastatic breast cancer in another study (McLachlan et al., 1998).
RESULTS
Depression
Group Comparisons
A significant group × time interaction was obtained for HDRS scores (p < .01; see Table 2 and Fig. 3). A priori contrasts conducted on this variable revealed a significant time effect in the treatment condition, t(23) = 6.88, p < .0001, but not in the control condition, t(22) = 1.84, p = .08. As shown in Table 2, the mean HDRS score decreased from 14.2 to 6.9 (d = −1.81) in the treatment condition, whereas it decreased only from 14.4 to 12.2 (d = −0.54) in the control condition. Findings for BDI scores were in the same direction, with a greater reduction in the treatment condition (from 21.1 to 11.5; d = −1.86) than in the control condition (from 20.4 to 15.9; d = −0.86). However, the group × time interaction obtained for this variable only approached statistical significance (p = .08). When somatic and cognitive/affective BDI items were analyzed separately (data not shown), a significant group × time interaction was found on somatic items, F(1,26) = 6.69, p < .05, but not on cognitive/affective items (p = .33). Finally, the group × time interaction obtained for the HADS-D scores was not significant (p = .32), although the effect size estimated for time effects was much greater for the treatment condition (d = −1.82) than the control condition (d = −1.30).
Results of group comparisons: adjusted means (and SE) and effect sizes for each group and results of the group × time interaction


Group comparisons on depression scores.
Pooled Analyses
Significant reductions from pre- to posttreatment were obtained for all depression measures, namely the HDRS, BDI, and the HADS-D (all ps < .0001; see Table 3 and Fig. 4). As for the maintenance of these gains over time, no significant differences were obtained between posttreatment and follow-up assessments on BDI (p = .13) and HADS-D scores (p = .24), although the means indicated some further reduction in depressive symptoms during the follow-up period. Indeed, the mean BDI score further decreased from 12.0 at posttreatment to 9.5 at the 6-month follow-up, t(73) = 3.60, p = .06, and the mean HADS-D score decreased from 4.4 to 3.4 during the same period, t(69) = 2.11, p = .15. An additional significant reduction of HDRS scores was obtained from posttreatment to the follow-up period (p < .01). A priori contrasts showed that this significant effect mainly occurred between the 3- and 6-month follow-up, with mean HDRS scores decreasing from 8.2 to 4.1 during that time, t(48) = 9.71, p < .01.
Adjusted means, standard errors for each time assessment and time effects obtained from the pooled data set


Depression scores across time for both groups pooled together.
Other Psychological and Quality of Life Variables
Group Comparisons
No significant group × time interaction was found for any of the additional psychological variables, namely anxiety (p = .24), fatigue (p = .68), insomnia (p = .14), and global quality of life (p = .94; see Table 2). However, the effect sizes for time effects obtained were consistently greater in the treated group than the control group (HADS-A: −1.39 vs. −0.84; ISI: −0.96 vs. −0.09; MFI: −0.94 vs. −0.74).
Pooled Analyses
Significant reductions from pre- to posttreatment were obtained on anxiety, insomnia, and fatigue scores (all ps < .01). The mean HADS-A score decreased from 9.9 to 6.7, the mean MFI score decreased from 3.3 to 2.9, and the mean ISI score decreased from 11.5 to 5.5. These gains were maintained during the follow-up period as shown by the absence of significant differences between posttreatment and follow-up on HADS-A (p = .29), MFI (p = .55), and ISI (p = .18) scores. However, no significant difference was observed between pre- to posttreatment on the global quality of life, nor between posttreatment and the follow-up period.
Immunological Variables
Group Comparisons
Significant group × time interactions were found only for enumerative measures of CD3+ (p < .05), CD4+ (p < .05), and CD8+ cells (p < .05). However, as shown in Table 2, there were important between-group differences at baseline, with control patients having uniformly higher values for these immune parameters than patients in the treatment condition, differences that tend to disappear at posttreatment (or postwaiting). Thus, it would appear that the significant interaction effects observed are more likely related to a phenomenon of regression to the mean than a real treatment effect on immune functioning.
Pooled Analyses
Analyses revealed no significant differences between pretreatment and posttreatment. Comparisons between posttreatment and follow-up revealed a significant time effect only for NK cell activity (see Table 3) and a trend analysis revealed a significant quadratic effect, indicating that NK cell activity increased from posttreatment to 3-month follow-up but decreased to posttreatement level at the 6-month follow-up.
Clinical Significance
Among the total sample, 88% of the patients perceived that their mood was significantly improved at posttreatment. This proportion was 89% and 57% when the patient's degree of improvement was judged by the clinician and the significant other, respectively. Table 4 shows that the proportion of patients obtaining depression scores falling under the clinical cut-off score used was much higher in CT patients at posttreatment (from 73% to 87% of the patients) than WLC patients after their waiting period (from 25% to 58% of the patients). A majority of the patients attained this criterion at the 6-month follow-up (from 85% to 100% of the patients). Similarly, the proportion of patients showing at least a 50% reduction in depression scores was higher in CT patients at posttreatment (from 40% to 73% of the patients) compared to WLC patients after their waiting period (from 17% to 33% of the patients). Between 43% and 100% of the patients attained this criterion at the 6-month follow-up evaluation, with higher proportions obtained on HDRS. Finally, 43% of CT patients had a score greater than 67 on the global quality of life scale at posttreatment compared to only 20% of WLC patients at postwaiting. Between 33% and 42% attained that criterion at the 6-month follow-up evaluation.
Proportion of patients reaching clinical significance criteria by group and at each time assessment

DISCUSSION
The main goal of this randomized controlled study was to assess the efficacy of CT, administered individually, for the treatment of depressive symptoms in women with metastatic breast cancer. Although the group comparison was statistically significant on HDRS scores only, comparison of means obtained on other measures (BDI, HADS-D) at posttreatment consistently revealed a greater reduction in depression scores in treated patients compared to the control group. In addition, analyses conducted on the pooled data set indicated a significant reduction for all depression measures (BDI, HADS-D, HDRS) from pre- to posttreatment, gains that were sustained, and in some cases (HDRS) even further increased significantly, during the follow-up phase. CT for depression appeared to be associated with other positive outcomes, including decreased anxiety, fatigue, and insomnia symptoms, although the results were significant only when using the pooled data set. In addition, using various criteria of psychological functioning and quality of life, the treatment effects were found to be clinically significant in a considerable proportion of patients.
Thus, the general pattern of findings supports the efficacy of CT for depression in this population. However, the failure to find significant group × time interactions at posttreatment for several variables including the BDI and the HADS-D, two key dependent variables, deserves comment. Although one could argue that the only possible conclusion is that CT did not impact significantly on those variables, we think this argument would overlook the magnitude of effect sizes obtained. Indeed, large effect sizes were obtained in the treated group at posttreatment (BDI: d = −1.86; HADS-D: d = −1.82), which were much larger than those found in the control group (BDI: d = −0.86; HADS-D: d = −1.30). Thus, it seems reasonable to conclude that with a slightly larger sample size and increased statistical power, those findings would have become statistically significant. Unfortunately, as in other clinical studies conducted in metastatic patients (Edelman et al., 1999a), recruitment was a major challenge in this study and had to be stopped before attaining the projected sample size (n = 80) because of insufficient funding. It is also interesting to note that despite the fact that patients were selected on the basis of a minimal depression score and that most of them were actually suffering from a depressive disorder (73%), on average patients were not severely depressed at study entry, thus leaving less room for improvement. Previous studies that have assessed the efficacy of CT for depression in the general population included patients with BDI scores varying from 28 to 30 and HDRS scores varying from 18 to 24 at pretreatment (Murphy et al., 1984; Hollon et al., 1992), which is much higher than the scores of this study's participants (BDI: 17.5; HDRS: 12.5).
Treatment effects obtained in this study were of a greater magnitude for the HDRS relative to the BDI or the HADS-D. This is consistent with previous data indicating that the HDRS is more sensitive to clinical change than the BDI (Edwards et al., 1984; Lambert et al., 1986; Sayer et al., 1993), a finding that may be due to differences in measure content. The HDRS contains a larger proportion of somatic and behavioral items than the BDI, whereas the HADS-D contains no somatic item whatsoever. In this study, when cognitive/affective and somatic items of the BDI were analyzed separately, larger treatment effects were obtained for somatic items. This suggests that somatic and behavioral symptoms of depression improved more rapidly than other symptoms in this study, a hypothesis that is supported by previous data showing a more rapid decline of HDRS scores than BDI scores with depression treatment (Lambert et al., 1988). This may be related to the fact that CT begins with behavioral strategies (e.g., activity scheduling) that can have a strong beneficial effect on somatic and behavioral symptoms (e.g., sleep, fatigue, psychomotor retardation).
In general, this study supports the clinical significance of treatment effects. As much as 88% of patients perceived their mood improvement at posttreatment as clinically significant according to the criterion used. In addition, between 85% and 100% of women had a depression score falling under the clinical range at 6-month follow-up. The proportion of patients with at least a 50% reduction of depression scores relative to pretreatment data was somewhat lower, which is not surprising given that patients were generally not severely depressed at study entry. Finally, although fairly weak, the proportion of patients attaining the quality of life criterion (score > 67) at the 6-month follow-up evaluation ranging from 33% to 42% was much higher than the proportion of 0% obtained at pretreatment.
A secondary goal of this study was to assess the effect of CT for depression on immune function. No treatment effect was found on any of the immune variables. This finding contrasts with the results of previous studies, which have shown that psychological interventions can improve immunological functioning of patients with early-stage breast cancer (Gruber, 1993; Schedlowski et al., 1994; Larson et al., 2000; Andersen et al., 2004; McGregor et al., 2004). On the other hand, this finding is consistent with most studies that have failed to show any increase in survival associated with psychotherapy in women with metastatic breast cancer (Cunningham et al., 1998; Edelman et al., 1999b; Goodwin et al., 2001). It is likely that the important number of missing data on these variables has weakened the statistical power to detect an intervention effect. It is also possible that the potential influence of the metastatic disease itself on immune function goes beyond that of a psychological intervention in this population. Moreover, although we did everything possible to control for potential confounding variables that could affect immune function, these patients receive such a significant amount of treatment and medication for their disease that it becomes extremely difficult to effectively control for all of these factors.
This study is characterized by several strengths, including the use of a randomized design, selection of distressed patients, use of a theory-driven treatment, and use of a treatment manual. On the other hand, the small sample size and the associated lack of statistical power for several analyses is an important study limitation. However, it is increasingly argued that statistical significance is not the only or necessarily the best way to test hypotheses and that other indices such as effect sizes that are not influenced by sample size provide more useful information (Kline, 2004). Other limitations, including the fact that participants were all Caucasian, mostly well educated, were all breast cancer patients, and constituted a small proportion of all patients that were screened for the study, may limit the generalization of the results. The study is further limited by the use of a waiting-list control condition that did not control for nonspecific therapeutic ingredients. It is therefore impossible to determine whether the changes observed are really attributable to CT or to other ingredients common to all psychotherapeutic approaches (e.g., therapist empathy, treatment expectancies). Additionally, although it was advantageous from an ethical point of view, the fact that all patients received the intervention at some point limited the assessment of between-group differences in the long term.
In sum, this study supports the efficacy of CT for treating depressive symptoms in women with metastatic breast cancer. The notable maintenance of mood improvements over time obtained in this study, which is impressive considering the evolutive nature of the disease, compares favorably to a previous study conducted in the same population that failed to show sustained posttreatment gains at the 3- and 6-month follow-up evaluations (Edelman et al., 1999a). The better maintenance of gains in this study may be attributable to the use of individual treatment sessions, which allows the intervention to be tailored to the patient's needs. Alternatively, it may be due to the administration of three booster sessions following the end of treatment. Indeed, a continuation phase has been found to significantly reduce the relapse rate following acute CT for depression in the general population (Fava et al., 1994; Jarrett et al., 1998).
This study has important clinical implications. It suggests that short-term CT can effectively treat depression symptoms in women with metastatic breast cancer, in spite of the seriousness of the disease. Systematic screening of depression should therefore be implemented in this population, which could be followed by short-term CT. However, given the evolutive nature of metastatic disease, it may be necessary to offer individualized interventions and booster sessions, as needed or at fixed intervals, to decrease the risk of depression relapse. Future studies could assess more directly the differential efficacy of group and individual interventions, as well as compare the maintenance of gains over time of a short-term intervention with or without booster sessions.
ACKNOWLEDGMENTS
The preparation of this article was supported in part by an operating grant from the Canadian Breast Cancer Research Initiative (010436) and salary support from the Canadian Institutes of Health Research awarded to the first author. The authors wish to acknowledge the important contribution of the administrative and medical staff of the Centre des maladies du sein Deschênes-Fabia, L'Hôtel-Dieu de Québec, and l'Hôtel-Dieu de Lévis, Mylène Lévesque, Lucie Casault, Séverine Hervouet, Aude Caplette-Gingras, Dominique Rioux, Véronique Dupéré, Marie-Hélène Savard, Catherine Gonthier, Francine Matte, David Olivier, Geneviève Bossé, Serge Côté, Diane Langlois, Diane Bisson, Nancy Roberge, Monique Pelletier, Hélène Drombrowski, and Denise L'Héreault.










