Introduction
Hydatigera Lamarck, 1816 (Cestoda: Taeniidae) is a recently resurrected genus (Nakao et al., Reference Nakao, Lavikainen, Iwaki, Haukisalmi, Konyaev, Oku, Okamoto and Ito2013) comprising four valid species: Hydatigera taeniaeformis (Batsch, 1786), Hydatigera parva (Baer, Reference Baer1924), Hydatigera krepkogorski Schulz and Landa, 1934 and the recently discovered Hydatigera kamiyai Lavikainen et al., Reference Lavikainen, Iwaki, Haukisalmi, Konyaev, Casiraghi, Dokuchaev, Galimberti, Halajian, Henttonen, Ichikawa-Seki, Itagaki, Krivopalov, Meri, Morand, Näreaho, Olsson, Ribas, Terefe and Nakao2016. Adult Hydatigera tapeworms occur in the small intestine of felid and viverrid definitive hosts and are characterized by large rostellar hooks. Larval taeniid stages are broadly known as metacestodes and specifically named strobilocerci for Hydatigera species. These develop in tissues and body cavities of rodents as intermediate hosts and feature prominent segmented strobilae (Nakao et al., Reference Nakao, Lavikainen, Iwaki, Haukisalmi, Konyaev, Oku, Okamoto and Ito2013; Lavikainen et al., Reference Lavikainen, Iwaki, Haukisalmi, Konyaev, Casiraghi, Dokuchaev, Galimberti, Halajian, Henttonen, Ichikawa-Seki, Itagaki, Krivopalov, Meri, Morand, Näreaho, Olsson, Ribas, Terefe and Nakao2016).
Human-mediated introductions, in addition to ancestral migratory and colonization events, of hosts and their taeniid parasites have made the geographical distribution of Hydatigera spp. cosmopolitan, with reports and molecular data generated from intermediate and definitive hosts worldwide (Jones and Pybus, Reference Jones, Pybus, Samuel, Pybus and Kocan2001; Lavikainen et al., Reference Lavikainen, Iwaki, Haukisalmi, Konyaev, Casiraghi, Dokuchaev, Galimberti, Halajian, Henttonen, Ichikawa-Seki, Itagaki, Krivopalov, Meri, Morand, Näreaho, Olsson, Ribas, Terefe and Nakao2016). However, information on Hydatigera spp. from Africa remains limited. Aside from the description of adult H. parva in a common genet (Genetta genetta) from South Africa (Baer, Reference Baer1924) and in an African wildcat (Felis silvestris lybica) from the Democratic Republic of the Congo (Baer and Fain, Reference Baer, Fain and de Witte Mission1965), polycephalic strobilocerci identified as H. parva have been described in Nile rats (Arvicanthis niloticus) from Sudan (Elowni and Abu Samra, Reference Elowni and Abu Samra1988), in pygmy mice (Mus minutoides) from Nigeria (George et al., Reference George, Obiamiwe and Anadu1990), in greater Egyptian gerbils (Gerbillus pyramidum) from Tunisia (Bernard, Reference Bernard1963), in southern multimammate mice (Mastomys coucha) from South Africa (Julius et al., Reference Julius, Schwan and Chimimba2018) and in Guinea multimammate mice (Mastomys erythroleucus) from Sierra Leone and the Democratic Republic of the Congo (Southwell and Kirshner, Reference Southwell and Kirshner1937; Mahon, Reference Mahon1954). Larval stages of H. taeniaeformis have been observed in rats and other wild rodents from Egypt (Wanas et al., Reference Wanas, Shehata and Rashed1993), Nigeria (Udonsi, Reference Udonsi1989; Ivoke, Reference Ivoke2009), South Africa (Julius et al., Reference Julius, Schwan and Chimimba2018) and Sudan (Fagir and El-Rayah, Reference Fagir and El-Rayah2009), whereas Nelson and Rausch (Reference Nelson and Rausch1963) observed Cysticercus fasciolaris Rudolphi, 1808 (i.e. C. fasciolaris is a historical synonym of H. taeniaeformis (Nakao et al. (Reference Nakao, Lavikainen, Iwaki, Haukisalmi, Konyaev, Oku, Okamoto and Ito2013)) in the liver of black rats (Rattus rattus) in Kenya. Nevertheless, to our knowledge, the identity of Hydatigera isolates from the African continent has been molecularly confirmed only for specimens found in Rattus spp. from Ethiopia and South Africa (Lavikainen et al., Reference Lavikainen, Iwaki, Haukisalmi, Konyaev, Casiraghi, Dokuchaev, Galimberti, Halajian, Henttonen, Ichikawa-Seki, Itagaki, Krivopalov, Meri, Morand, Näreaho, Olsson, Ribas, Terefe and Nakao2016).
Studies on helminth communities of rodents in Senegal have enhanced our understanding of the impact of spatio-temporal factors on both biodiversity/abundance of rodent parasites (e.g. Brouat et al., Reference Brouat, Kane, Diouf, Bâ, Sall-Dramé and Duplantier2007; Sall-Dramé et al., Reference Sall-Dramé, Brouat, Bâ and Duplantier2010) and host population dynamics (e.g. Brouat and Duplantier, Reference Brouat and Duplantier2007; Diagne et al., Reference Diagne, Ribas, Charbonnel, Dalecky, Tatard, Gauthier, Haukisalmi, Fossati-Gaschignard, Bâ, Kane, Niang, Diallo, Sow, Piry, Sembène and Brouat2016). Nevertheless, knowledge gaps on rodents and their parasites still exist in Senegal as in the rest of sub-Saharan Africa (Bordes et al., Reference Bordes, Blasdell and Morand2015). We surveyed wild small mammal populations of the Senegal River Basin as potential intermediate hosts of larval taeniids. Our aim was to evaluate host–parasite associations between small mammal species and larval taeniids, and whether any transmission patterns relative to habitat and host characteristics could be observed.
Materials and methods
Trapping of small mammals
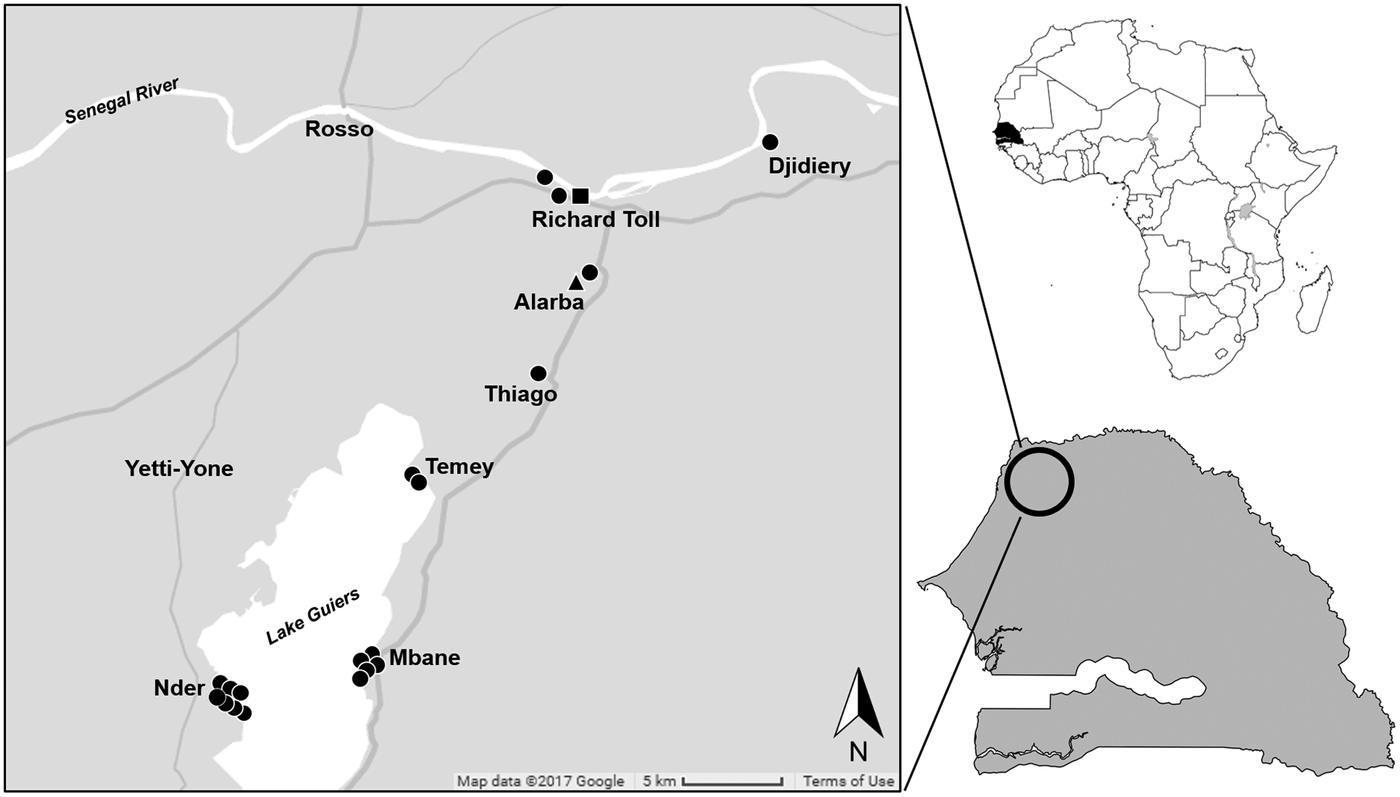
This study was conducted in sites in and around the town of Richard Toll (16°27′N, 15°41′W) and on the shores of Lake Guiers (16°15′N, 15°51′W), Senegal. Between May 2016 and April 2017, small mammals were trapped in the spring and autumn following methodologies previously described (Catalano et al., Reference Catalano, Sène, Diouf, Fall, Borlase, Léger, Bâ and Webster2018). Briefly, locally made wire-mesh live traps were used, and trapping sites were classified into two types of habitats: (i) crop fields near human dwellings and (ii) riparian habitat (i.e. habitat associated with bodies of water, dependent on the existence of perennial, intermittent or other forms of water drainage) primarily composed by thick reeds (Typha sp.). Each evening, the traps were baited with peanut butter and set in lines over a period of two to three nights per site. Each morning, we inspected the traps and recorded captures, misfires (i.e. any trap found sprung, missing or not triggered) and by-catch (i.e. any non-target species captured).
Laboratory analyses
The trapped small mammals were returned live to the laboratory and humanely euthanized by intraperitoneal injection with sodium thiopental (300 mg kg−1 body weight). Death of the animal was confirmed by cervical dislocation and the absence of pedal withdrawal reflex. At post-mortem, species, gender, sexual maturity and anatomical measurements of each individual were recorded. Age classification of rodents as juveniles or adults was based on the combined body weight, body length and reproductive status (Granjon and Duplantier, Reference Granjon and Duplantier2009; Herbreteau et al., Reference Herbreteau, Jittapalapong, Rerkamnuaychoke, Chaval, Cosson and Morand2011). During dissection, any cysts present in the thoracic and/or abdominal cavity was isolated and preserved in 95% ethanol at −20 °C until DNA extraction.
After rehydration in nuclease-free water, DNA from individual specimens was extracted using the Epicentre® MasterPure™ Complete DNA and RNA Purification Kit (Epicentre Biotechnologies, Madison, WI, USA) following the manufacturer's instructions. DNA extracts were eluted in 30 µL TE buffer and amplified for a segment of the cytochrome c oxidase subunit 1 (COI) gene of the mitochondrial DNA (mtDNA) using primers 2575 and 3021 (Bowles et al., Reference Bowles, Blair and McManus1992). Enzymatic amplification and thermocycling protocol for polymerase chain reaction (PCR) were performed in a 25 µL reaction mixture including PuReTaq™ Ready-To-Go™ PCR Beads (GE Healthcare UK Limited, Little Chalfont, UK), 0.5 µmol L−1 of each primer and 2 µL of DNA template. Cycling parameters consisted of an initial nucleic acid denaturation at 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, 52 °C for 1 min and 72 °C for 1 min, with a final 7 min extension at 72 °C. PCR products were sequenced using the original PCR primers in a 3730xl DNA Analyzer system by GATC Biotech (Konstanz, Germany). Assembly and editing of contigs were performed with a CodonCode Aligner (CodonCode Corporation, Centerville, MA, USA). The obtained COI sequences were compared by alignment with data available in the National Center for Biotechnology Information (NCBI) GenBank database.
Statistical analyses
Relative abundance of small mammals was assumed to be reflected in their effective capture rate, recorded for each trapping session and site as the number of captured animals as a whole per number of trap nights (Brouat et al., Reference Brouat, Kane, Diouf, Bâ, Sall-Dramé and Duplantier2007; Liccioli et al., Reference Liccioli, Kutz, Ruckstuhl and Massolo2014). Furthermore, we calculated the proportion of adult animals out of the total number of captures, since older animals are predicted to be more likely infected by Hydatigera metacestodes (Burlet et al., Reference Burlet, Deplazes and Hegglin2011). Variations in the proportion of adult rodents among seasons were tested using Pearson's chi-squared (χ 2) test. Logistic regression was performed to understand the association between gender (females vs males), age (adults vs juveniles), season (autumn vs spring), habitat (crop field vs riparian vegetation) and locality (Lake Guiers vs Richard Toll), included as dichotomous independent variables, on the occurrence of Hydatigera spp., included as the dichotomous response variable (with 0 for negative and 1 for infected individual). Statistical tests were implemented in R version 3.1.2 ‘Pumpkin Helmet’ (https://www.r-project.org) and were considered significant when P ⩽ 0.05.
Results
Trapping of small mammals
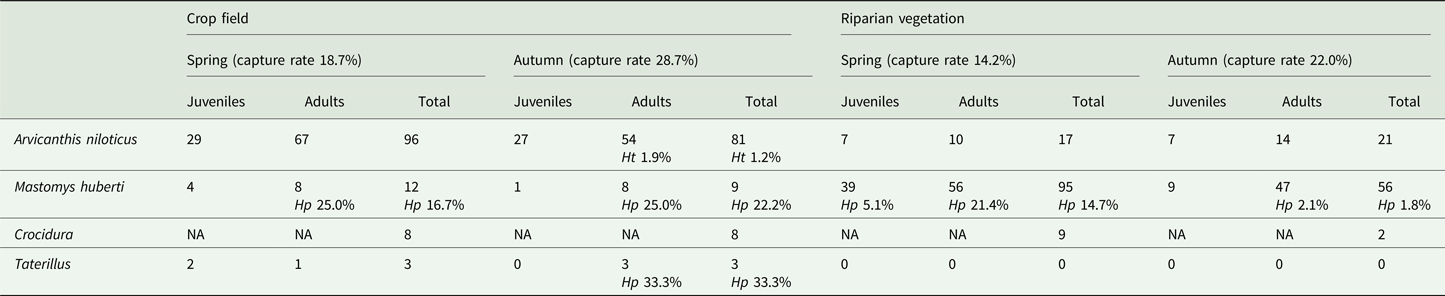
We captured 420 small mammals including 215 Nile rats (A. niloticus), 172 Hubert's multimammate mice (Mastomys huberti), 27 shrews (Crocidura sp.) and six gerbils (Taterillus sp.). Identification of Taterillus gerbils and Crocidura shrews was made to the genus level given the presence of sympatric species that are morphologically undistinguishable (Granjon and Duplantier, Reference Granjon and Duplantier2009; Galan et al., Reference Galan, Pagès and Cosson2012). We set 2531 trap-nights for an overall capture rate of 20.1% when accounting for misfires (n = 2043).
Laboratory data
One to three translucent cysts with the diameter of approximately 10 mm were observed in the abdominal cavity of infected individuals (Fig. 1), with the exception of a M. huberti in which two cysts were present in the thoracic cavity. Based on the molecular analysis of the mtDNA COI gene (396 base pairs), we identified H. parva in 19 out of 172 M. huberti (11.0%) and one out of six Taterillus sp. (16.7%), while H. taeniaeformis sensu stricto was detected in one out of 215 A. niloticus (0.5%) (Fig. 2). Alignment of the COI sequences showed the presence of two different haplotypes of H. parva (identity ⩾99.50%) isolated in M. huberti. Table 1 summarizes the results of trapping activities and parasitological analyses. Pairwise comparisons of the generated H. parva COI sequences with NCBI GenBank data available from Spain (EU544580) showed 98.74% identity; comparisons between our H. taeniaeformis sensu stricto COI sequence and data from Ethiopia (KT693060 and KT693063) and South Africa (KT693064) showed 99.24–99.50% identity (Table 2). The COI sequence data from individual specimens were deposited in the GenBank database under the accession numbers MH036503-MH036508 for H. parva and MH036509 for H. taeniaeformis sensu stricto. Representative specimens of Hydatigera spp. were archived in the collection of the Natural History Museum (London, UK) under the accession numbers 2018.3.7.1-32.

Fig. 1. Cysts containing polycephalic strobilocerci of Hydatigera parva (indicated by white arrows) isolated during the post-mortem of a Hubert's multimammate mouse (Mastomys huberti) before (A, B) and after (C) dissection in a 90 mm diameter Petri dish.
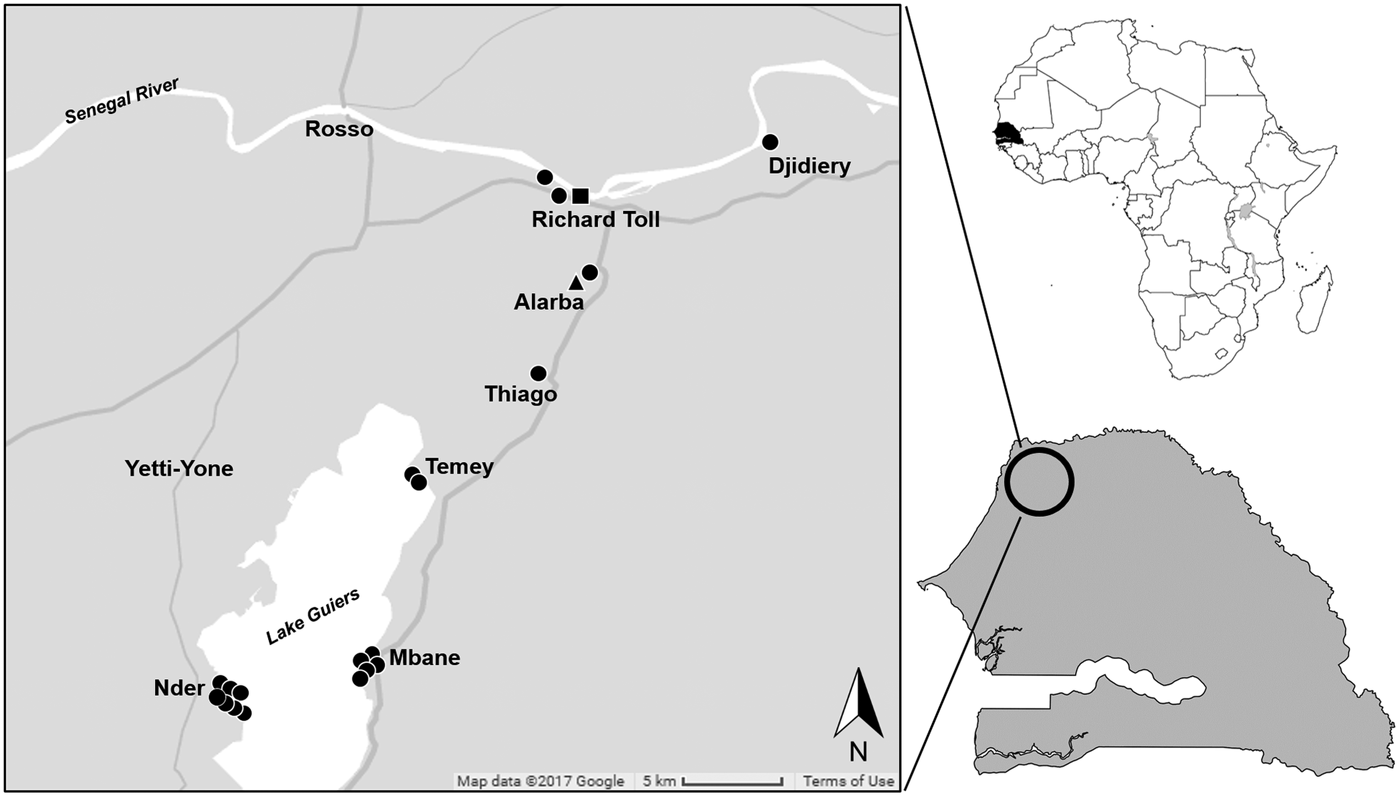
Fig. 2. Map of trapping localities in northern Senegal and occurrence of Hydatigera parva in 19 Mastomys huberti mice (black circles) and one Taterillus gerbil (black triangle) and of H. taeniaeformis sensu stricto in one Arvicanthis niloticus rat (black square).
Table 1. Number of captured Nile rats (Arvicanthis niloticus), Hubert's multimammate mice (Mastomys huberti), shrews (genus Crocidura) and gerbils (genus Taterillus), and percentage of hosts harbouring Hydatigera parva (Hp) and H. taeniaeformis sensu stricto (Ht) per habitat type, season and small mammal age class (NA, not applicable)
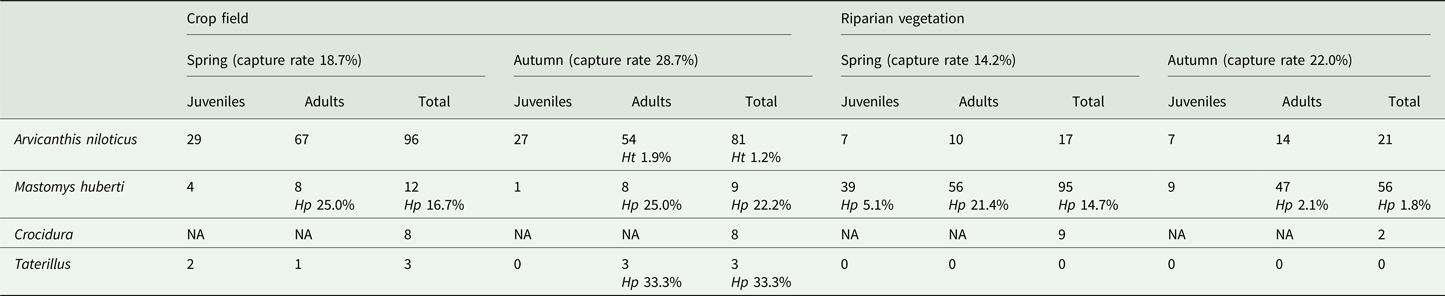
Table 2. Range of pairwise similarity scores (%) for the partial sequence (396 base pairs) of the mitochondrial COI gene within and between Hydatigera species (H. taeniaeformis sensu stricto is reported as H. taeniae s.s.)

The sequences included are MH036504, MH036507, MH036508 (our study) and EU544580 for H. parva; MH036509 (our study), AB745096, KT693044, KT693060, KT693063, KT693064 and KT693072 for H ydatigera taeniae s.s.; AB731761, AB745098, EU544596 and KT693093 for H. kamiyai; AB731762 and MF281972 for H. krepkogorski.
Statistical analyses
Capture rates for each trapping season and habitat varied markedly (Table 1). Statistical comparisons showed that the proportion of trapped adult rodents (viz., A. niloticus, M. huberti and Taterillus sp.) significantly differed across season (χ 2 = 4.85; d.f. = 1; P = 0.028), with a peak in autumn (74.1% adults out of 170 trapped rodents) and a decrement in spring (63.7% adults out of 223 trapped rodents). Logistic regression was performed only on H. parva occurrence in M. huberti due to the small number of infected Taterillus sp. and A. niloticus. Age demonstrated a significant association with probability of infection (P = 0.017), where 17 out of 19 infected M. huberti (89.5%) were adults. Likewise, season was significantly associated with infection probability (P = 0.011), where 16 out of 19 infected M. huberti (84.2%) were captured during the spring. The prevalence of H. parva did not significantly vary when tested against host gender, habitat or locality (P > 0.05).
Discussion
We identified rodent populations from the Senegal River Basin as intermediate hosts of Hydatigera taeniids. To our knowledge, this is the first study using genetic tools to characterize H. parva and H. taeniaeformis in autochthonous rodents of the African continent. Molecular diagnostic approaches, alongside comparative morphology and a range of field data, provide solid bases to identify and revise geographical distribution, host spectrum and evolutionary hypotheses of taeniids and other helminths of medical and veterinary importance (McManus, Reference McManus2006; Nadler and Pérez-Ponce de León, Reference Nadler and Pérez-Ponce de León2011; Zhang et al., Reference Zhang, Chen, Yang, Liu, Jiang, Gu, Wang and Wang2014). In fact, recent molecular and phylogenetic evidence has demonstrated that H. taeniaeformis represents a cryptic species complex (Jia et al., Reference Jia, Yan, Lou, Ni, Dyachenko, Li and Littlewood2012; Nakao et al., Reference Nakao, Lavikainen, Iwaki, Haukisalmi, Konyaev, Oku, Okamoto and Ito2013; Lavikainen et al., Reference Lavikainen, Iwaki, Haukisalmi, Konyaev, Casiraghi, Dokuchaev, Galimberti, Halajian, Henttonen, Ichikawa-Seki, Itagaki, Krivopalov, Meri, Morand, Näreaho, Olsson, Ribas, Terefe and Nakao2016). DNA sequence comparisons with the specimen we isolated in a Senegalese Nile rat show identity to what is described as H. taeniaeformis sensu stricto, a lineage that might have originated in Southeast Asia and rapidly invaded Australia, the Americas, Europe and Africa, where it has been identified in Ethiopia and South Africa from Rattus spp. (Lavikainen et al., Reference Lavikainen, Iwaki, Haukisalmi, Konyaev, Casiraghi, Dokuchaev, Galimberti, Halajian, Henttonen, Ichikawa-Seki, Itagaki, Krivopalov, Meri, Morand, Näreaho, Olsson, Ribas, Terefe and Nakao2016; Mello et al., Reference Mello, Furtado, Rabelo and Pinto2018). In contrast, the origin of H. parva is hypothesized in the African continent (see Alvarez et al., Reference Alvarez, Iglesias, Bos, Tojo and Sanmartin1990), since both its main definitive hosts (i.e. viverrids of the genus Genetta) and intermediate hosts (i.e. rodents of the genera Aethomys, Arvicanthis and Mastomys) are native to Africa (Jones and Pybus, Reference Jones, Pybus, Samuel, Pybus and Kocan2001; Granjon and Duplantier, Reference Granjon and Duplantier2009). The occurrence of H. parva in Europe (see Jones and Pybus, Reference Jones, Pybus, Samuel, Pybus and Kocan2001) could be the consequence of multiple, successful introductions of the common genet from Maghreb to Europe, likely between the end of the Upper Palaeolithic (c. 10 000 years ago) and the end of the Phoenician influence in the Mediterranean (300 BC) (Gaubert et al., Reference Gaubert, Del Cerro, Centeno-Cuadros, Palomares, Fournier, Fonseca, Paillat and Godoy2015). Host phylogeography suggests that H. parva has followed its native host to Mediterranean Europe, where the parasite has found wood mice (Apodemus sylvaticus) as suitable intermediate hosts (Alvarez et al., Reference Alvarez, Iglesias, Bos, Tojo and Sanmartin1990; Lavikainen et al., Reference Lavikainen, Haukisalmi, Lehtinen, Henttonen, Oksanen and Meri2008).
In our study, the presence of Hydatigera strobilocerci was related to the age of the rodents, with H. parva prevalence significantly higher in adult M. huberti. Similar studies on H. taeniaeformis in deer mice (Peromyscus maniculatus) from California, USA (Theis and Schwab, Reference Theis and Schwab1992), in water voles (Arvicola terrestris) from Switzerland (Burlet et al., Reference Burlet, Deplazes and Hegglin2011) and in common voles (Microtus arvalis) from France (Fichet-Calvet et al., Reference Fichet-Calvet, Giraudoux, Quéré, Ashford and Delattre2003) further supported the positive relationship between metacestode prevalence and older rodent hosts. In addition, we found a significantly higher relative abundance of adult rodents trapped during the autumn, which appears to be in contrast with the higher H. parva prevalence in M. huberti observed during the spring season. However, such differences may be explained by complex host population dynamics, including reproductive patterns driving age structures and density-dependent effects between definitive and intermediate hosts, which all play important roles in the exposure to Hydatigera spp. infectious stages (Fichet-Calvet et al., Reference Fichet-Calvet, Giraudoux, Quéré, Ashford and Delattre2003; Deter et al., Reference Deter, Berthier, Chaval, Cosson, Morand and Charbonnel2006; Burlet et al., Reference Burlet, Deplazes and Hegglin2011). Furthermore, the development of H. parva strobilocerci in M. huberti and Taterillus sp., while A. niloticus harboured H. taeniaeformis sensu stricto, may indicate specific predator-prey dynamics between definitive hosts (i.e. viverrids and felids) and rodents in our study area. Such trophic interactions are applicable to the transmission of Hydatigera spp., but they could also be used as a proxy for any rodent-borne parasite with similar life-cycle mechanisms. The zoonotic protozoan Toxoplasma gondii (Nicolle and Manceaux, 1908) is a particularly relevant example considering its public health importance and the limited publicly accessible data on T. gondii infections in West Africa (Keats Shwab et al., Reference Keats Shwab, Zhu, Majumdar, Pena, Gennari, Dubey and Su2014).
Rodents are an abundant and diverse vertebrate order, predicted as the most important reservoir of infectious diseases of public health concern, particularly in tropical regions and ever-growing urban areas (Han et al., Reference Han, Schmidt, Bowden and Drake2015, Reference Han, Kramer and Drake2016; Young et al., Reference Young, McCauley, Dirzo, Nunn, Campana, Agwanda, Otarola-Castillo, Castillo, Pringle, Veblen, Salkeld, Stewardson, Fleischer, Lambin, Palmer and Helgen2017). The synanthropic habits and resilience to anthropogenic disturbance of some rodent species, together with their wide geographical distribution and invasive potential, makes long-term surveys on the ecology of rodents and rodent-borne diseases a priority in many areas worldwide (Meerburg et al., Reference Meerburg, Singleton and Kijlstra2009; Bordes et al., Reference Bordes, Blasdell and Morand2015). In West Africa, initiatives are being taken to address the knowledge gap that still exists in our understanding of ecological dynamics driving transmission risks of rodent-borne infectious organisms (e.g. Lecompte et al., Reference Lecompte, Fichet-Calvet, Daffis, Koulémou, Sylla, Kourouma, Doré, Soropogui, Aniskin, Allali, Kan, Lalis, Koivogui, Günther, Denys and ter Meulen2006; Garba et al., Reference Garba, Dalecky, Kadaoure, Kane, Hima, Veran, Gagare, Gauthier, Tatard, Rossi and Dobigny2014; Catalano et al., Reference Catalano, Sène, Diouf, Fall, Borlase, Léger, Bâ and Webster2018). Herein, we report epidemiological and molecular information on H. parva and H. taeniaeformis sensu stricto, further supporting the phylogeographic hypothesis on the African origin of H. parva. Our results highlight that future field investigations of host population ecology and parasite communities of small mammals in West Africa have the potential to shed light on host–parasite associations at different temporal and spatial scales, and to identify significant relationships contributing to pathogen transmission in the region.
Acknowledgements
We thank Boubacar Bâ, Alassane Ndiaye, Alima Thiam, Cheikh Thiam and Mariama Sène for their outstanding support during fieldwork, and Kathryn Berger for her valuable comments on both the paper and statistical analyses. We are grateful to the Senegalese communities involved in the project for their friendly collaboration and hospitality. Finally, we acknowledge the immense contribution of all the wild small mammals trapped and sacrificed during this study.
Financial support
This study was supported by the Royal Veterinary College and the Zoonoses and Emerging Livestock Systems (ZELS) research initiative including the Department for International Development, Biotechnology and Biological Sciences Research Council, Economic and Social Sciences Research Council, Medical Research Council, Natural Environment Research Council, and Defence Science and Technology Laboratory (grant numbers BB/L018985/1 and BB/N503563/1).
Conflicts of interest
None
Ethical standards
The species of small mammals involved in the study are classified as ‘Least Concern’ by the International Union for Conservation of Nature Red List. Animals were treated in compliance with the guidelines of the American Veterinary Medical Association Council (https://www.avma.org/KB/Policies/Documents/euthanasia.pdf) and the Animals (Scientific Procedures) Act as implemented by the Home Office in Great Britain (https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/535574/working-with-wild-animals-160706.pdf). Trapping activities commenced after explicit approval from local authorities and land owners. Approval for live trapping and euthanasia of small mammals was obtained from the Clinical Research Ethical Review Board of the Royal Veterinary College, University of London (reference number: 2016 1505).