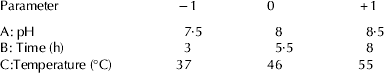Caseinophosphopeptides (CPPs) derived from bovine caseins are well studied as multifunctional peptides due to their mineral binding, antioxidant (Diaz et al. Reference Diaz, Dunn, McClements and Decker2003; Sakanaka et al. Reference Sakanaka, Tachibana, Ishihara and Juneja2005) and cytomodulatory potential (Hata et al. Reference Hata, Ueda and Otani1999; Otani et al. Reference Otani, Kihara and Park2000). Their capacity to form complexes with mineral elements through the clusters (SpSpSpEE) (three phosphoseryl groups followed by two glutamic acid residues), plays an important role in mineral bioavailability. Some biological functions such as promotion of calcium uptake (Hansen et al. Reference Hansen, SandstroÈm and LoÈnnerdal1996; Bennet et al. Reference Bennet, Desmond, Harrington, McDonagh, FitzGerald, Flynn and Cashman2000), calcium absorption (Erba et al. Reference Erba, Ciapplellano and Testolin2002), calcium retention (Sato et al. Reference Sato, Masanori, Gunshin, Noguchi and Naito1991), bone calcification (Tsuchita et al. Reference Tsuchita, Goto, Shimizu, Yonehara and Kuwata1996), hypotensive effect (Kitts & Yuan, Reference Kitts and Yuan1992) and anticariogenicity (Reynolds et al. Reference Reynolds, Cain, Webber, Black, Riley, Johnson and Perich1995) of CPP could be attributed to their chelating activity for transition metal ions. These CPPs can be released enzymatically, e.g. by tryptic cleavage, resulting in general tryptic casein phosphopeptides; [αs1-CN 2P (f43-58), αs1-CN 5P(f59-79), αs1-CN 1P (f106-119), αs2-CN 4P (f2-21), αs2-CN 4P (f46-70), αs2-CN 2P (f126-136), αs2-CN 1P (f138-149), β-CN 4P (f2-25) and β-CN 1P (f33-48)]. All these peptides showed calcium binding capacity and characteristic inhibitory effect on calcium phosphate crystallisation and precipitation (Schmidt et al. Reference Schmidt, Both, Visser, Slangen and van Rooijen1987; Naito, Reference Naito, Yoshida, Naito, Niiyama and Suzuchi1990; Schlimme & Meisel, Reference Schlimme and Meisel1993; Holt, Reference Holt1996). To date, the actions of trypsin (Wei et al. Reference Wei, Zhimin and Deqing2003), chymotrypsin (McDonagh & FitzGerald, Reference McDonagh and FitzGerald1998), plasmin (Andrews et al. Reference Andrews, Williams, Brownsell, Isgrove, Jenkins and Kanekanian2006), Lactobacillus proteinase (Corsetti et al. Reference Corsetti, Massitti, Minervini, Fox and Gobbetti2003), pancreatin (Adamson & Reynolds, Reference Adamson and Reynolds1995), and simulated gastrointestinal proteases (Miquel et al. Reference Miquel, Gomez, Alegria, Barbera, Farre and Recio2006) on bovine caseins, Na-caseinates or milk have been typically studied for the CPPs production.
India has the highest livestock population in the world which includes 50% of the buffaloes and 20% of the world's cattle population. Out of 92 140 146 tonnes world buffalo milk production in 2009, India contributes about 62 860 000 tonnes (FAO, 2011). Buffalo milk is mainly used for preparations of dairy products and ingredients similar to those of bovine milk. It also can be used for preparation of various functional ingredients like bioactive peptides. There are very few reports on production of bioactive peptides from buffalo milk (El - Shibiny et al. Reference El - Shibiny, Metwally, El - Dieb and Assem2001, Petrilli et al. 1987). Various buffalo milk caseins like αs1, αs2, β and κ differ at certain positions in their primary structure from respective cow milk caseins (Ferranti et al. Reference Ferranti, Scaloni, Caira, Chianese, Malorni and Addeo1998). These sequences indicate that αs1, αs2, β and κ casein differ in their amino acid sequence at 10, 10, 5 and 13 different sites, respectively (Cosenza et al. Reference Cosenza, Pauciullo, Feligini, Coletta, Colimoro, Berardino and Ramunno2009, Mishra et al. Reference Mishra, Mukesh, Kataria, Kumar, Pandey and Ahlawat2005, Mukesh et al. Reference Mukesh, Mishra, Kataria, Sobti and Ahlawat2006, Sukla et al. Reference Sukla, Bhattacharya, Venkatachalapathy, Kumar and Sharma2006, Reference Sukla, Bhattacharya, Venkatachalapathy, Kumar and Sharma2007). It is well documented that the mineral binding ability of CPPs is due to the highly anionic phosphorylated regions present in them and the amino acid sequence around anionic hydrophilic domain seems to play a significant role in mineral binding (McDonagh & FitzGerald, Reference McDonagh and FitzGerald1998). Furthermore, the extent of hydrolysis may be different in buffalo milk caseins vis-à-vis cow milk caseins because of differences in their primary structure and it has been reported that yield of the CPPs vary with extent of hydrolysis (Adamson and Reynolds, Reference Adamson and Reynolds1995). So there is a need to optimise the conditions of hydrolysis of buffalo caseins for better yield of CPPs, to study their calcium solubilising and binding capacity and to identify the sequence of these peptides.
Materials and methods
Materials
Trypsin (EC 3·4·21·4, Sigma-Aldrich, St.Louis, MO, USA, Cas No. 9002077), PhosphoQuant™ phosphoprotein phosphate estimation kit (GBiosciences, St.Louis, MO, USA). All other reagents were procured from Sisco Research Laboratories (SRL) Pvt Ltd., India.
Preparation of CPPs
Buffalo milk casein was prepared by acid precipitation using skim milk. Sodium caseinate was prepared by dissolving wet casein in water and adjusting to pH 7·0 using 2 m NaOH with continuous stirring (Fox, Reference Fox, Webb and Whittier1970).
CPPs were prepared by hydrolysing buffalo sodium caseinate with trypsin. The hydrolysis conditions were optimised by using Response Surface Methodology. The software Design Expert 7.0 (Stat-Ease Inc., Minneapolis, Minn., USA) was employed for experimental design, data analysis and model building. A central composite rotatable design (CCRD) with 3 variables was used to determine the response pattern and then to establish a model. Three variables used in this study were pH (A), time (B) and temperature (C) of hydrolysis, with 3 levels of each variable (Table 1), while the dependent variable was yield of CPPs (Y). The design consisted of 20 experimental points (8 factorial, 6 axial and 6 central). These 20 experiments were carried out in random order. Fifty ml sodium caseinate (5%, w/v in distiled water) was taken for hydrolysis and trypsin (E: S; 1 : 100) was added and kept in waterbath maintained at required temperature. The pH of the solution was maintained throughout the hydrolysis using 1 m NaOH manually. After hydrolysis, the enzyme was inactivated by heating at 80 °C for 5 min and the hydrolysate was adjusted to pH 4·6 with 1 m HCl. The unhydrolysed caseinate was removed by centrifugation at 3000 g (Kubota centrifuge, Tokyo, Japan) for 10 min at 4 °C. Supernatant was collected and adjusted to pH 7 with 1 m NaOH. Calcium chloride (11 mg/ml, w/v) was added to the supernatant and allowed to aggregate for 1 h at room temperature. Ninety five percent ethanol (v/v) was then added to get 50% (v/v) final concentration and resultant precipitate was collected by centrifugation at 6000 g for 10 min (McDonagh & FitzGerald, Reference McDonagh and FitzGerald1998). The precipitates were freeze dried and stored at −20 °C for further analysis. The protein content of the sample was analysed by method given by Lowry et al. (Reference Lowry, Rosebrough, Farr and Randall1951).
Table 1. Coded settings for the process parameters for hydrolysis, according to central composite rotatable design
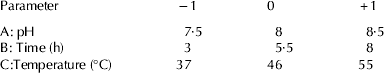
Degree of hydrolysis
The degree of hydrolysis (DH) was calculated by using pH-stat technique formula which gives a direct measurement of the percentage of hydrolysed bonds (Adler-Nissen, Reference Adler-Nissen1986).
Where, B=volume of NaOH used for maintaining the pH; N B=normality of the base; 1/α=average degree of dissociation of the α-amino groups related with the pK of the amino groups at particular pH and temperature; h tot (meq/g)=sum of millimoles of individual amino acids per gram of protein associated with the source protein. (8·2 for casein); M p=amount of the protein in reaction mixture (g).
Calcium solubilising capacity of CPPs
The calcium solubilising capacity of CPPs was determined by using the method of McDonagh & FitzGerald (Reference McDonagh and FitzGerald1998) with slight modification. Two hundred microgram (protein equivalent) of CPPs (demineralised by Amberlite IR120) was incubated at 37 °C with test solution (0·5 ml) containing 0–3·0 mg/ml calcium at pH 8·0. Excess phosphate (i.e. 0·5 ml of 60 mm Na2HPO4/NaH2PO4, pH 8·0 buffers) was added after 60 min incubation. The calcium concentration of the supernatant arising from the mixed and centrifuged (6000 g for 1 min) samples was then determined by atomic absorption spectrometer (Polarized Zeemann AAS, Hitachi Z-5000, Japan). The calcium solubilised at pH 8·0 by one mg CPPs preparation in the presence of excess phosphate was expressed as mg of Ca2+/mg of CPPs.
Calcium binding capacity of CPPs
The calcium binding capacity of CPPs was determined by using the method of McDonagh & FitzGerald (Reference McDonagh and FitzGerald1998) with some modification. One mg (protein equivalent) of CPP preparation (de-mineralised on Amberlite IR120) was incubated with 5 mg of Ca2+at pH 7·5, 37 °C in a total volume of 1·0 ml. After 60 min incubation, the test solution was placed in the upper chamber of filtration unit (PALL life sciences, USA) membrane (1000 Da cut-off). The assembly was then centrifuged for 30 min at 2450 g at 10 °C (Herasus Centrifuge). The calcium concentration in the retentate was determined by using atomic absorption spectrophotometer (Polarized Zeemann AAS, Hitachi Z-5000, Japan), relative to a blank solution without CPP. The calcium-binding abilities of the CPPs were expressed as mg of Ca2+ bound/mg of CPP.
Analysis of CPPs by RP-FPLC and identification of peptides by LC-MS/MS
The sample was separated by using RP-FPLC (AKTA purifier, GE Healthcare biosciences, Hongkong) with a fraction collector attached. Freeze-dried sample (10 mg) was dissolved in 1 ml trifluoro acetic acid (TFA) (0·1 ml/100 ml) and 500 μl of this solution was injected and separated on RESOURCE RPC 3 ml (Reverse phase column). Detection was performed at 214 nm with UV Detector. The mobile phases were solvent A, containing TFA (0·037 ml/100 ml (v/v)) in HPLC grade water, and solvent B, containing TFA (0·027 ml/100 ml (v/v)) in 80% (v/v) acetonitrile. A linear gradient from 100–40% A and 0–60% B was applied in 70 min; the amount of B was then increased in 5 min to 90%, which was held constant for another 10 min, and finally reduced to 0% B, all at a flow rate of 1·0 ml/min. The fractions were collected and analysed for protein by Lowry method (Lowry et al. Reference Lowry, Rosebrough, Farr and Randall1951) and phosphorus content by phosphate estimation kit. The fractions having higher protein and phosphorus content were pooled and freeze dried. The sample was then sequenced at Proteomics International Pvt. Ltd., Western Australia through Technoconcept Pvt Ltd., New Delhi. Samples were analysed by electrospray ionisation mass spectrometry using the Ultimate 3000 nano HPLC system [Dionex] coupled to a 4000 Q TRAP massspectrometer [Applied Biosystems]. Tryptic peptides were loaded onto a C18PepMap100, 3 mm [LC Packings] and separated with a linear gradient of water/acetonitrile/0·1% formic acid (v/v). Spectra were analysed to identify proteins of interest using Mascot sequence matching software [Matrix Science] with taxonomy set to all entries.
Results and discussion
Process optimisation for the preparation of CPPs
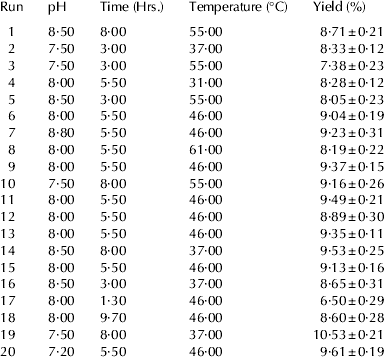
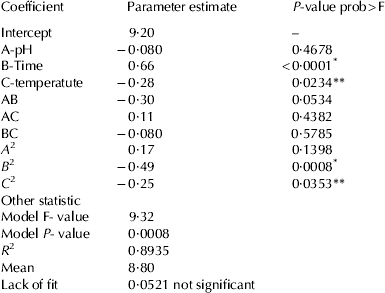
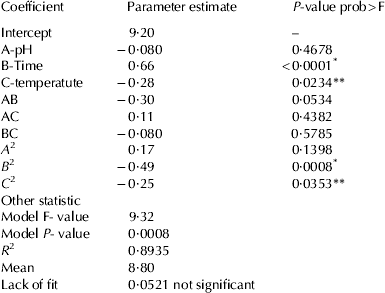
The process for the preparation of CPPs was optimised using response surface methodology. Sodium caseinate prepared from buffalo milk was hydrolysed using trypsin. Total of 20 runs for optimising the three individual parameters by the current design, including 6 replicates, were obtained. The results of 20 experiments are presented in Table 2. At different hydrolysing conditions, the yield of CPPs varied from 7·38±0·23 to 10·53±0·21%. The regression coefficient and their P values for central composite randomised design are shown in Table 3. The coefficient of determination (R 2) is defined as the ratio of the explained variation to the total variation and is a measurement of the degree of fitness. The closer the value of R 2 is to 1·00, the better is the model. The R 2 of present model as calculated by ANOVA was 0·8935, indicating that the sample variation of 89·35% could be attributed to the independent variables. The ANOVA also showed that there was non-significant (P>0·05) lack of fit, which further validates the model (Khuri & Cornell, Reference Khuri and Cornell1987).
Table 2. Experimental design showing different hydrolysis conditions for the optimisation of yield of CPPs in central composite rotatable design by trypsin

Figures are the mean±se of three replicates
Table 3. Regression coefficients of the perdicted quadratic model
* P<0·01, ** P<0·05, significance
The P values were used as a tool to check the significance of each coefficient, which in turn may indicate the pattern of the interactions between the variables. Smaller the value of P, more significant is the corresponding variable. The results showed that the yield of CPPs was significantly affected by two parameters i.e. time and temperature of hydrolysis, as indicated in Table 3 (P<0·01 and P<0·05 respectively). The effect of hydrolysis time was more as indicated by the high value for regression coefficient (Table 3); the positive sign indicates that as the hydrolysis time increases, the yield also increased within the experimental conditions and similar results are predicted from the response surface plot (Fig. 1b, c). The sign for the regression coefficients for the pH and temperature of hydrolysis were negative which shows the yield of CPPs has negative correlation with these two variables. Similarly, it was observed from the response surface plot that with the increase in the temperature and pH of hydrolysis, the yield of CPPs decreased (Fig. 1). For the hydrolysis time and temperature, the quadratic effects were highly significant which indicates that optimal values for hydrolysis time and temperature exist within the experimental area. ANOVA analysis showed that the P value for the model is significant (P<0·01) (Table 3), which means it is suitable for the prediction of the yield of CPPs with respect to hydrolysis conditions (Table 4). The yield of CPPs from the optimised method was 10·04±0·24% which was slightly less than that of the predicted value (Table 4). McDonagh & FitzGerald (Reference McDonagh and FitzGerald1998) reported that the yield of CPPs from the original protein varies from 3·4 to 16·0% with various commercially available proteases, while yield by trypsin is 8·3%. But Corsetti et al. (Reference Corsetti, Massitti, Minervini, Fox and Gobbetti2003) reported much lower yield of CPPs i.e. 0·6–1·86% with lactobacillus proteases. The yield may be depending on the type of proteases and the hydrolysis conditions used.

Fig. 1. Response surface plots (3-D) a, b and c showed the effects of variables (pH, Temperature, °C; Time, hrs) on the response yield.
Table 4. Optimised hydrolysis conditions and calcium binding and solubilising activity of CPPs

DH measured for trypsin was 17·93±1·02%. DH was determined by taking into account the amount of alkali/acid used to maintain the constant pH during hydrolysis of Na caseinate. Adamson & Reynolds (Reference Adamson and Reynolds1995) studied the relationship between casein hydrolysis and phosphopeptides release. They found that the highest yields for Novo trypsin and pancreatin were obtained at DH 17% and DH 19–23% respectively, while McDonagh & FitzGerald (Reference McDonagh and FitzGerald1998) reported 8% DH for trypsin.
Calcium solubilising and binding capacities
The method used for the calcium solubilising ability of CPPs is related to the ability of CPPs to form soluble complexes with calcium which is prevented from precipitating in presence of excess phosphate. The calcium solubilising ability of CPPs was 1·03±0·08 mg of Ca/mg of CPPs (Table 4). McDonagh & FitzGerald (Reference McDonagh and FitzGerald1998), and Perich et al. (Reference Perich, Kelly and Reynolds1992) reported that differences in the calcium solubilising activity of CPPs may be related to the sequences of amino acids flanking the phosphoseryl rich region of the peptides i.e. the sequence containing the SpSpSpEE motif or part of it.
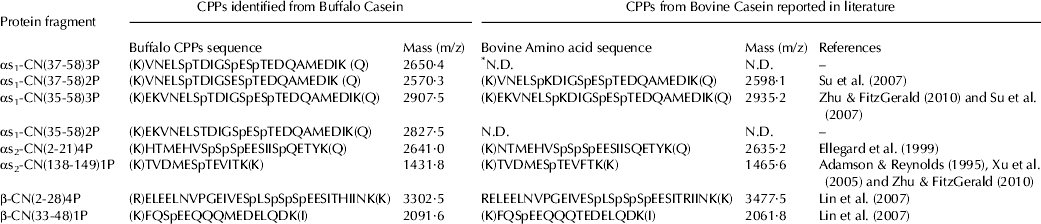
The calcium binding ability of CPPs was 0·934±0·05 mg calcium/mg CPPs (Table 4). Corsetti et al. (Reference Corsetti, Massitti, Minervini, Fox and Gobbetti2003) observed that the calcium binding ability of CPPs isolated from various species varies from 0·4 to 1·4 mg calcium/mg CPPs. Their results also showed that bovine, goat and buffalo hydrolysates have higher calcium binding activity than human and sheep milk. The highly polar acidic domains confer mineral binding abilities to phosphopeptides. Dephosphorylated peptides do not bind minerals (Sato et al. Reference Sato, Noguchi and Naito1983; Gerber & Jost, Reference Gerber and Jost1986; Berrocal et al. Reference Berrocal, Chanton, Juillerat, Pavillard, Scherz and Jost1989). The phosphorylated residues play an important role in mineral binding which was demonstrated by Yoshikawa et al. (Reference Yoshikawa, Sasaki and Chiba1981). They observed that chemical phosphorylation of αs1 and β casein increased the binding capacity. The structure of the phosphopeptides identified in the present study indicates that calcium binding ability may be due to the presence phosphoseryl residues i.e. β-CN f (2-28)4P and f (33-48) 1P; αs2-CN f (2-21)4P and f (138-149)1P and αS1-CN f (37-58)3P, f (37-58)2P, f (35-58)3P, f (35-58)2P (Table 6).
Identification of CPPs
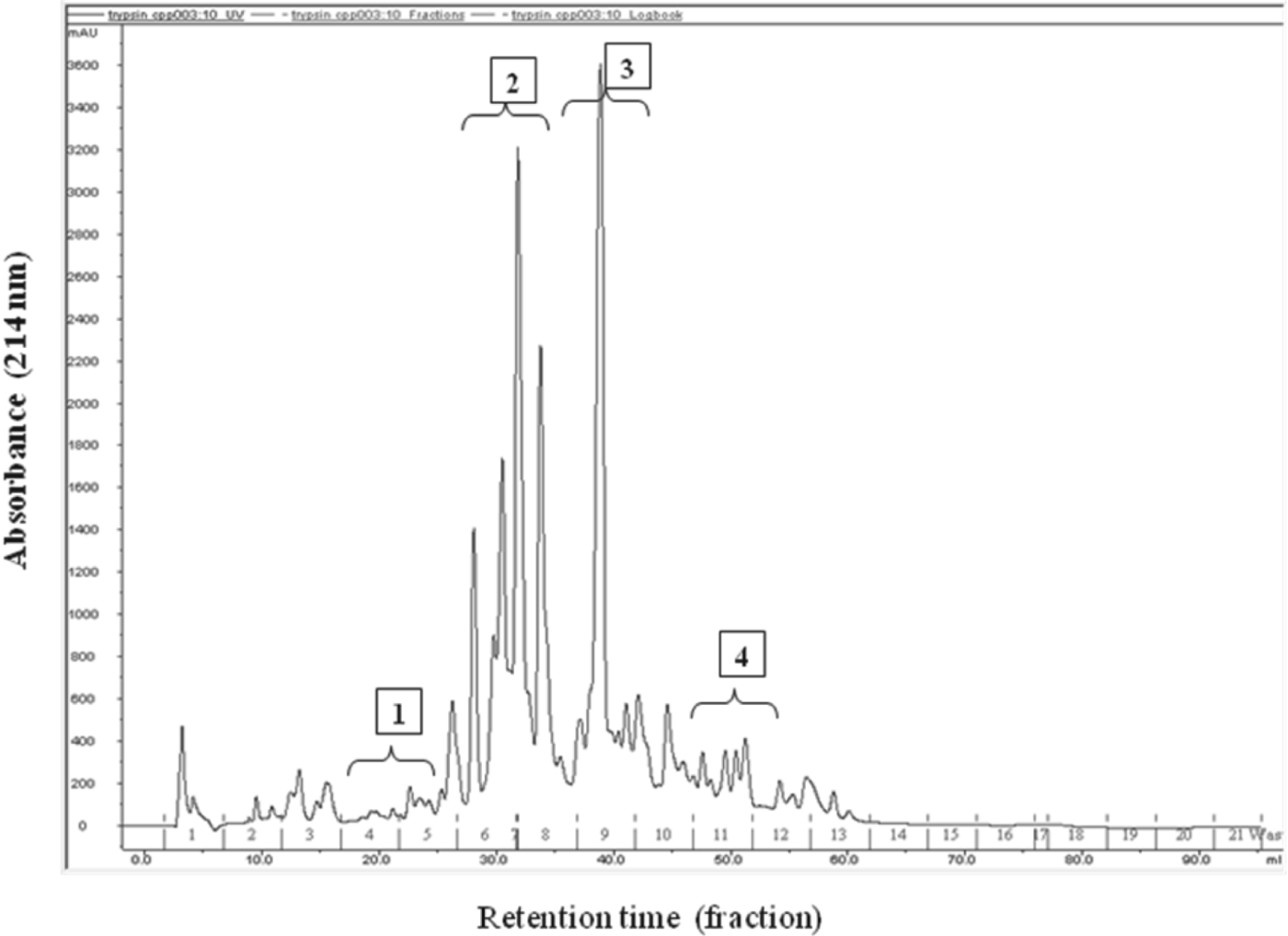
The separation of CPPs was done by preparative scale RP-FPLC and presented in Fig. 2. The whole chromatograph has been divided into four fractions. These fractions were collected and analysed for their protein and phosphorus contents (Table 5). The fraction No. 2 and 3 showed the highest phosphorus and protein contents which indicated the presence of phosphopeptides in these fractions. These two fractions were pooled and freeze dried. These fractions were further subjected to ESI-MS/MS. Eight caseinophosphopeptides have been identified in the FPLC fractions 2 and 3 (Table 6). These peptides were identified as fragments of αs1-casein f (37-58 and 35-58), αs2-casein f (2-21 and 138-149) and β-casein f (2-28 and 33-48). All these eight peptides showed different level of phosphorylation. The peptides αs2-CN f (138-149) and β-CN f (33-48) had one, αs1-CN f (37-58 and 35-58) had two, αs1-CN f (37-58 and 35-58) had three and β-CN f (2-28) and αs2-CN f (2-21) had four phosphorylated serine residues. The sequences of CPPs identified in this study were similar to those reported by the earlier workers. The phosphoseryl segments that can be released by tryptic digestion of bovine casein CPPs are αs1-CN f (43-58) 2P; αs1-CN f (59-79) 5P; αs1-CN f (106-119) 1P; αs2-CN f (2-21) 4P; αs2-CN f (46-70) 4P; αs2-CN f (126-136) 2P; αs2-CN f (138-149) 1P; β-CN f (2-25) 4P and β-CN f (33-48) 1P, all having the calcium binding capacity (Naito, Reference Naito, Yoshida, Naito, Niiyama and Suzuchi1990; Schlimme & Meisel, Reference Schlimme and Meisel1993). Ellegard et al. (Reference Ellegard, Gammelgard-Larsen, Sorensen and Fedesov1999) identified that the N-terminal sequence analysis of these fractions results in identification of the phosphorylated peptides αs1-CN f (43-58) 2P; αs2-CN f (1-21)1P; αs2-CN f (2-21)4P; αs2-CN f (46-70)4P; αs2-CN f (46-70)3P; β-CN f (1-25) 2P; β-CN f (1-25) 3P; β-CN f (30-48) 1P and β-CN f (33-48) 1P from the tryptic hydrolysates of bovine casein. Similarly Zhu & FitzGerald (Reference Zhu and FitzGerald2010) reported a number phosphopeptides in tryptic hydrolysate of bovine casein i.e. αs1-CN f (35-58) 3P; αs1-CN f (43-58) 2P; αs1-CN f (43-59) 2P; αs1-CN f (104-119) 1P; αs1-CN f (106-119) 1P; αs1-CN f (126-136) 2P; αs2-CN f (126-137) 2P; αs2-CN f (138-149) 1P; αs2-CN f (130-150) 1P; β-CN f (32-48) 1P; β-CN f (33-48) 1P and β-CN f (33-52) 1P. In the present study, no phosphopeptides were identified as a fragment of κ-casein. There may be a number possible reasons which may account for this finding as reported by Zhu & FitzGerald (Reference Zhu and FitzGerald2010) that κ-casein represents only 10% of the total casein content and has fewer tryptic cleavage sites. Moreover, κ-casein being a phosphoglycoprotein may result in the release of tryptic peptides having greater solubility in the presence of calcium leading to their resistance to aggregation during calcium and ethanol precipitation.

Fig. 2. RP-FPLC chromatogram of CPPs prepared from buffalo casein.
Table 5. Table showing protein and phosphorus content in RP-FPLC fraction

Table 6. Comparision of CPPs identified from buffalo casein and CPPs from Bovine Casein (reported in literature) with respect to their sequences and phosphorylation

* N.D.: Not detected
The sequence of all the eight peptides reported in this study indicates that CPPs were released due to the C-terminal cleavages of R and K residues which are attributed to the specific activity of trypsin (Allen, Reference Allen, Burdon and van Knippenberg1989; Antal et al. Reference Antal, Pal, Asbóth, Buzás, Patthy and Gráf2001; Su et al. Reference Su, Qi, He, Yuan and Zhang2007). Two peptides β-CN (f 2-28) and αs2-CN (f 2-21) have well defined structural characteristics of the CPPs which is the presence of phosphorylated cluster (SpSpSpEE) and specific amino acids located around it. The presence of the phosphorylated cluster in the CPPs enhanced absorption of mineral (Ferraretto et al. Reference Ferraretto, Gravaghi, Fiorilli and Tettamanti2003; Kibangou et al. Reference Kibangou, Bouhallab, Henry, Bureau, Allouche, Blais, Guerin, Arhan and Bougle2005). Miquel et al. (Reference Miquel, Gomez, Alegria, Barbera, Farre and Recio2006) indicated that CPPs containing the cluster sequences could be good candidate for incorporation as functional ingredients in mineral fortified foods.
The molecular mass of the peptides ranges from 1431·8–3302·5 as presented in Table 6. Slight difference in the molecular mass was observed when compared with similar peptides sequence reported in literature (Table 6). This difference is due to the difference in the amino acid composition of the peptides previously reported. The present study was conducted with buffalo casein while the previous worker identified the peptides bearing the same sequence of bovine casein (Table 6) (Adamson & Reynolds, Reference Adamson and Reynolds1995; Ellegard et al. Reference Ellegard, Gammelgard-Larsen, Sorensen and Fedesov1999; Xu et al. Reference Xu, Lu, Ma, Mohammadi and Neubert2005; Lin et al. Reference Lin, Jean-Marc, Sarah and Alain2007; Su et al. Reference Su, Qi, He, Yuan and Zhang2007). The phosphoseryl fragments of αS1 –CN f (37-58 and 35-58) have threonine at position 42 while the bovine milk CPPs have lysine at same position (Ferranti et al. Reference Ferranti, Scaloni, Caira, Chianese, Malorni and Addeo1998; Su et al. Reference Su, Qi, He, Yuan and Zhang2007). Similarly in the CPPs identified as fragment of αS2 –CN f (2-21 and 138-149), have histidine at position 2 instead of aspargine and isoleucine at 147 position instead of phenylalanine (Sukla et al. Reference Sukla, Bhattacharya, Venkatachalapathy, Kumar and Sharma2006) while the fragments of β-casein f (2-28 and 33-48) have histidine at 24 and methionine at 41 positions in place of arginine and threonine in bovine β-casein phosphopeptides (Adamson & Reynolds, Reference Adamson and Reynolds1995; Ellegard et al. Reference Ellegard, Gammelgard-Larsen, Sorensen and Fedesov1999; Xu et al. Reference Xu, Lu, Ma, Mohammadi and Neubert2005; Lin et al. Reference Lin, Jean-Marc, Sarah and Alain2007).
Conclusion
The result revealed that response surface methodology is an effective tool to optimise the conditions for hydrolysis of buffalo casein by trypsin to obtain maximum yield of CPPs. The CPPs enriched ingredient prepared from buffalo casein has similar calcium solubilising and binding ability as that of the CPPs reported from bovine casein. The peptides identified by mass spectroscopy contain the motif for binding of minerals and are well defined as mineral binding peptides in the literature. These CPPs can be used as a functional ingredient in foods designed for increase mineral absorption.