Introduction
Cicadas are Hemipteran insects mainly characterized by the presence in males of cuticle membranes (tymbals) placed dorsolaterally in the first and second segments of the abdomen. Tymbals can be distorted by the action of powerful muscles producing loud airborne acoustic signals (e.g., Pringle, Reference Pringle1954; Popov, Reference Popov1975; Young & Bennet-Clark, Reference Young and Bennet-Clark1995; Bennet-Clark, Reference Bennet-Clark1997, Reference Bennet-Clark1998a, Reference Bennet-Clark1999). Males produce different kinds of acoustic signals, but the calling song involved in mate attraction is typically species-specific and females are only attracted to the calls of the conspecific males (e.g. Magicicada spp.: Cooley & Marshall, Reference Cooley and Marshall2001).
Paterson (Reference Paterson and Vrba1985) pointed out that species are primarily characterized by unique specific mate recognition systems (SMRSs). These serve to ensure the mating of conspecifics and result secondarily in reproductive isolation (Claridge & de Vriijer, Reference Claridge, de Vrijer, Denno and Perfect1994). It is common to find that the most striking differences between closely related sympatric species concern characters used in mate recognition (e.g. Maynard-Smith, Reference Maynard-Smith1989); thus, SMRSs should allow the discrimination of species. As such, calling songs constitute distinct specific-mate recognition systems (SMRSs) and have been commonly used to recognize cicada species in both natural and farmed ecosystems, especially when no other so distinctive characters are available, such as in the genera Tosena, Pomponia and Lyristes (e.g. Boulard, Reference Boulard1988, Reference Boulard, Drosopoulos and Claridge2006), Cicadetta (e.g. Gogala & Trilar, Reference Gogala and Trilar2004) or in Tettigetta (e.g. Boulard, Reference Boulard1982; Quartau & Boulard, Reference Boulard1995; Sueur et al., Reference Sueur, Puissant, Simões, Seabra, Boulard and Quartau2004). Paterson's (Reference Paterson and Vrba1985) recognition concept of species emphasizes that SMRSs and, therefore, the calling song should be maintained relatively constant by stabilizing selection across the distribution range of the species.
Cicada barbara Stål is known to occur in the Iberian Peninsula, Italy and in North Africa (Nast, Reference Nast1972; Boulard, Reference Boulard1982; Quartau, Reference Quartau1988) in scattered high temperature environments and is usually associated with Mediterranean trees and shrubs, and frequently in farmed environments as olive tree orchards. This cicada produces a typical sound composed of a continuous series of pulses without pauses (Boulard, Reference Boulard1982, Reference Boulard1995; Fonseca, Reference Fonseca1991; Quartau & Rebelo, Reference Quartau, Rebelo, Almada and Correia1994). Boulard (Reference Boulard1982), based on small differences in the male genitalia and the female ovipositor, created a new subspecific taxon for C. barbara from Portugal, i.e. C. barbara lusitanica Boulard, in comparison with the nominal subspecies from North Africa. An investigation of the genetic differentiation also supports the splitting of this species into C. barbara lusitanica and C. barbara barbara Stål (see Pinto-Juma et al., this journal); however, their geographical boundaries may be questionable since a population in Ceuta (North Africa) has been found to cluster with the Iberian Peninsula specimens.
It should be pointed out that the discrimination of subspecies has been a source of controversy (e.g. Phillimore & Owens, Reference Phillimore and Owens2006); and, indeed, biological information from different sources should be used when defining species (Lee, Reference Lee2004). At the subspecific level, the use of complementary data should also assist on classification.
Intraspecific variation in the calling song can occur at a variety of scales and geographical population divergence can lead to reproductive isolation and subsequent speciation (Zuk et al., Reference Zuk, Rotenberry and Simmons2001). Otte (1994, inShaw & Herlihy, Reference Shaw and Herlihy2000) suggested that acoustic features of the calling song of swordtail crickets in Hawaii are among the earliest characters to change in the speciation process. The same was also referred to Drosophila willistoni complex (Gleason & Ritchie, Reference Gleason and Ritchie1998). Boulard (Reference Boulard1995) also referred to the potential importance of comparing the calling songs of C. barbara barbara and C. barbara lusitanica, but no such comparison has been made yet. On the other hand, the courtship song of both subspecies has been compared by Boulard (Reference Boulard1995), who stated that C. barbara lusitanica from Portugal has shorter phrases with higher periodicity in comparison with C. barbara barbara from North Africa. However, no statistical significance tests have ever been accomplished. The courtship song has been described as a continuous amplitude modulated sound, presenting also modulation in frequency and in syllable period (Fonseca, Reference Fonseca1991; Boulard, Reference Boulard1995).
In this paper, the acoustic variation of the calling song, as well as of an amplitude modulated signal of C. barbara are assessed and compared in both the Iberian Peninsula and North Africa populations. Moreover, the usefulness of the present acoustic data in the splitting of C. barbara into subspecies, C. barbara barbara and C. barbara lusitanica, is evaluated.
Material and methods
Specimens of Cicada barbara Stål were sampled in the summer (July–September) over several years (1996–2002) from different localities of the Iberian Peninsula (C. barbara lusitanica) and North Africa (C. barbara barbara) (fig. 1, table 1). Males were identified in the field by their calling songs, located and recorded on a digital Sony DAT recorder (TCD-D10 ProII and TCD-D8 at frequency ranges of 20–22,000 Hz and 20–20,000 Hz, respectively and at a sampling frequency of 44.1 kHz) connected to a dynamic Sony F-780 microphone with frequency responses of 50–18,000 Hz. All recordings were made approximately at the same distance from the cicada (within at least 30 cm to 1 m), to avoid distance interference on the frequency measurements. Air temperature was measured at most collecting sites at the place where cicadas were singing.

Fig. 1. Recording sites in the Iberian Peninsula and North Africa of the Cicada barbara specimens studied.
Table 1. Sampled populations of Cicada barbara.

Temperature intervals refer to the range of temperature within each collecting site at the place and time where cicadas were singing; N, number of males acoustically recorded.
Acoustic analysis
The software Avisoft-SASLab Pro (Specht, Reference Specht2002) was used to digitize sound recordings as in Pinto-Juma et al. (Reference Pinto-Juma, Simões, Seabra and Quartau2005). A sound fragment of about 60 s was used to produce oscillograms, sonagrams (or spectrograms) and mean amplitude spectra (using Fast Fourier transformation with a resolution of 512 points and a Hamming Window and 50% overlap for temporal resolution), allowing temporal and spectral analyses. The frequency variables measured included: peak frequency; minimum and maximum frequencies; bandwidth; quartiles 25, 50, 75% and quartile 75%–quartile 25%. The number of syllables (a syllable being a unitarian group of pulses, e.g. Fonseca, Reference Fonseca1991) was counted per unit of time – 30 fragments of about 0.1 s were analysed and the average number of syllables was calculated for each specimen.
Statistical analyses were performed using MINITAB version 14 software (Minitab Inc., 2004). Anderson-Darling Normality tests were performed to verify the distribution for each variable; and, since the null hypothesis of a normal distribution was always rejected, all the subsequent analyses followed non-parametric statistics.
Following Boulard's (Reference Boulard1982) subspecific classification, the new mtDNA data (see Pinto-Juma et al., this journal) and the fact that no apparent barriers exist between the populations from Portugal and Spain the Iberian Peninsula specimens were considered as C. barbara lusitanica. Similarly, North African specimens were considered as C. barbara barbara. However, since the specimens from Ceuta in northern Africa revealed the same genetic pattern as the Iberian ones (see Pinto-Juma et al., this journal), several tests were carried out grouping Ceuta with the remaining North African localities and, on the other hand, with the Iberian ones.
Spearman's rank correlations were analysed between time and frequency variables and the temperature in the field. Descriptive statistics of each variable for each region were also analysed, including the coefficient of variation. The coefficients of variation (CV) for each variable were calculated within populations (CVpop) and within regions (CVreg). Since these coefficients are the standard deviation expressed as a percentage of the mean of each variable, it was possible to compare the variation between different sets of data. Mann-Whitney U (MW) and Kruskal-Wallis (KW) non-parametric tests were used to compare regions and populations within each region, respectively. The significance of multiple tests was adjusted according to Dunn-Sidák method (Dytham, Reference Dytham2003), by reducing the critical P value from 0.05 to 1–(0.95)1/k, where k is the number of tests performed. The correlation between each variable and the latitude was also assessed in order to test any variation gradient within the geographical distribution of the specimens.
Frequency measurements and the syllable rate were used to assess differences between specimens and regions using multivariate methods. A Principal Component Analysis (PCA) was performed in order to reduce the variables to a small number of components, as well as to assess the correlation between the variables and those components (component loadings). Kruskall-Wallis and Mann-Whitney tests were then used to compare the component scores obtained for the individuals between regions. Discriminant Function Analysis (DFA), with two and three regions defined a priori, was also performed to determine statistical significant discriminant functions that might separate these groups.
Besides the more generally known calling song, amplitude modulated signals were analysed in five males of C. barbara lusitanica from Portugal (one from Moura, two from Portel and one from Sousel, all recorded in 2001; and one from Crato, recorded in 1997) and in eight males of C. barbara barbara from North Africa (three from Meknès, three from Fès and one from Fès South, all recorded in 2001; and one from Ceuta, recorded in 1999). Recordings and digitizing were performed as described above. Ten phrases per male, each composed of one high amplitude section (Section I) and one low amplitude section (Section II) were analysed whenever possible. The peak frequency and duration of each section in each phrase were obtained using Avisoft and the number of syllables in three fragments of about 0.1 s was counted for each section of each phrase and an average was obtained for each section per specimen.
Non-parametric tests were used to compare the two phrasal sections (Wilcoxon test for two related samples) and to compare African and Iberian samples (Mann-Whitney U test for two independent samples). Correlations between the acoustic variables and the temperature were calculated using the Spearman correlation coefficient.
Results
Acoustic analyses were based on the recordings of a total of 119 specimens from 14 localities, four from North Africa and ten from the Iberian Peninsula (fig. 1, table 1).
Calling song
The calling song of Cicada barbara consists of a continuous emission of pulses produced by the tymbals (fig. 2). Each tymbal is responsible for the repetitive production of a group of pulses which we call syllable. The tymbals alternate their sound production, but slightly overlap (Fonseca, Reference Fonseca1991). In the calling song, each syllable lasts about 5 ms (ca. 200 syllables s−1; table 2). In the males analysed (N=119), two were apparently either using only one tymbal or there was a synchronous action of both tymbals, as revealed by the oscillograms with half of the number of syllables produced per unit of time. In this case, the frequency values were not significantly different from when both tymbals were functioning (MW test, P>0.05), but the variable ‘syllable rate’ had to be discarded.

Fig. 2. Oscillograms (time vs. amplitude) and sonagrams (time vs. frequency) of the calling songs of (a) one male of Cicada barbara lusitanica from Crato, Portugal and (b) one male of C. barbara barbara from Fès, Morocco. A fragment of 0.2 seconds is shown, with an individual syllable pointed out, which is further magnified in (a) to show the pulses.
Table 2. Descriptive statistics of the acoustic variables of Cicada barbara.

SD, standard deviation; CV, coefficient of variation.
The peak frequency for all the specimens analysed varied from 5160 to 7660 Hz. The bandwidth ranged between 5160 and 15,330 Hz, and the difference between upper and lower quartiles ranged between 1300 and 4040 Hz (table 2).
No correlation between frequency variables and temperature was detected. However, the syllable rate was slightly correlated with temperature: rS=0.34 (P<0.001) when considering all specimens (see fig. 3).

Fig. 3. Variation of the syllable rate with the temperature in specimens of Cicada barbara. ◆, Fès south; ■, Fès; ▲, Meknès; ●, Ceuta; ![]() , Sevilla; ○, Cordoba; +, Moura; −, Portel; □, Sousel; ◊, Crato.
, Sevilla; ○, Cordoba; +, Moura; −, Portel; □, Sousel; ◊, Crato.
The calling songs differed significantly between populations for all variables (KW tests, P<0.001), except for the mean syllable rate (P=0.06). For all population pairwise comparisons within the Iberian Peninsula, MW tests (P<0.05) showed that Moura and Sousel had higher maximum frequencies (see appendix I). Within North African populations, including Ceuta samples, the variables peak frequency, minimum frequency, and quartiles 25, 50 and 75% differed significantly. Ceuta specimens seem responsible for most of these differences, since significantly lower values for peak frequency and quartiles 25% and 50% were found in Ceuta when compared to the Morocco samples (MW tests in table 3, boxplots in fig. 4).

Fig. 4. Boxplots of the acoustic variables analysed for Cicada barbara at two regions (Iberian Peninsula+Ceuta and Morocco) and three regions (Ceuta, Iberian Peninsula and Morocco). Boxplots represent the sample distribution: the rectangular box corresponds to approximately the middle 50% of the data, the top of the box is the third quartile (75%), the bottom is the first quartile (25%) and the horizontal line is the median; vertical lines extending to either side indicate the general extent of the data; *, outlier.
Table 3. Mann-Whitney U tests comparing two regions for each acoustic variable in Cicada barbara.

Significant differences highlighted in grey are according to the method of Dunn-Sidák (Dytham, Reference Dytham2003) (P<1–(0.95)1/k, where k=number of tests performed, in this case k=45, so P<0.001).
When temperature was controlled by performing ANCOVA (temperature as covariate), the differences between North African populations were still present for most variables, except for minimum frequency. This does not necessarily contradict the non-correlation between frequency variables and temperature. In fact, ANCOVA is the only test which allows controlling the temperature, but it is a parametric test, so the minimum frequency result might be a simple statistical artefact due, for instance, to a violation in the test criteria.
The influence of temperature could not be tested to explain the low syllable rate values found in Alvor, as this information was missing. However, when excluding specimens for which there was no temperature data, there was no significant differences between populations in syllable rate (KW test, P>0.05).
A negative significant correlation between each frequency variable of all populations and their latitude was found (P<0.05), indicating that there is a decreasing gradient on frequency parameters from south to north (table 4, appendix I). The syllable rate did not show a significant correlation with latitude when all populations were analysed.
Table 4. Spearman correlation (r S) between latitude and acoustic variables analysed for Cicada barbara.
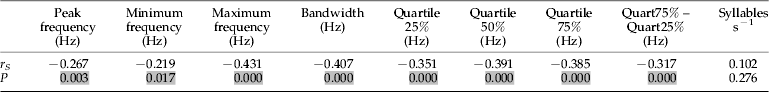
P is the level of significance; for P<0.05, the correlation is significant. Significant differences are highlighted in grey.
Calling song and the differentiation between regions
Corroborating molecular analyses, the Ceuta population revealed considerable acoustic differences in comparison with the remaining North African populations, so multiple MW tests (with the significance level corrected via Dunn-Sidák method) were performed in order to evaluate different subregions (table 3). The mean syllable rate and the minimum frequency were not significantly different for any of the subregions compared, but all other frequency variables showed differences depending on the regions.
Ceuta revealed more highly significant differences when compared to Morocco (peak frequency, quartile 25% and quartile 50%) than when compared to the Iberian Peninsula (only bandwidth). MW tests between Morocco vs. Iberian Peninsula+Ceuta also revealed more significant differences than North Africa (Morocco and Ceuta) vs. Iberian Peninsula (see table 3). All comparisons where Ceuta was included suggested less differentiation between this population and the Iberian Peninsula than with Moroccan populations.
Similar levels of coefficients of variation (CV) were found within populations in the Iberian Peninsula+Ceuta and within populations in Morocco (table 5). However, within-region CV were generally higher in Iberian Peninsula+Ceuta than in Morocco; only the CV of the minimum frequency and of the bandwidth for Morocco was higher than the CV of Iberian Peninsula+Ceuta. The variable presenting the highest CV of all was bandwidth, while the lowest CV was found for quartile 50%, for both regions.
Table 5. Mean, standard deviation (SD) and coefficient of variation (CV) for each acoustic variable of Cicada barbara on each region.

CVpop (%), average of the CV between populations in each region; CVreg (%), CV between individuals within the region.
PCA did not reveal non-overlapping groups between regions, however Moroccan specimens predominantly aggregated in the upper and lower right quadrants of the diagram, while Iberian specimens were mostly spread along the upper and lower left quadrants (fig. 5). KW tests showed significant differences for the first two components between the scores of specimens of Morocco, Iberian Peninsula and Ceuta (P<0.001). MW tests between Morocco and Iberian Peninsula+Ceuta also revealed highly significant differences for the first two components (same level of significance, P<0.001). Comparing Morocco and the Iberian Peninsula alone, MW tests revealed even stronger significant differences for component 1 with P<0.0001; Morocco compared to Ceuta also had significant differences (P<0.005), while the Iberian Peninsula compared to Ceuta did not show significant differences (P=0.650).

Fig. 5. Component scores obtained from the Principal Component Analysis (PCA) based on a correlation matrix between all nine acoustic variables analysed for Cicada barbara: (a) ◆, Alc; ![]() , Alv;
, Alv; ![]() , Arr; △, Ceu;
, Arr; △, Ceu; ![]() , Cor; ◃, Cra; ▼, Fès; +, FèsS; ×, Mek;
, Cor; ◃, Cra; ▼, Fès; +, FèsS; ×, Mek; ![]() , Mon; ◊, Mou;
, Mon; ◊, Mou; ![]() , Por;
, Por; ![]() , Sev;
, Sev; ![]() , Sou population level and (b)
, Sou population level and (b) ![]() , Iberian Peninsula+Ceuta;
, Iberian Peninsula+Ceuta; ![]() , Morocco region level.
, Morocco region level.
The proportion of the total variance (table 6a) accounted for by the first component was 52%, the first two combined components accounted for 71%, and 82.5% for the first three components. The cumulative variance accounted by consecutive components decreased rapidly, which is indicative of significant inter-correlation between the variables. Most variables (excluding the syllable rate which had the lowest loading attributed, 0.011) were almost equivalently responsible for the first component, the loadings varying from 0.196 (minimum frequency) to 0.439 (quartile 75%). Even though most frequency variables were cross-correlated, the score plot of the PCA showed a better separation of the regions than other plots using only non-correlated variables (data not shown).
Table 6. Multivariate analyses based on acoustic variables for Cicada barbara.
a) Principal Component Analysis (PCA) based on nine acoustic variables: eigen analysis and component loadings.

PC, principal component.
b) Discriminant Function Analysis (DFA) results based on seven acoustic variables.

The frequency variables Q75%–Q25% and bandwidth were highly correlated with other predictors and had to be discarded from this analysis.
For DFA, two different groupings were tested: one separating Morocco from the Iberian Peninsula+Ceuta and the other separating three regions, Morocco, Iberian Peninsula and Ceuta (table 6b). The frequency variables Q75–Q25% and bandwidth had to be discarded from this analysis, as they were highly correlated with other variables (predictors).
DFA separating Morocco from the Iberian Peninsula+Ceuta had a global proportion of correct classification of 64.7%; after cross validation, the proportion was 60.3%. Morocco specimens were less misclassified than the ones from Iberian Peninsula+Ceuta since Morocco alone had 70.8% of correct classification, while the Iberian Peninsula+Ceuta had 63%. The overall correct categorization of DFA for the three regions, considering Ceuta as a separate region, was 54.3% and 45.7% after cross validation. For each region separately, Morocco had 66.7% of correct categorization, the Iberian Peninsula had the lowest proportion of correct categorization (49.4%) and Ceuta had 63.6%. The lower overall proportion of correct classification observed suggests that the two regions defined in the first test, Morocco and the Iberian Peninsula together with Ceuta, are more adequate to explain the differentiation between the specimens.
Amplitude modulated signal
The amplitude modulated signal analysed here is similar in pattern to the one referred by Fonseca (Reference Fonseca1991) and Boulard (Reference Boulard1995) as a courtship song. However, from our field observations, we cannot corroborate that it is truly a courtship song, being instead generally associated with the disturbance caused by the approaching of the operator during the field recordings. However, this is an ethological issue that requires further clarification.
In the amplitude modulated signal, Section I of the phrase had higher amplitude (fig. 6) and also significantly lower duration and higher syllable rate than Section II (Wilcoxon test, P=0.001 for both) (table 7). On the other hand, peak frequency was not significantly different between Sections I and II (Wilcoxon test, P=0.701). Temperature had no significant correlation with any of the acoustic variables measured in this modulated signal (Spearman correlation, P>0.1). There was no significant difference between African and Iberian samples for each variable or between Morocco samples and Iberian and Ceuta samples taken together (MW tests, P>0.1). The acoustic variable with the highest coefficients of variation was the temporal variable duration (CV from 21.3% to 32.7%). On the other hand, the peak frequency and the syllable rate were more constant (CV from 5.2% to 10.8%) (table 7). Also, the intra-individual coefficients of variation of duration were, on average, 14.5% for Section I and 29.4% for Section II and were lower for peak frequencies and syllable rate (2% and 3.7% for the first; 4.4% and 8% for the latter).

Fig. 6. Oscillogram and sonagram of the amplitude modulated signal of (a) one male of Cicada barbara lusitanica from Moura and (b) one male of C. barbara barbara from Fès. Sections I and II of the signal are signalled; and a fragment of 0.3 seconds of the transition between sections is shown enlarged on top, depicting the clear difference in syllable rates between Section I and II.
Table 7. Descriptive statistics for the acoustic variables measured in the phrases of the amplitude modulated signal of Cicada barbara barbara and C. barbara lusitanica.

SD, standard deviation; CV, coefficient of variation.
Discussion
Acoustic variation
The values found for the acoustic variables of the calling song in the present study are within the range found by other authors. Fonseca (Reference Fonseca1991) reported syllable rates from 162 s−1 to 238 s−1 on specimens of C. barbara lusitanica (recorded in the field with free tethered animals, but unfortunately without reference to the locality) and a peak frequency of 5.5–6.5 kHz, a minimum frequency of 2–3 kHz and a maximum of 9.5–11 kHz. Boulard (Reference Boulard1995) described the calling song of a male of C. barbara barbara from North Africa with a peak frequency at 6.2 kHz. If we consider that the two regions analysed in this study, Iberian Peninsula+Ceuta and Morocco, correspond to the subspecies C. barbara lusitanica and C. barbara barbara, respectively, the two subspecies showed different means but with overlapping values for these acoustic variables: C. barbara lusitanica had a mean (±SD) peak frequency of 6.3±0.4 kHz, a minimum frequency of 2.8±0.3 kHz, a maximum frequency of 11±1.7 kHz and a syllable rate of 200±19.1 s−1; while C. barbara barbara had a mean peak frequency of 6.6±0.2 kHz, a minimum frequency of 2.9±0.5 kHz, a maximum frequency of 12.4±1.8 kHz and a syllable rate of 203±17.6 s−1.
Temperature had a significant effect on the syllable rate, which increased with air temperature, as observed for this species previously (Fonseca, Reference Fonseca1991) and for other cicadas (e.g. Tettigeta argentata, T. josei and Tympanistalna gastrica, in Fonseca & Revez (Reference Fonseca and Revez2002a)).
The syllable rate did not show any significant differences between populations and regions in our study, although most other acoustic characters revealed significant differences. However, variability between individuals (within populations) was relatively high (see appendix II), increasing the complexity of the results. According to Gerhardt (Reference Gerhardt1991), the properties of the calling songs of frogs and insects can be categorized as static, responsible for the quality of calls, mainly concerning species recognition; or dynamic, determining the quantity of signalling, and more related with mating success. Static characters should be highly stereotyped within and between males, whereas dynamic characters can vary considerably (Gerhardt, Reference Gerhardt1991). We did not assess within-male variability as we would need several calling songs from the same male in the field, but variability between males can also show this pattern. In fact, for C. barbara in both regions studied, the peak frequency, quartiles 25, 50 and 75% seem to be static characters, according to Gerhardt (Reference Gerhardt1991) classification, usually with less than 5% variability. The minimum and maximum frequency, bandwidth and quartiles 75–25% varied in most cases more than 12%, corresponding, therefore, to the Gerhardt dynamic characters. However, these particular characters depend considerably on recording settings, like distance, and the variability found might also reflect some recording heterogeneity. The syllable rate showed coefficients of variation between 5–8%, slightly higher than static properties but lower than most dynamic characters. Nonetheless, Fonseca & Revez (Reference Fonseca and Revez2002b) proved that the temporal pattern in C. barbara lusitanica can influence long-range communication; so, this character might be constrained by specific selective pressures and perform as static properties. Cicadas are difficult to keep in a laboratory, and females are particularly difficult to observe in the field. Therefore, female preference' studies are hard to develop and, for C. barbara, there is no literature available. Fonseca & Revez (Reference Fonseca and Revez2002b) studied the males' response to natural and modified songs and managed to successfully determine the relative importance of song parameters in song discrimination. Besides the importance of the temporal pattern, they also recognized that the most attractive peak frequency was 6 kHz and that C. barbara males can discriminate peak frequencies differing by 1–2 kHz. The amplitude modulated signal analysed in our study had similar low coefficients of variation for the peak frequency and the syllable rate of the calling song, corroborating the significance of these parameters on within species communication. However, future female choice experiments are needed to elucidate this significance.
The generally higher acoustic variability among populations in the Iberian Peninsula compared to Morocco might have been due to sampling effort, since more populations were analysed in Iberia. Habitat heterogeneity might also have had some influence, since topographic and latitudinal differences between sampling sites in the Iberian Peninsula were higher than in Morocco. Furthermore, all populations studied from Morocco occur in olive tree orchards, while in the Iberian Peninsula (and Ceuta) some of the populations analysed were also found in pine and even in eucalyptus trees (table 1).
All populations analysed here revealed significant differences for most acoustic variables. This differentiation might be partially due to a ‘chorusing’ effect; cicadas tend to sing in groups apparently to increase the chance of attracting conspecific females and to avoid predators (Villet, Reference Villet1992; Cooley & Marshall, Reference Cooley and Marshall2001; Fonseca & Revez, Reference Fonseca and Revez2002b) or as a result of inter-male competition (Greenfield et al., Reference Greenfield, Tourtellot and Snedden1997; Sueur & Aubin, Reference Sueur and Aubin2002), which might have supported a scattered distribution favouring divergence between different populations, as also hypothesised for C. orni populations in Europe (Pinto-Juma et al., Reference Pinto-Juma, Simões, Seabra and Quartau2005). Moreover, sound propagation depends not only on the insects’ anatomy and physiology but also on the physical environmental conditions (Bennet-Clark, Reference Bennet-Clark1998b), so cicadas may need to adjust some qualities of the calling songs to local environmental conditions in order to maximise sound communication.
Regional variation and subspecies
Although most acoustic characters revealed significant differences between populations and regions, the statistical analyses performed did not completely separate any region or subspecies and an overlap of specimens from different regions was often observed. In addition, the amplitude modulated signal on which Boulard (Reference Boulard1995) found support for the splitting of the species into C. barbara barbara and C. barbara lusitanica did not show any significant differences between these subspecies in our study.
The low but significant correlation between most acoustic variables and latitude showed that there is a slightly gradient, with all frequency variables decreasing from south to north. This gradient and some high coefficients of variation for some variables probably complicated the effective separation of regions. Nonetheless, the significant differences of most acoustic variables between regions do suggest a partitioning within this species between Iberian Peninsula+Ceuta and Morocco. The higher level of variation in the Iberian Peninsula compared to Morocco certainly influenced the results obtained for the DFA, as shown by the high proportion of misclassified samples in the Iberian Peninsula. The Ceuta population tended to separate from the other regions, but the split of this population from the Iberian Peninsula did not have statistical support. On the other hand, the separation between Morocco and the remaining populations was supported by all analyses performed. Because these calling songs are likely to be constrained by species-specific morphological/physiological characteristics, significant differences at higher levels, such as at the regional level, might indicate partitioning within species; and, as such, the present data support the subspecific division of C. barbara of Boulard (Reference Boulard1982) with C. barbara lusitanica present in the Iberian Peninsula and Ceuta and C. barbara barbara present in Morocco. The fact that all static properties of the calling song, excluding the syllable rate, had significant differences between these two regions reinforces this supposition since these variables are probably more associated to species recognition (Gerhardt, Reference Gerhardt1991).
The Ceuta population, in spite of being located in North Africa, seems to be more similar to the Iberian Peninsula populations than to those located in the Moroccan mainland south of the Rif Mountains. This might be expected since the Rif Mountains might have caused the segregation of Ceuta and the Moroccan populations analysed and, thus, increased their divergence. On the other hand, the strait of Gibraltar, separating the Iberian Peninsula from Ceuta, is relatively short and does not appear to have acted as an effective barrier. These results corroborate and complement the analyses of the populations at the molecular level (Pinto-Juma et al., this journal) since the same separation between regions was achieved.
Even though the acoustic signals analysed are variable at the inter-individual and population levels, the acoustic divergence observed at the regional level seems more consistent, as observed also in Cicada orni (Pinto-Juma et al., Reference Pinto-Juma, Simões, Seabra and Quartau2005), and may be useful to distinguish between closely related taxa. However, determining the level of differences between two groups of populations of a given species in order to split it into independent subspecies is highly complex.
In the present study, C. barbara presented a highly stereotyped calling song, which, notwithstanding this, allowed some discrimination between regions and, therefore, supported its splitting into two subspecies. But it should be noted that had this marker been applied alone it would not be sufficient to sustain such subspecific differentiation. However, the combination of the acoustic data with the molecular analyses for the same populations have reinforced and strengthened the delimitation of the two different subspecies within C. barbara.
Acknowledgements
We are very grateful to Genage André, Paula Simões, Teresa Rebelo, Mónica Ribeiro and Teresa Fernandes (Faculty of Science, University of Lisbon) for their help with field work. Moreover, we thank Paula Simões, Octávio Paulo and Michael Bruford for having discussed some topics analysed here with us. This research was supported by two PhD grants (PRAXIS BD/18229/98 and SFRH/BD/1027/2000) from the Fundação para a Ciência e Tecnologia (Lisbon, Portugal).

Appendix I. Boxplots of the acoustic variables analysed for Cicada barbara at the sampled sites.
Boxplots represent the sample distribution: the rectangular box corresponds to approximately the middle 50% of the data, the top of the box is the third quartile (75%), the bottom is the first quartile (25%) and the horizontal line is the median; vertical lines extending to either side indicate the general extent of the data; *, outlier; FèS, Fès south; Mek, Meknès; Ceu, Ceuta; Sev, Sevilla; Cor, Cordoba; Alc, Alcalar; Alv, Alvor; Mou, Moura; Por, Portel; Sou, Sousel; Mon, Monforte; Cra, Crato; Arr, Arrábida.
Appendix II. Values of average±standard deviation and coefficient of variation for each acoustic variable per population.

FèS, Fès south; Mek, Meknès; Ceu, Ceuta; Sev, Sevilla; Cord, Cordoba; Alc, Alcalar; Alv, Alvor; Mou, Moura; Port, Portel; Sou, Sousel; Mon, Monforte; Cra, Crato; Arr, Arrábida.


















