Introduction
The introduction of non-indigenous species (NIS) has been identified as a major threat to aquatic ecosystems, which may lead to loss of biodiversity with environmental, economic and social effects (Carlton, Reference Carlton1996). The ‘science of invasion’ supports regulatory structures that protect human health and local and/or global economies (Bax et al., Reference Bax, Carlton, Mathews-Amos, Haedrich, Howarth, Purcell, Rieser and Gray2001; Simberloff et al., Reference Simberloff, Martin, Genovesi, Maris, Wardle, Aronson, Courchamp, Galil, García-Berthou, Pascal, Pyšek, Sousa, Tabacchi and Vilà2013). The introduction of harmful aquatic organisms and pathogens to new environments via ballast water and other vectors, such as hull fouling and imports for aquaculture and aquariums, has been identified as one of the four greatest threats to the world's oceans (Darrigan & Damborenea, Reference Darrigan and Damborenea2005; Davidson & Simkanin, Reference Davidson and Simkanin2012). Recently, there is a growing increase in opportunities to translocate fish species among different areas by different means, such as in ballast water that is discharged from ships that cross oceans and transport species as eggs and larvae a long way from their natural range of distribution area (Hewitt et al., Reference Hewitt, Gollasch, Minchin, Rilov and Crooks2009). The increase of invasive fish species occurrences in estuarine areas is a major threat to the local fish communities with potential to cause considerable losses in biodiversity (Lopes & Villac, Reference Lopes, Villac, Lopes, Coradin, Pombo and Cunha2009; Britton et al., Reference Britton, Gozlan and Copp2010). In this context, knowledge of biodiversity and identifying species are essential to protect against global threats, among them, invasive species (Luypaert et al., Reference Luypaert, Hagan, McCarthy, Poti, Jungblut, Wegener, Liebich and Bode-Dalby2020).
The toadfish Opsanus beta (Goode & Bean, Reference Goode and Bean1880) has its natural distribution in the Western Central Atlantic, from the Gulf of Mexico to Palm Beach, Florida, including the Little Bahamas (Collette, Reference Collette and Carpenter2002; Froese & Pauly, Reference Froese and Pauly2020) and it is one of the most abundant fish in South Florida estuaries (Serafy et al., Reference Serafy, Hopkins and Walsh1997). Recent records of O. beta species on the south-eastern and southern Brazilian coast near to port areas suggest this species was able to reach these regions via ships' ballast waters (Caires et al., Reference Caires, Pichler, Spach and Ignácio2007; Ribeiro et al., Reference Ribeiro, Caires, Mariguela, Pereira, Hanner and Oliveira2012; Tomás et al., Reference Tomás, Tutui, Fagundes and Souza2012; Carvalho et al., Reference Carvalho, Júnior, Fávaro, Artoni and Vitule2020; Cordeiro et al., Reference Cordeiro, Bertoncini, Abrunhosa, Corona, Araújo and dos Santos2020).
Opsanus beta belongs to the family Batrachoididae, which is represented by 23 genera and 83 species, widely distributed throughout the Atlantic, Indian and Pacific Oceans (Greenfield et al., Reference Greenfield, Winterbottom and Collette2008). The great majority of species of Opsanus are marine, while some are found in brackish and freshwater environments (Nelson et al., Reference Nelson, Grande and Wilson2016). It reaches a maximum size of 300–324 mm standard length (SL) (Serafy et al., Reference Serafy, Hopkins and Walsh1997) and is a polyphagic lurking predator, feeding compulsively, mainly on fish, crustaceans and molluscs, with nocturnal feeding behaviour (Yáñez-Arancibia et al., Reference Yáñez-Arancibia, Lara-Domínguez and Day1993). Potential predators of this species in their original habitat are marine mammals (e.g. Sotalia guianensis and Tursiops truncatus), some grouper species, such as Alphestes afer (Bloch, 1793), Dermatolepis inermis (Valenciennes, 1833) and Epinephelus striatus (Bloch, 1792), the great barracuda Sphyraena barracuda (Edwards, 1771) and the lemon shark Negaprion brevirostris (Poey, 1868) (Robins & Ray, Reference Robins and Ray1986; Barros & Wells, Reference Barros and Wells1998).
In the present study, we report the occurrence of Opsanus beta at the Ilha da Madeira, in the Sepetiba Bay harbour area. This is the second record of this species on the coast of Rio de Janeiro State, with the first occurrence recorded in Guanabara Bay (Cordeiro et al., Reference Cordeiro, Bertoncini, Abrunhosa, Corona, Araújo and dos Santos2020). In addition to the morphological identification, we applied a barcoding approach to confirm the identity of the species. Lastly, we investigated whether the species may be using Sepetiba Bay as a breeding area by describing some stages of its developmental germinative cells.
Materials and methods
Study area
Sepetiba Bay (22°54′–23°40′S 43°34′–44°10′W) is located in Rio de Janeiro State, south-eastern Brazil, and has an area of 450 km2, which encompasses a wide range of habitats, including mangroves, sandbanks and small estuarine areas. The bay supports a rich and diversified fish fauna and is used as rearing grounds by several coastal fish species (Araújo et al., Reference Araújo, Teixeira, Guedes, Azevedo and Pessanha2018). The Port of Sepetiba (named Port of Itaguai since 2006) was constructed in the Ilha da Madeira, which was formerly separated from the mainland by narrow deltaic estuarine channels and mangrove areas (Leal Neto et al., Reference Leal Neto, Legey, Gonzalez-Araya and Jablonski2006). This area is located in the north-east part of Sepetiba Bay (22°55′S 43°50′W), and is ~80 km west of Rio de Janeiro City. The port, active since 1982, was initially built with a single pier to provide a bulk import terminal for coal and alumina. Since 1998, the port has a new wharf used for the import and export of various cargos, including rolled steel, vehicles, containers and sulphur products (Clarke et al., Reference Clarke, Hilliard, Junqueira, Leal Neto, Polglaze and Raaymakers2004).
Specimens
Thirteen individuals of Opsanus beta were collected in the Sepetiba Bay harbour area. The two smallest (73.2–100.4 mm SL) were collected by beach seine and the 11 largest (120.3–265.0 mm SL) by recreational anglers fishing with hook-and-line gear in July and November 2018 and July 2019. Morphometric, meristic and staining data were obtained from the left side of each specimen (N = 13). Observations were performed under stereomicroscope, and the morphometric measurements were taken with a digital calliper following Collette (Reference Collette1974). Vouchers were deposited in the Fish Ecology Laboratory (LEP-UFRRJ) of the Universidade Federal Rural do Rio de Janeiro. The captured individuals in this study were registered on a digital platform (https://www.bioinvasaobrasil.org.br, 25 November 2019), which is a digital platform developed with the objective of making available records of invasive alien species in Brazil.
In addition to the 13 individuals sampled in the present study (size range = 73.2–265.0 mm standard length – SL), information obtained from the literature of another 48 specimens (size range = 30.3–250.0 mm SL) were included (Table 1). The latter individuals are deposited in the ichthyological collection of the Museum of Zoology of the University of São Paulo (MZUSP) and were used for comparisons and confirmation of the species (Table 1). These specimens were recorded from the Cedar Keys (Florida, USA), Western Caribbean Sea, southern Mexico to the Brazilian coast (states of São Paulo and Paraná).
Table 1. List of examined specimens of Opsanus beta and its junior synonym in the present study

DNA extraction and genetic analyses
We extracted the genomic DNA from fin clips using the Wizard Genomic DNA Purification Kit (Promega) following the manufacturer's instructions. Subsequently, the 650-bp barcode region of COI was amplified under standard conditions using the primers FishF1 5′-TCAACCAACCACAAAGACATTGGCAC-3′ and FishR1 5′-TAGACTTCTGGGTGGCCAAAGAATCA-3′ (Ward et al., Reference Ward, Zemlak, Innes, Last and Hebert2005). PCR products were visualized on a 1% agarose gel and sequenced in both directions using the BigDye Terminator v. 3.1 Cycle Sequencing Ready Reaction Kit (Applied Biosystems). Sequences were deposited on GenBank (https://www.ncbi.nlm.nih.gov/genbank/, 20 November 2019) under the accession numbers MT348381–MT348384.
Consensus sequences were generated using the Geneious v9.05 software and then queried (blastn) against two public databases: (i) the NCBI-Nucleotide collection, and (ii) the Barcode of Life Datasystems in April 2020. Considering the sequences that produced significant alignments in the NCBI collection, we retrieved all the Opsanus sequences (N = 17) and aligned with our data using the MUSCLE algorithm (Edgar, Reference Edgar2004). Sequence divergence values in the within and between species levels were calculated using the Kimura-2 Parameter (K2P) distance model of nucleotide substitution and a Neighbour-joining (NJ) tree of K2P distances was created to provide a graphic representation of divergence, with 1000 bootstrap replications using the MEGA X software (Stecher et al., Reference Stecher, Tamura and Kumar2020).
Reproduction
Some fish specimens (N = 9) were stored in ice and transported to the laboratory for histological analyses, where they were measured (standard length, SL, nearest 1 mm) and weighed (total weight, TW nearest 0.01 g). A ventral incision was made to expose gonads for determination of the sex and the gonadal development phases. Gonads were removed and a portion (<0.05 g) of each ovary and testis were taken from the middle part of the gonad, weighed to the nearest 0.01 g and fixed in formalin 10% solution for histological analyses. Gonads samples were then transferred to 70% ethanol for preservation. Afterwards, the gonads were dehydrated and embedded in paraffin wax. Cross-sections, 4–6 μm thick, were cut in a rotary microtome (Leica RM 2135, Wetzlar, Germany), stained with hematoxylin-eosin (HE) and mounted on glass slides for light microscopy scrutiny (Spector & Goldman, Reference Spector and Goldman2006). Microphotographs were taken with a MOTICAM 2300 3.0 megapixels camera coupled to an Olympus BX41 microscope. Identification of the gonadal maturation phases were made following the criteria in Brown-Peterson et al. (Reference Brown-Peterson, Wyanski, Saborido-Rey, Macewicz and Lowerre-Barbieri2011).
Results
We report the first occurrence of Opsanus beta in Sepetiba Bay, near the area of Sepetiba Port (22°55′S 43°50′W) (Figure 1). Examination of the O. beta specimens collected from the Brazilian coast demonstrated that morphometric, meristic and colour characteristics overlapped (Tables 2 and 3) with other individuals recorded elsewhere, especially in areas of its natural distribution as reported by Collette (Reference Collette2001, Reference Collette and Carpenter2002).

Fig. 1. Front, back and side views of the copy of Opsanus beta LEP-UFRRJ 2466 (100.4 mm SL) and LEP-UFRRJ 2473 (67.9 mm SL) captured from the Sepetiba Bay (Rio de Janeiro State, Brazil) in 2018.
Table 2. Morphometrics data of Opsanus beta (LEP-UFRRJ and MZUSP)

N, number of individuals.
a Additional information from Collette (Reference Collette2001).
Table 3. Meristic data of Opsanus beta (LEP-UFRRJ and MZUSP).

N, number of individuals.
a Additional information from Collette (Reference Collette2001).
The main diagnostic features of this group are the presence of several dark brown bars on the caudal and pectoral fins; bars on the pectoral fins irregularly joined, and small white spots of various sizes in the shape of a rosette along the body (Figure 1). First dorsal with three thorns. Second dorsal with 25–27 rays, usually 26, and pectoral fin with usually 20–21, occasionally 19 rays (Table 3). Dorsal and opercular spines solid and not connected to venom glands. Presence of small barbels wrapped around the mouth and eyes. Canine teeth and missing photophores.
COI gene-based identification revealed that specimens studied here exhibited >99.9% similarity to other O. beta specimens deposited in public databases (Table 4), showing low mean K2P divergence values of 0.032%, 0.032% and 0.02% between our samples and those collected in Ubatuba, Brazil, Alabama, USA and Gulf of Mexico, USA, respectively. Congeneric mean K2P comparisons evidenced 7.0% (O. tau) and 7.6% (O. pardus) divergence values, establishing a barcode gap of ~2.3 times between intra- and interspecific variation, providing a starting point for future studies of O. beta species/populations differentiation. Interestingly, considering only O. beta species, we observed a split of three subclusters (sc1: Brazil/Alabama/Gulf of Mexico; sc2: Mexico; sc3: Florida) (Figure 2) with 3% of within-species mean K2P genetic divergence; however, it must be noted that a single specimen of O. beta collected in Florida, USA (accession number FJ583769.1) showed a considerable K2P divergence value from our O. beta samples, with a remarkable maximum divergence value of 6.5%, while K2P divergence of this same sample from the congeneric O. tau is 4.26% (Table 5).

Fig. 2. K2P distance Neighbour-joining dendrogram of 23 COI sequences of different Opsanus species. Numbers at each branch indicate bootstrap values. Samples collected from Sepetiba Bay (Rio de Janeiro State, Brazil) are green coloured.
Table 4. Identification of sampled Opsanus beta using GenBank and BOLD databases

Table 5. Means and ranges of K2P distance values considering Opsanus beta species

Note that a minimum K2P divergence between species is lower than the maximum intraspecies divergence, depicting a taxonomic issue.
In the present study, only one male individual had immature gonads whereas all the other eight individuals were in maturation process (Figure 3). According to the histological analyses, most individuals were in the spawning capable phase, indicated by the late stages of vitellogenesis, with large amounts of lipid accumulation, yolk coalescence and the presence of the zona pellucida in females (Figure 3A, B). In addition, the males had the presence of spermatozoa in the lumen of the lobules and in the sperm ducts (Figure 3C, D).
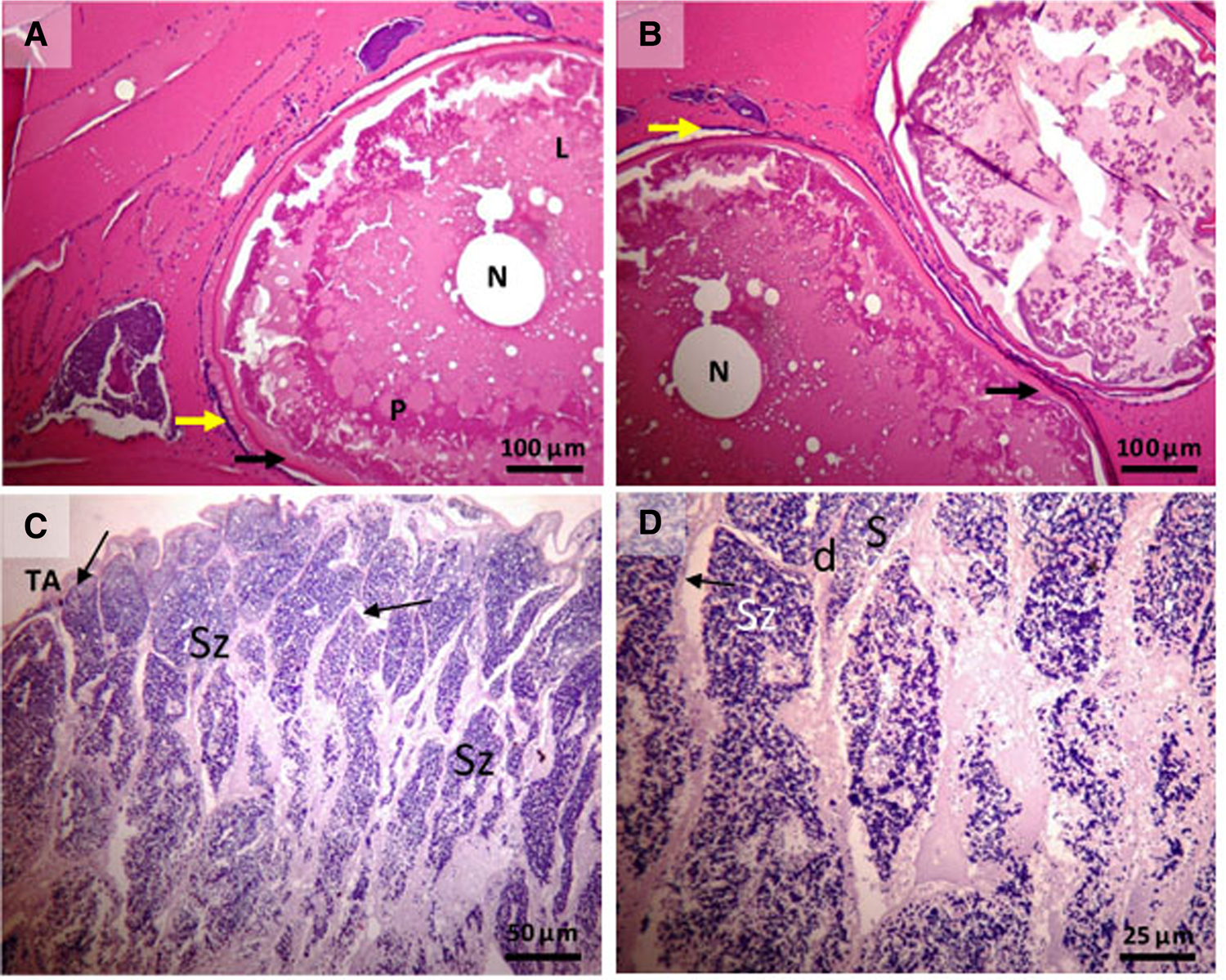
Fig. 3. Histology of Opsanus beta reproductive organs. (A and B) Females. The spawning capable stage is characterized by the appearance of vitellogenic oocytes, the amount of lipid accumulation, follicular cells and zona pellucida (or zona radiata). (C and D) Males. The spawning capable stage is identified by the presence of spermatozoa (Sz) in the lumen of the lobules and in the sperm ducts and the presence of seminiferous tubules. N, nucleus; yellow arrow, follicular cells; black arrow, zona radiata; L, lipids; P, proteins; TA, tunic albuginea; d, seminiferous ducts.
Discussion
This study reports the first occurrence of Opsanus beta in Sepetiba Bay, south-eastern Brazil. A comprehensive description of the morphological characteristics and molecular diversity has been provided, together with comparisons with other specimens from elsewhere, as well as from their area of natural distribution in the Central-Western Atlantic. In addition, histological analyses were conducted to determine the reproductive status of this species. These different approaches are crucial to make sure that the target studied species met with the original characteristics of the species in its original distribution area, and to indicate the potential risk of introductions of NIS.
It is not uncommon to have misleading descriptions of ‘new species’ for NIS, thus adding confusion, uncertainties and risks in programmes aiming at environmental management. Incomplete knowledge of species diversity has serious implications for marine conservation. Therefore, a careful study of NIS is very important for managing species introductions and for the assessment of invasion impacts, since this information is evaluated by international conservation boards, such as the International Union for Conservation of Nature (IUCN, 2018; Luypaert et al., Reference Luypaert, Hagan, McCarthy, Poti, Jungblut, Wegener, Liebich and Bode-Dalby2020). In the present study, we identified Opsanus beta using multiple methods (i.e. morphology, genetic structure) and to detect the establishment of a population in the area, since most of the examined individuals were in the spawning capable gonadal phase.
Examination of the O. beta specimens collected from the Brazilian coast demonstrated overlapping morphometric, meristic and colour characteristics with other individuals recorded elsewhere, especially in areas of its natural distribution as reported by Collette (Reference Collette2001, Reference Collette and Carpenter2002). Although Rotundo et al. (Reference Rotundo, Spinelli and Zavala-Camin2005) described Opsanus brasiliensis, the supposed differences in the number of pre-caudal vertebrae and colour pattern between the specimens of the Brazilian coast and other Opsanus species, including O. beta, were misleading. They did not consider the first pre-caudal vertebra that is under the developed supraoccipital crest present in Opsanus spp. (Collette, Reference Collette2001). For this reason, Opsanus brasiliensis is a junior synonym of O. beta (Caires et al., Reference Caires, Pichler, Spach and Ignácio2007) with 10–11 pre-caudal vertebrae and, therefore, within the expected range for O. beta (Collette, Reference Collette2001). Likewise, the colour patterns of Brazilian specimens (including the O. brasiliensis holotype) are similar to those individuals recorded between Belize and Florida by Collette (Reference Collette and Carpenter2002), especially the presence of several dark brown bars on the caudal and pectoral fins, the bars on the pectoral fins irregularly joined, and the small white spots of various sizes in the shape of a rosette along the body.
The use of DNA barcode-based delimitation of species has become an important alternative as a quick start for the taxonomic process (Ribeiro et al., Reference Ribeiro, Caires, Mariguela, Pereira, Hanner and Oliveira2012). Here, our molecular analyses showed that specimens collected in Sepetiba Bay are genetically similar (>99.9%) to O. beta samples from Alabama/USA and Gulf of Mexico/USA, confirming their identity. However, the intraspecific split of three subclusters, with a maximum K2P divergence of 6.5%, might indicate taxonomic problems in this group, such as cryptic species or even misidentification of species (Ribeiro et al., Reference Ribeiro, Caires, Mariguela, Pereira, Hanner and Oliveira2012; Gangan et al., Reference Gangan, Pavan-Kumar and Jaiswar2019). In this context, a deeper taxonomic and population knowledge about this group is necessary, since multiple records of O. beta in the Brazilian coast were reported in the last few years (Ribeiro et al., Reference Ribeiro, Caires, Mariguela, Pereira, Hanner and Oliveira2012; Tomás et al., Reference Tomás, Tutui, Fagundes and Souza2012; Carvalho et al., Reference Carvalho, Júnior, Fávaro, Artoni and Vitule2020; Cordeiro et al., Reference Cordeiro, Bertoncini, Abrunhosa, Corona, Araújo and dos Santos2020) and whether these samples represent unique taxonomic units remains to be answered.
Our observations indicate that this species is using Sepetiba Bay as a breeding area. In general, males are in charge of protecting the spawning eggs that are more vulnerable to capture (Gray & Winn, Reference Gray and Winn1961). When reproductively active, males settle in a nest (Gray & Winn, Reference Gray and Winn1961) and create a tonal boat whistle sound to attract females (Møhl et al., Reference Møhl, Wahlberg, Madsen, Miller and Surlykke2000). Notably, it is known that vessel noises and dolphin sounds are detected by male toadfish that depressed calling rate during sound exposure (Krahforst et al., Reference Krahforst, Sprague and Luczkovich2016). Although the repetitive boat noise might reduce mating success in busy shipping channels, the warm waters around mangrove areas in Sepetiba Bay, together with the lack of possible predators such as marine mammals (e.g. Sotalia guianensis and Tursiops truncatus) and some species of groupers – Alphestes afer, Dermatolepis inermis, Epinephelus striatus (Robins & Ray, Reference Robins and Ray1986), are indicators of adequate conditions for the establishment and increase of new populations of O. beta. Opsanus beta is an important component of trophic food webs in Florida's and Veracruz's subtropical estuarine communities, with a diet based mainly on fish (Gobionellus oceanicus (Pallas, 1770) and Citharichthys spilopterus Günther, 1862) (Serafy et al., Reference Serafy, Hopkins and Walsh1997) and crustaceans such as Callinectes spp. (López et al., Reference López, González, Arenas, Sánchez, Escorcia, Pérez, Rodríguez and Legorreta2017). These prey species are also present in Sepetiba Bay (Araújo et al., Reference Araújo, Teixeira, Guedes, Azevedo and Pessanha2018). Because these consumed species are common in estuarine areas, this suggests that O. beta has a generalist feeding habit with a broad impact on the food webs.
Opsanus beta has territorial and aggressive behaviour, high predatory potential and is considered to be resistant to environmental changes, thus its potential impact where it has been introduced is a concern (Gray & Winn, Reference Gray and Winn1961; Ferreira et al., Reference Ferreira, Junqueira, Villac, Lopes, Rilov and Crooks2009). Barimo et al. (Reference Barimo, Serafy, Frezza and Walsh2007) show that O. beta was locally most abundant adjacent to the mangrove fringe of Florida Bay (USA) where the highest ammonia fraction was found, with numerous possible nitrogen sources including guano from avifauna and an accumulation of seagrass detritus and drift algae. These habitats offer the toadfish refugia and are suitable spawning substrates. It may use anthropogenic litter and man-made structures as shelter and for a nest, so they are prone to inhabit artificial hard substrates like ship hulls, pilings in ports, etc. (Barimo et al., Reference Barimo, Serafy, Frezza and Walsh2007).
Occurrences of O. beta in the south-eastern Brazilian coast have been recorded in close proximity to port areas, such as Santos Port (23°59′S) (Rotundo et al., Reference Rotundo, Spinelli and Zavala-Camin2005; Ribeiro et al., Reference Ribeiro, Caires, Mariguela, Pereira, Hanner and Oliveira2012; Tomás et al., Reference Tomás, Tutui, Fagundes and Souza2012), Paranaguá Bay Port (25°33′S) (Caires et al., Reference Caires, Pichler, Spach and Ignácio2007), Guanabara Bay Port (22°49′S) (Cordeiro et al., Reference Cordeiro, Bertoncini, Abrunhosa, Corona, Araújo and dos Santos2020) and Sepetiba Bay Port (22°55′S, present study). In addition to ports providing appropriate spawning habitats, it is likely that the presence of O. beta near port areas is associated with the discharges of ballast water from ships. Several studies have postulated that the introduction of this species was from ships' ballast water (e.g. Caires et al., Reference Caires, Pichler, Spach and Ignácio2007; Tomás et al., Reference Tomás, Tutui, Fagundes and Souza2012), but we have yet to find concrete evidence to support this hypothesis (e.g. yet to be confirmed from ballast water specimens or eDNA). In addition, the construction of static maritime structures (SMS, e.g. oil and gas platforms, offshore wind farms, navigational buoys, non-cargo barges and dry docks) can be used as ‘stepping stones’ by marine non-indigenous species (Hewitt et al., Reference Hewitt, Gollasch, Minchin, Rilov and Crooks2009). Most SMS are characterized by their large and complex wetted surface area, providing space for fouling organisms, which may attract predators (Hewitt et al., Reference Hewitt, Gollasch, Minchin, Rilov and Crooks2009; Lacarella et al., Reference Lacarella, Davidson and Dunham2019). Habitat provided by reefs placed in areas devoid of natural hard bottom or structure may be colonized by NIS propagules dispersed from natural or anthropogenic sources (Glasby et al., Reference Glasby, Connell, Holloway and Hewitt2007). In the following text we review the linkages between NIS and constructed reefs, and recommend approaches for anticipating, assessing and controlling introductions of toadfishes.
An essential first step is that countries should have and keep up-to-date lists of non-indigenous species (NIS) recorded for their region. The digital platform https://www.bioinvasaobrasil.org.br was developed with the objective of making available records of invasive alien species in Brazil. We registered in this platform the individuals of O. beta captured in Sepetiba Bay. The Brazilian Federal Government adopted scientific criteria validated by experts to classify exotic species into three categories (detected, established and invasive) according to their population status after initial introduction, and their ecological, economic or health impact (Lopes & Villac, Reference Lopes, Villac, Lopes, Coradin, Pombo and Cunha2009). In this context, a recent report indicates that there are 76 non-indigenous species established that threaten native species, habitats or ecosystems in Brazil to date (Teixeira & Creed, Reference Teixeira and Creed2020). Opsanus beta in Brazil can be considered established, because it is an ‘introduced species detected on a recurring basis, with a complete life cycle in the wild and evidence of population increase over time in a restricted or wide region, but without apparent ecological or socioeconomic impacts’ (Lopes & Villac, Reference Lopes, Villac, Lopes, Coradin, Pombo and Cunha2009). Although it is possible that O. beta will never become an invasive species (‘invasive’ category) in Brazil, its observation in the ‘established’ modality as a precursor to biological invasion must be considered with due attention when implementing programmes for prevention and control, according to the precautionary principle (Lopes et al., Reference Lopes, Coradin, Pombo and Cunha2009).
The records of O. beta in Brazil demonstrate the necessity of conducting studies that allow understanding of the use of new environments by this non-indigenous species. Thus, it is important to generate biological and ecological information for this species, which will allow a more detailed knowledge about how individuals interact and participate in the different processes that take place in estuarine environments. For instance, efforts to detect areas where invasive fish species have been introduced might help to elaborate mitigation measures and such information would also be valuable to fine-tune the proposals for sustainable management and use of the coastal ecosystems. The management problems include predatory, competitive or displacement effects on indigenous fishes (Levin et al., Reference Levin, Achord, Feist and Zabel2002); general homogenization of fish assemblages across areas that were previously distinct (Rahel, Reference Rahel2000); the erosion of genetic biodiversity in otherwise isolated populations (Douglas & Brunner, Reference Douglas and Brunner2002); and substantial structural and functional alterations to aquatic food webs (Townsend, Reference Townsend1996). In this context, we suggest that further studies should be directed to the search of effective alternatives for the management of fish introduction. Recently, Species Distribution Models (SDMs) have demonstrated their potential to become powerful and valuable tools in identifying high-risk areas and species and developing mitigation strategies (Seebens et al., Reference Seebens, Schwartz, Schupp and Blasius2016). However, it is critical to characterize and quantify the invasion vectors, and gather non-indigenous species records that are made publicly available. In this sense, it is very important to include information in digital platforms on the records of invasive and non-indigenous species, and verify morphological identification with genetic analysis and reproductive activity with histology of gonads or other means, as we have provided herein for O. beta in Sepetiba Bay. Moreover, it is important to keep monitoring programmes for biological communities in regions considered critical for the introduction of marine and estuarine species, such as port areas and localities close to mariculture farms of exotic species. Prevention is the most effective risk reduction approach because controlling marine NIS after introduction is expensive and offers limited probability for success (Carlton, Reference Carlton1996; Sheehy & Vik, Reference Sheehy and Vik2010). Information from these programmes can help a precise diagnosis and early warning of the introduction of marine NIS and is crucial to develop monitoring and mitigating initiatives to deal with these concerns.
Acknowledgements
We thank Sergio Hiroshi fishermen from Ilha da Madeira for providing some of the individuals analysed here. We thank Denis Carvalho Leite da Silva for helping in fieldwork. We also thank Iracema David Gomes and Aparecida Alves do Nascimento for the histological analyses. Grants for M.F. de Andrade-Tubino from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). This research was conducted under SISBIO Collection of Species Permit number 10707 issued by ICMBio, Brazilian Environmental Agency.
Financial support
This study was partially supported by the Project Pesquisa Marinha e Pesqueira, a compensatory measure established by Conduct Adjustment Term responsibility of the Chevron Company, conducted by the Federal Public Ministry – MPF/RJ, with the implementation of the Fundo Brasileiro para a Biodiversidade – Funbio, Proc.16/2017.










