Introduction
Organochlorines (OC) include industrial compounds such as polychlorinated biphenyls (PCBs) and chlorinated pesticides. They bioconcentrate in fatty tissues and biomagnify in the environment.Reference Muir, Wagemann and Hargrave 1 Due to these properties and because direct exposure can lead to diverse pathologies, many OC have been restricted or banned since the 1990s. 2 High levels of OC, however, persistently contaminate the Arctic food chain.Reference Van Oostdam, Donaldson and Feeley 3 As a consequence, Inuit populations have 25-fold higher OC body burdens than people from southern Canada.Reference Bjerregaard, Dewailly and Ayotte 4 Because pregnant mothers consume contaminated fish and seal blubber, infants are also exposed in utero across the placental barrier and after birth during lactation.Reference Ayotte, Muckle and Jacobson 5
Reduced sperm quality has been reported in OC-exposed Inuit men.Reference Bonde, Toft and Rylander 6 Exposure of men to the OC pesticide, p,p'-dichlorodiphenyltrichloroethane, in MexicoReference De Jager, Farias and Barraza-Villarreal 7 and South AfricaReference De Jager, Aneck-Hahn and Bornman 8 is associated with endocrine changes and compromised sperm quality. Bioaccumulation leads to high levels as men age,Reference Medehouenou, Ayotte and Carmichael 9 because males do not effectively eliminate OC. Yet, few studies address the effects of prenatal exposure to environmental OC concentrations on the health of adult males.
Our laboratory has reported that prenatal exposure to environmentally relevant OC disrupts sperm motility in adult rats at postnatal day (PND) 90, which are approximately equivalent of a 30-year-old man,Reference Flurkey, Currer and Harrison 10 whereas younger PND 60 males (representative of a 20-year-old man), showed similar sperm quality as unexposed controls.Reference Anas, Guillemette and Ayotte 11 These observations and gaps in the literature led us to use a rat model to test the hypothesis that prenatal exposure to an OC mixture, resembling that which contaminates the traditional foods of Inuit, induces age-related systemic impacts that compromise male reproductive capacity and health.
Materials and methods
Chemicals
Unless specified otherwise, all chemicals were purchased from Sigma-Aldrich, Oakville, ON, Canada. Organochlorine suppliers are indicated in Table 1. Gibco®Media-199 without phenol red was from Life Technologies, Grand Island, NY. Propidium iodide (PI) was from Life Technologies. The testosterone EIA kit was from Cayman Chemical, Ann Arbor, MI, and isoflurane was from Baxter, Mississauga, ON, Canada.
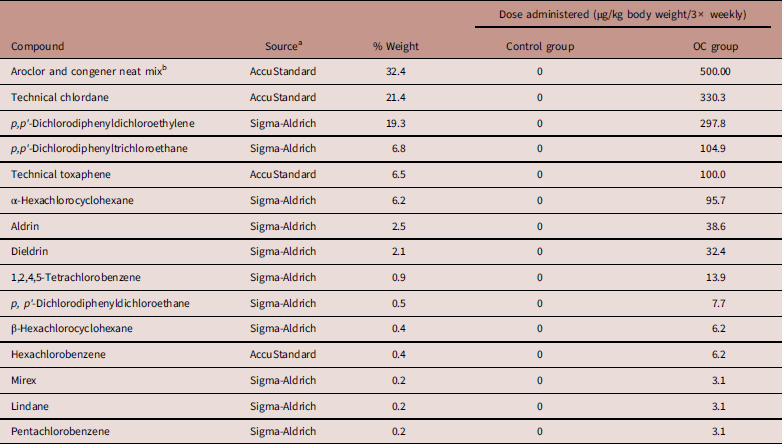
Table 1 Composition of the organochlorine (OC) mixture used in this study

a -Sigma-Aldrich (Oakville, ON); AccuStandard (New Haven, CT).
b Mix containing Aroclor 1260 (58.9%), Aroclor 1254 (39.3%), 2,4,4'-trichlorobiphenyl (PCB 28; 1.0%), 2,2',4,4'-tetrachlorobiphenyl (PCB 47; 0.8%), 3,3',4,4',5-pentachlorobiphenyl (PCB 126; 0.02%), and 3,3',4,4'-tetrachlorobiphenyl (PCB 77; 0.004%). CAS number of each substance are presented in previous studyReference Auger, Park and Zoungrana 22 .
Prenatal treatment
Before starting the study, all protocols were pre-approved in accordance with the guidelines of the institutional committees for animal use, chemical safety and ethics. The Université Laval committee for the ethical use of live animal certificate was CPA-2009124. Pure OC compounds or technical mixtures were dissolved in corn oil to obtain 5 mg/ml (stock solution) (Table 1).Reference Anas, Guillemette and Ayotte 11 Five-week-old female (F0 founders dams), 10-week-old male (untreated F0 sires) and 10-week-old female Sprague–Dawley rats were purchased as required from Charles River Canada Ltd. (St. Constant, Québec). Animals were acclimatized for 10 days before experimentation and housed with a 12 L–12 D photoperiod, temperature range of 22±1°C and humidity range of 46±10%. Water and standard commercial rat chow were provided ad libitum.
Prenatal exposure of F1 males
Five-week-old female rats were randomly assigned to two groups (n=4 per group; two per cage) and treated by gavage (1 ml) with corn oil (Control) or the organochlorine mixture (OC; 500 µg/kg body weight) thrice weekly for 5 weeks. The 500 µg/kg/day dosage was confirmed in a previous studyReference Anas, Guillemette and Ayotte 11 to be environmentally relevant, because the serum contaminant levels in the F0 dams and F1 pups approximate serum levels in Inuit people. The body weight of each female was assessed the morning of gavage to determine the quantity of OC to be administered. Each pair of females was housed with an unexposed male until mating was confirmed by the presence of sperm in vaginal smears, after which females were housed one per cage. Gavage continued until parturition of the F1 litters. The day of mating was considered as gestational day (GD) and the day of parturition was designated as postnatal day (PND) 0.
Male fertility analysis
At PND 90, 15 F1 males from four litters in each treatment (3–4 males per litter) were individually housed overnight with two unexposed 10-week-old virgin females and mating was confirmed by the presence of sperm in the vaginal smears. Males and females were housed for mating during the nights only for a maximum of seven consecutive nights. For PND 90 males, the week after the last mating, animals were euthanized by cardiac puncture under isoflurane anesthesia following by CO2 asphyxia. At PND 365, 10 OC males (2–3 males per litter and different animals than PND 90) and 10 Control males (2–3 males per litter and different animals than PND 90) were mated in the same way as the PND 90 males. One female coupled with a PND 365 male appeared to have not been mated, so she was removed from the study. One week after the mating, PND 365 males were anesthetized then euthanized by decapitation because to avoid vasoconstriction in the lungs due to isoflurane exposure. Fertility rate was calculated as follows: % fertility=(number of corpora lutea−number of live fetuses at GD 19.5)/number of corpora lutea×100. Sperm and reproductive organ were collected for reproductive assessment.
Lung histology
At PND 365, five males were sacrificed by decapitation and lungs were instilled under constant pressure of 10 cm3 4% paraformaldehyde for 15 min to minimize atelectasia.Reference Burri, Dbaly and Weibel 12 – Reference Boucher, Provost and Plante 14 After instillation, the lungs were removed from the chest cavity, and immersed in toto in the same fixative for at least 24 h at 4°C. The pressure was maintained during fixation.Reference Weibel 15 , Reference Weibel 16 Lungs were individually embedded using paraffin inclusion and serial step sections of 4–5 μm were taken along the longitudinal axis of the lobe. The sections were stained with hematoxylin–eosin and were examined using an Axioskop2 Plus microscope (200× magnification, Carl Zeiss, Toronto, ON), Qimaging Retiga 2000R camera (Qimaging, Surrey, BC) and Image-Pros Plus (MediaCybernetics, Rockville, MD). The paraffin sections of the middle lobe were projected onto a screen containing a single point test system. The sections were moved in a stepwise manner in the x and y directions, while categorizing the underlying structures hit by the test point. A total of 50–100 counting events per structure from five sections per rat were counted and the cavalieri principle was used to estimate the volume of lung structure as described previously.Reference Nyengaard and Gundensen 17 The volume of parenchyma (air spaces and tissue) and of non-parenchyma (bronchi, bronchioli, capillary and larger connective tissue strips) were measured using the whole lung as reference space.
Reproductive assessment of PND 90 and PND 365 F1 males
For PND 90 and PND 365 aged rats, reproductive organ and body weights were recorded during necropsy. Epididymal sperm assays were performed on the same day whereas testes were snap frozen in liquid nitrogen for storage at −80°C for later use.
Daily testicular spermatid production was performed on one thawed testisReference Burri, Dbaly and Weibel 12 for each male used for mating at PND 90 (n=15) and at PND 365 (n=10). The testis without tunica was weighed then homogenized in 10% DMSO/0.9% NaCl using a Polytron VDI 12 (VWR International, Radnor, PA). Samples were sonicated at 40% for 1 min (Sonic Dismembrator, Model 500; Fisher Scientific, Pittsburgh, PA) and 0.1% trypan blue was added to color spermatid heads, counted using a hemacytometer. Daily testicular spermatid production was calculated according to the equation:Reference Seung, Wolfe and Rocca 18
((Mean count of hemacytometer/0.00004 µl volume of secondary square in hemacytometer)×100.5 ml of total number of rat testis suspension)/6.10 days for spermatogenesis cycle.
The caudal epididymis was excised at necropsy on all males used for mating at PND 90 (n=15) and at PND 365 (n=10), trimmed of fat, sliced and placed in a 35 mm Petri dish containing 5 ml Gibco®Media-199 without phenol red with 0.5% fatty acid-free BSA at 37°C for 15 min in a humidified 5% CO2 incubator to release the fresh sperm. For all reproductive assessments, two replicates per animal were conducted.
A sperm aliquot was diluted to 20×106 sperm/ml in phosphate buffered saline (PBS; 1.5 mm Kh2PO4, 8.1 mm Na2HPO4, 137 mm NaCl, 2.7 mm KCl; pH 7.4) then treated with 48 µm PI and 1 µg/ml of FITC-labeled peanut-agglutinin (FITC-PNA; fluoroisothianocynate-labeled Arachis hypogaea lectin) to detect plasma membrane and acrosome integrity, respectively, and incubated in the dark (10 min, 37°C). A total, 10,000 sperm were analyzed using a Guava EasyCyte Plus flow cytometer with Guava ExpressPro sofware (Guava Technologies/IMV Technologies, L’Aigle, France).
The chlortetracycline fluorescence (CTC) assay was used to evaluate the physiological status (capacitation, spontaneous acrosome reactions) of the sperm as described previously for the rat with some modifications.Reference Oberländer, Yeung and Cooper 19 In brief, the CTC stock solution contained 750 µM (final concentration) CTC–HCl, 130 mm NaCl, 5 mm l-cycteine and 20 mm Tris acid (pH 7.8) was prepared freshly and shielded from light at 10°C before using. A total of 10 µl of spermatozoal suspension were mixed 10 µl CTC stock solution, 2 µl of 12.5% glutaraldehyde in 20 mm Tric-HCl (pH 7.4) and 25 µl of 1.4-diaza-byciclo (2.2.2) octane (0.22 m) on a clean slide at room temperature. Finally, a drop of glycerol was added to retard the fading of CTC fluorescence. The sample (two replicates per slide) were covered with coverslips and stored in the dark at 4°C overnight. For evaluation of the CTC patterns, the slides were observed within 24 h under an Optiphot-2 microscope (Nikon Canada Inc., Mississauga, ON) equipped with phase contrast and epifluorescence optics with a 10× ocular and 40× objective using a BV filter. At least, 200 spermatozoa per sample were classified according to one of three CTC staining patterns as described by Oberländer et al.Reference Oberländer, Yeung and Cooper 19 : F pattern=uncapacited cell with uniform bright fluorescence over the head; B pattern=intermediate pattern with a dark band (arrow) in the postacrosomal region of the sperm head and AR pattern=acrosome-reacted cell with dark head except for the tip, which retained some fluorescence.
Another aliquot of fresh epididymal sperm from all males used for mating at PND 90 (n=15) and at PND 365 (n=10) was diluted 1/10 with PBS 1× to obtain 2×106 sperm/ml in 100 µm deep Rat Toxicology Slides (Leja Compagny, Nieuw-Vennep, The Netherlands). A minimum of 200 sperm from seven different fields was assessed. The percentages of motile and progressively motile sperm, average path velocity (VAP), straight-line velocity (VSL), curvilinear velocity, amplitude of lateral head displacement (ALH), beat cross frequency (BCF), straightness and linearity were measured using a Hamilton-Thorne CEROS II Analyzer (version 14; Beverly, MA) with a 4× objective. CEROS settings were: frame rate 60 Hz; frames acquired 30; minimum contrast 80; minimum cell size 8; cell size 25; cell intensity 80; path velocity 50 µ/s; straightness 25%; slow cells motile; VAP cut-off 10 µ/s; VSL cut-off 10 µ/s; intensity 2300; photometer 2; magnification 0.7.
Serum testosterone measurements
Serum was isolated from blood by centrifugation (4°C) at 1200 g for 20 min then stored at −80°C. Total serum testosterone levels were determined in 50 µl of sample using the Testosterone EIA kit following the manufacturer’s instructions. Each sample was assessed in triplicate. The intra- and inter-assay coefficients of variation (CV=s.d./mean×100) were less than 15% as per manufacturer recommendations. The IC50 and the detection limit of the testosterone assay were both 32 and 6 pg/ml.
Statistical analyses
Values are presented as means±s.e.m. Data were analyzed using SPSS version 22.0 (SPSS Inc., Chicago, IL). Data for body weight, reproductive assessments and reproductive organ weights were analyzed by one-way ANOVA followed by a post-hoc Tukey’s test to assess differences due to OC exposure. When tests for assumption of homogeneity of variance and normality failed, data were log-transformed and retested. An ANOVA followed by Tukey’s test was performed for the testosterone assay using the software package JMP 10 (SAS Institute, Cary, NC). Differences between OC and Controls were regarded as statistically significant at P<0.05.
Results
Pregnancy outcomes of F0 dams
Throughout the gavaging, F0 dams treated with OC showed the same body weight, mating success, fertility rate and pregnancy index as Controls (Table 2). Also, the number of pups per litter and the sex ratio were not different due to OC treatment (Table 2). As previously reported, this is an environmentally relevant OC level to Inuit peoples and did not induce apparent systemic toxicity in dams or alter their fertility parameters.Reference Anas, Guillemette and Ayotte 11
Table 2 Pregnancy outcomes of F0 females

OC, organochlorine.
a Number of females mated/number of female cohabited×100.
b Number of pregnant females/number of mated females×100.
c Number of females delivering live pups/numbers of pregnant females×100.
Health of rats prenatally exposed to OC
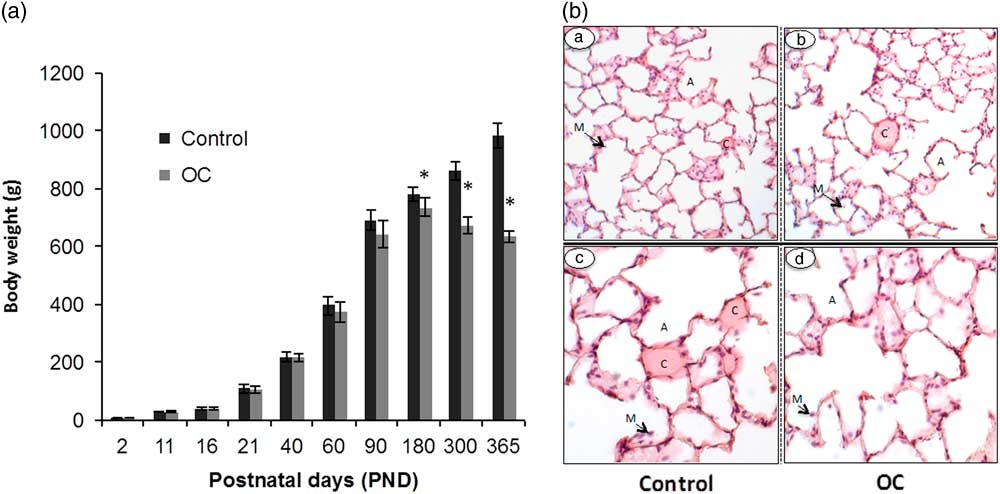
From PND 2 to PND 90, the body weights of the F1 males did not differ due to OC exposure (n=15; Fig. 1A). Between PND 90 and PND 180, however, prenatally exposed OC males gained less weight than Controls. From PND 180 on, all OC males weighed less than Controls (n=10; P<0.05) and after PND 200, OC males lost weight (n=10; P<0.05). At PND 350, one OC male died with apparent respiratory distress (Table 3) and all rats that were prenatally exposed to OC showed similar breathing difficulties breath and observable inactivity. Nevertheless, no major change in lung histology could be observed between Control and OC-exposed males, with the exception of a possible thinning of the alveolar barrier (Fig. 1B). No differences in lung volume, parenchymal volume and non-parenchymal volume were observed between Controls and prenatally OC exposed males on PND 365 (Table 4).
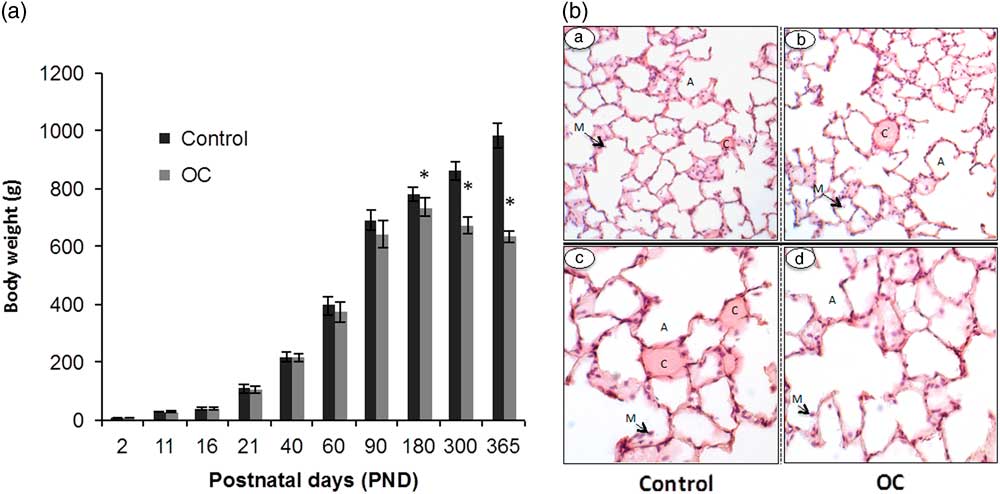
Fig. 1 Impact of early life exposure to an environmentally relevant concentration of the Arctic organochlorines (OC) mixture on the body weights and lung histology of rats at different ages. (Panel A) The body weights (g) of male rats from postnatal day (PND) 2 to PND 365 varied due to treatment as the males aged (n=15 until PND 90 and then n=10 onwards). (Panel B) Lung histology in aged male rats at PND 365 (n=5; representative images are presented). Tissue sections showing lung architecture from a Control male, 100× (a) and 200× magnifications (c) and an OC male at 100× (b) and 200× magnifications (d). There were no major differences in morphology between treatment groups. A, alveoli; C, capillary; M, macrophage. *Significantly different body weights due to early life OC exposure (P<0.05).
Table 3 Impact of prenatal exposure to the organochlorine (OC) mixture on the fertility parameters and relative organ weights of male rats at postnatal day (PND) 90 and PND 365

GD, gestational day; CTC, chlortetracycline; VAP, smoothed path velocity; VSL, straight line velocity; VCL, track velocity; ALH, amplitude of lateral head displacement; BCF, beat cross frequency; STR, straightness; LN, linearity.
All values are mean±s.e.m.
a Ratio of the organ weight to body weight (g/g).
b (Number of viable fetuses/number of corpora lutea)×100.
c (Number of corpora lutea−number of implantation sites)/number of corpora lutea×100.
*Significantly different within each age due to early life OC exposure (P<0.05).
Table 4 Lung measurements in F1 males that were prenatally exposed to organochlorines (OC) and Controls on postnatal day 365

All values are mean±s.e.m.; n=5.
The relative reproductive organ weights (adjusted for body weight) did not differ among groups at PND 90 due to prenatal OC (n=15; Table 3). In contrast, the relative weights of the testes, epididymides and seminal vesicles were lower for OC males than for Controls at PND 365 (n=10; P<0.05). Relative prostate weights did not differ due to treatment at PND 365. At PND 90, no difference in serum testosterone level was detected between treatments (n=15), however, at PND 365, these were lower in OC rats v. Controls (n=10; P<0.05; Table 3).
Male fertility and sperm quality
At PND 90, OC males were less fertile than Controls (n=15), whereas at PND 365, all OC males were infertile, despite the presence of sperm in vaginal smears of their mating partners (n=10; Table 3). Females mated with OC males at PND 90 had fewer fetuses at GD 19.5 and more preimplantation loss (n=30 females; Table 3); preimplantation loss was defined as: (number of corpora lutea−number of implantation sites)/number of corpora lutea×100.
Sperm quantity and quality could play roles in the compromised fertility of the OC males (Table 2). The preimplantation loss was greater for OC males compared with Controls at PND 90 (n=30 females) and 365 (n=20 females) indicating fertilization/embryonic failure. Daily spermatid production per testis and caudal epididymal sperm concentrations were lower for OC males compared with Controls at both PND 90 (n=15) and 365 (n=10; P<0.05). The percentages of live sperm with intact acrosomes did not differ due to treatment at PND 90 (n=15). At PND 365, however, more sperm from OC males than Controls had intact acrosomes (n=10; P<0.05; Table 3). The percentage of live sperm with intact acrosomes is a specific population combining both dyes, negative PI and PNA. At PND 365, 75% of live control sperm and 80% the live OC sperm had intact acrosomes (n=10). The CTC assay estimated whether sperm were spontaneously capacitated or acrosome reacted (Table 3). The percentage of pattern F (non-capacitated) OC sperm was always higher than in Controls (P<0.05), whereas fewer OC sperm appeared to be capacitated (pattern B; P<0.05). More live sperm from OC males than Controls had intact acrosomes according to the PI/PNA-FITC assay. Together, the CTC and PI-PNA assays indicate that sperm from OC males are less responsive to the environment. Sperm motility parameters were also affected by early life OC exposure at both PND 90 and 365 (Table 3). The percentages of motile and progressively motile sperm were consistently lower in the OC males relative to Controls at PND 90 (n=15) and 365 (n=10; P<0.05). The ALH and BCF were similarly reduced for the sperm of the OC males v. Controls at both ages (P<0.05).
Discussion
There is a major health discrepancy between Inuit in the Arctic and non-Aboriginal Canadians, such that the life expectancy of the Inuit is 10 years shorter.Reference Donaldson, Van Oostdam and Tikhonov 20 The leading causes of premature death in the Canadian Inuit population are cancers, cardiovascular diseases and chronic respiratory diseases.Reference Donaldson, Van Oostdam and Tikhonov 20 Many factors contribute to this discrepancy, however, exposure to contaminants has been implicated as a significant influence.Reference Donaldson, Van Oostdam and Tikhonov 20 Although Greenland Inuit had high exposure to OC, their fertility and semen quality seem not to be reduced, a criterion of this study was that the men studied were fertile, as evidenced by their partner being pregnant.Reference Toft, Axmon and Giwercman 21 A major source of OC contamination for Inuit is through traditional foods, including marine mammals. It has been suggested that the negative impact of OC is compensated by the positive health effects of antioxidants and polyunsaturated fatty acids present in marine-based food.Reference Toft, Axmon and Giwercman 21 Also, the high fertility rates in Inuit may be the result of a higher percentage of fathers under 30 years of age, in contrast with other populations.Reference Donaldson, Van Oostdam and Tikhonov 20 A population of OC-exposed Swedish fishermen displayed lower sperm quality and subfertility compared with a control population, which supports our results that northern OC harm male fertility.Reference Toft, Axmon and Giwercman 21 Although there are no existing data on sperm quality or male fertility in Inuit populations in northern Canada, it is well documented that Inuit people suffer a higher rate of stillbirth due to poor fetal growth, placental disorders and congenital anomalies in comparison with non-Aboriginal Canadians.Reference Auger, Park and Zoungrana 22 In this pilot study, we demonstrate for the first time that prenatal exposure to an environmentally relevant level of an OC mixture designed to mimic that which contaminates the Arctic food chain, reduces fertility and the body condition in an aging rat model.
Prenatal exposure alters the health of aged males
Prenatal exposure to this Arctic OC mixture does not appear to have harmful systemic effects, as reflected by the normal average body weights of the male rats up to PND 90.Reference Anas, Guillemette and Ayotte 11 The marked shift in body weight observed in the older rats was unexpected. The reasons for the weight loss in the F1 males during aging are unclear, however, it could be associated with acute illness, pain, constipation and confusion, eating or swallowing problems, poor oral health, reduced appetite and low food intake.Reference Stajkovic, Aitken and Holroyd-Leduc 23 The prevalence of overweight and obese people in Inuit has risen, presumably to contemporary lifestyle changes.Reference Boucher, Provost and Plante 14 In addition, Inuit men have a shorter life expectancy (63 years), a higher percentage of chronic respiratory (11%) and cardiovascular diseases (26%) in comparison with non-Aboriginal Canadians.Reference Donaldson, Van Oostdam and Tikhonov 20 Although health status is multifactorial, in light of our findings with a rat model, it is tempting to speculate that prenatal exposure to OC is involved in the reduced life expectancy of Inuit people. Future studies should assess food intake, metabolic function and detailed pathological analyses in prenatally exposed animals to clarify the cause of the weight loss and the premature death reported here.
Following the unexpected respiratory problems in OC rats, they were quickly euthanized as well as the Controls. However, no remarkable change in pulmonary structure and morphology could be observed between the two groups. Because our euthanasia method was not optimal for pulmonary histology,Reference Ochs and Mühlfeld 24 we were unable to conduct accurate lung stereology. Nevertheless, we cannot assert that a difference does not exist between OC and Control lungs. It has been reported that exposure to OC is associated with asthma and chronic bronchitis in non-Aboriginal Canadian adultsReference Ye, Beach and Martin 25 as well as otitis media, pneumonia, pertussis, asthma, and upper and lower respiratory tract infection in children.Reference Karmaus, Kuehr and Kruse 26 , Reference Gascon, Vrijheid and Martinez 27 In Inuit populations, prenatal exposures to PCBs and DDE have been strongly associated with incidence of acute respiratory infections as acute otitis media and upper and lower respiratory tract infections in preschool children.Reference Dallaire, Dewailly and Muckle 28 , Reference Dallaire, Dewailly and Vézina 29 Knowing that Inuit men in Arctic Canada are at a greater risk to die prematurely from a respiratory diseases than non-Aboriginal Canadians,Reference Donaldson, Van Oostdam and Tikhonov 20 it is possible that prenatal exposure to OC is responsible for respiratory dysfunction in our F1 male rats. A dyspnea such as heart failure could also be a factor of the respiratory distress, although heart histology was not conducted in this study.Reference Bozkurt and Mann 30 Reduced immune response due to prenatal exposure to OC is another hypothesis, as such animals could develop chronic pathologies that would slowly limit physical ability and food intake, resulting in slow weight loss and respiratory congestion.Reference Scanga, Verde and Paolone 31 Weight loss in obese patients increases the concentration of OC compounds in their blood and decreases the amount of leukocytes and lymphocytes, thereby suppressing natural killer cell activity.Reference Scanga, Verde and Paolone 31 These symptoms were observed for all the aged OC males. To support this hypothesis, prenatal exposure to the Arctic OC mixture has been reported to depress immune responses in swine.Reference Bilrha, Roy and Wagner 32 Similarly, prenatal exposure to OC in Inuit populations is correlated with a high level of infection and respiratory diseases.Reference Sheppard and Hetherington 33 High levels of chronic respiratory disease, cancers and infectious diseases are major contributors to the high mortality rate in Inuit adults.Reference Donaldson, Van Oostdam and Tikhonov 20 As observed in our animal model, a slow decrease of body weight and overall decline of health seem to be related to the prenatal exposure to the Arctic OC because none of the Controls animals demonstrated observable health issues. Prenatal exposure to OC could affect Inuit health, however, further research is required to understand these links.
Fertility decline due to prenatal exposure
Gradually with age, the OC rats in this study lost weight, likely liberating the sequestered OC into the blood.Reference De Roos, Ulrich and Sjödin 34 The Arctic OC mixture is known to be anti-androgenic.Reference Anas, Guillemette and Ayotte 11 Increased blood OC concentrations in aged rats could contribute to decreased testosterone levels and weights of androgen-sensitive organs (testes and epididymides) as observed in the present study. These reduced reproductive organ weights might contribute to the observed infertility of the aged PND 365 OC rats (approximating 50-year-old menReference Flurkey, Currer and Harrison 10 ). We have previously reported that young adult male rats at PND 60 (approximating 18-year-old menReference Flurkey, Currer and Harrison 10 ) had normal sperm following prenatal exposure to the same OC mixture, whereas sperm quality declined at PND 90 (approximating 27-year-old menReference Flurkey, Currer and Harrison 10 , Reference Anas, Guillemette and Ayotte 11 ). Here, we report that PND 90 rats are subfertile due to prenatal exposure and that the older PND 365 rats are infertile, indicating that fertility declines gradually with age. Normally, sperm quality in men decreases gradually from 35 years old and fertility does not decline before the age of 40–50 years (approximating PND 365 in ratsReference Flurkey, Currer and Harrison 10 ).Reference Kidd, Eskenazi and Wyrobek 35 Most Inuit fathers are relatively young, often under 20 years, when they have their first children.Reference Donaldson, Van Oostdam and Tikhonov 20 Although high relative to other groups in Canada, Inuit fertility is gradually declining.Reference Donaldson, Van Oostdam and Tikhonov 20 However, no data are available on the sperm parameters in Canadian Inuit populations, although decreasing sperm count and motility was strongly associated with high PCB serum levels in Inuit men in Greenland.Reference Toft, Axmon and Giwercman 21 Whereas the fertility rate of the Inuit population is calculated by the number of live births per 1000 women,Reference Donaldson, Van Oostdam and Tikhonov 20 male fertility remains unknown. In our model, prenatally exposed male rats produced less sperm than Controls. In men, decreased sperm production is associated with infertility.Reference Kidd, Eskenazi and Wyrobek 35 , Reference Bailey 36 Moreover, fewer sperm from the OC rats were spontaneously capacitated and more had intact acrosomes relative to Controls, suggesting the OC sperm are less responsive to their environment and, therefore, less likely to fertilize. Indeed, the inability of sperm to respond to stimulators of capacitation or the acrosome reaction is associated with reduced male fertility.Reference Bailey 36 , Reference Liu and Baler 37 Alternatively, the infertility of the OC males at PND 365 might be explained by inefficient mating due to declining health, which would trigger pseudopregnancy. Nevertheless, subfertility and infertility have been reported to be increasing in men worldwide.Reference Skakkebaek, Jorgensen and Main 38 Our results with an animal model, therefore, suggest that prenatal exposure to environmentally relevant OC could be at least partly responsible for declining human fertility.
In conclusion, our animal model is the first to address the impact of a prenatal exposure to Arctic contaminants on male fertility and health at different times during adulthood. More research is necessary to determine if the prenatally exposed males have immune deficiencies, pulmonary abnormalities or heart defects.
Financial Support
This work was supported by the Fonds de Recherche du Québec – Nature et Technologies (PR-133831) Team Grant awarded to JL Bailey as the Principal Investigator.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of laboratory animals (Canadian Council on Animal Care) and has been approved by the institutional committee (Comité de protection des animaux de l'Université Laval).
Acknowledgements
The authors thank Drs Pierre Provost and Eric Boucher for advice on lung histology.