Introduction
Migratory birds connect ecosystems, with millions of individuals flying long distances between breeding and wintering grounds (Bauer and Hoye, Reference Bauer and Hoye2014). Due to their intercontinental dispersal capability, migrant birds are thought to play an important role in global disease transmission (Altizer et al., Reference Altizer, Bartel and Han2011). Migrating birds can disperse parasitic and pathogenic organisms, as well as vectors and their associated pathogens, such as ticks and tick-borne pathogens (Hasle, Reference Hasle2013). Therefore, migratory birds may play an important role in spreading zoonotic diseases across naïve host populations, increasing both parasite and vector geographic ranges (Hasle, Reference Hasle2013).
During migration, birds usually stopover locally to feed between breeding and wintering areas where they are exposed to different parasitic taxa (Figuerola and Green, Reference Figuerola and Green2000; Ricklefs et al., Reference Ricklefs, Medeiros, Ellis, Svensson-Coelho, Blake, Loiselle, Soares, Fecchio, Outlaw, Marra, Latta, Valkiūnas, Hellgren and Bensch2017). This behaviour might also increase parasite transmission at stopover grounds owing to the dense aggregation of conspecifics during foraging (Altizer et al., Reference Altizer, Bartel and Han2011). Therefore, migratory birds are expected to have higher infection rates and harbour a more diverse parasite fauna than resident species. However, parasite diversity and prevalence are not only influenced by avian host exposure and susceptibility in migratory species, but also by transmission dynamics and parasite life histories. For example, in several analyses, migratory birds had higher richness of endoparasites acquired by host's ingestion, such as helminths and nematodes, than resident birds (Koprivnikar and Leung, Reference Koprivnikar and Leung2015; Leung and Koprivnikar, Reference Leung and Koprivnikar2016; Gutiérrez et al., Reference Gutiérrez, Piersma and Thieltges2019), whereas migratory and resident birds showed similar richness of vector-transmitted blood parasites (Gutiérrez et al., Reference Gutiérrez, Piersma and Thieltges2019) or even higher diversity, but lower infection rates in migratory compared to the resident congeneric host species (Emmenegger et al., Reference Emmenegger, Bauer, Dimitrov, Marin, Zehtindjiev and Hahn2018).
Haemosporidian parasites of the genera Plasmodium, Leucocytozoon, Haemoproteus, and Parahaemoproteus comprise a diverse and cosmopolitan group of microparasites that infect avian blood cells for development and reproduction (Galen et al., Reference Galen, Borner, Martinsen, Schaer, Austin, West and Perkins2018). They are transmitted by mosquitoes (Culicidae), black flies (Simuliidae), hippoboscid flies (Hippoboscidae), and biting midges (Ceratopogonidae), respectively (reviewed by Santiago-Alarcon et al., Reference Santiago-Alarcon, Palinauskas and Schaefer2012a). The few studies assessing feeding preferences of haematophagous diptera have revealed low preference for specific bird species (e.g., Santiago-Alarcon et al., Reference Santiago-Alarcon, Havelka, Schaefer and Segelbacher2012b; Bobeva et al., Reference Bobeva, Zehtindjiev, Ilieva, Dimitrov, Mathis and Bensch2015; Chakarov et al., Reference Chakarov, Kampen, Wiegmann, Werner and Bensch2020). This reinforces that haemosporidian lineage sharing between unrelated avian species is mediated by their vectors, possible due to their generalist feeding preference, being capable of feeding on an enormous diversity of vertebrate host species, thus increasing host switching in any given environment (Chakarov et al., Reference Chakarov, Kampen, Wiegmann, Werner and Bensch2020).
By connecting avian host communities from distinct geographic regions, long-distance migratory birds can also influence local parasite shifting (Waldenström et al., Reference Waldenström, Bensch, Kiboi, Hasselquist and Ottosson2002; Ricklefs et al., Reference Ricklefs, Medeiros, Ellis, Svensson-Coelho, Blake, Loiselle, Soares, Fecchio, Outlaw, Marra, Latta, Valkiūnas, Hellgren and Bensch2017). For example, Nearctic passerine migrants harbour Plasmodium and Parahaemoproteus lineages from both temperate breeding and tropical wintering grounds, indicating host-switching between resident bird species across migratory ranges (Ricklefs et al., Reference Ricklefs, Medeiros, Ellis, Svensson-Coelho, Blake, Loiselle, Soares, Fecchio, Outlaw, Marra, Latta, Valkiūnas, Hellgren and Bensch2017). Because haemosporidians may produce life-long infections, these parasites can be transported by their avian hosts during migration to potentially colonize the local vector community and eventually infect new avian hosts in different regions. However, this local shifting in parasite lineages might depend on avian host compatibility and not only on vector exposure (Medeiros et al., Reference Medeiros, Hamer and Ricklefs2013). Therefore, migratory birds have played an important role in the evolutionary history of avian haemosporidians (Jenkins et al., Reference Jenkins, Thomas, Hellgren and Owens2012; Ricklefs et al., Reference Ricklefs, Medeiros, Ellis, Svensson-Coelho, Blake, Loiselle, Soares, Fecchio, Outlaw, Marra, Latta, Valkiūnas, Hellgren and Bensch2017).
The Chilean Elaenia (Elaenia chilensis) is a long-distance migrant species that breeds in Patagonia and overwinters in tropical South America (Bravo et al., Reference Bravo, Cueto and Gorosito2017). During the non-breeding season, this species shows intratropical displacement between the South American biomes of Caatinga, Atlantic Forest, and Cerrado (Bravo et al., Reference Bravo, Cueto and Gorosito2017). Chilean Elaenias follow a loop migration pattern, using different fall routes and only 1 spring route (Bravo et al., Reference Bravo, Cueto and Gorosito2017). In the Patagonian breeding grounds, the Chilean Elaenia is the most abundant bird species during the breeding season (Ippi et al., Reference Ippi, Anderson, Rozzi and Elphick2009; Cueto and Gorosito, Reference Cueto and Gorosito2018). This Neotropical austral migrant is known to harbour lineages of Plasmodium, Parahaemoproteus, and Leucocytozoon, with variable degrees of specificity, prevalence, and sharing between resident host species in their breeding range across temperate Chile (Merino et al., Reference Merino, Moreno, Vasquez, Martinez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and Mcgehee2008). However, the parasite turnover and transmission dynamics through the annual cycle of this migratory avian host remain unknown.
Spatial heterogeneity in prevalence and diversity of haemosporidian parasites within migratory host populations during their annual cycles may result from variation in both abundance and composition of sympatric avian species within the local host community, which promotes new opportunities for parasite host shifting between resident and migratory host populations. Variation in functional host traits that limit avian malaria infection can change in response to landscape conditions (Fecchio et al., Reference Fecchio, Lima, Bell, Schunck, Corrêa, Beco, Jahn, Fontana, Silva, Repenning, Braga, Garcia, Lugarini, Silva, Andrade, Dispoto, Anjos, Weckstein, Kirchgatter, Ellis, Ricklefs and De La Torre2021a). As spatial variation in avian host occurrences and competent vectors composition can alter host–parasite contact rates (Canard et al., Reference Canard, Mouquet, Mouillot, Stanko, Miklisova and Gravel2014), the arrival of migrant hosts could change local host community composition and vector compatibility leading to local changes in a parasite's host specificity. Changes in host specificity may subsequently influence parasite prevalence, distribution, and diversity.
Here, we investigated the beta diversity, prevalence, and host specificity of haemosporidian parasites hosted by Chilean Elaenia and other avian hosts occurring in sympatry during the annual cycle of this intracontinental austral migrant across South America. Because the similarity of haemosporidian parasites assemblages decreases in host avian communities with increasing geographic distance across South America (Fecchio et al., Reference Fecchio, Bell, Pinheiro, Cueto, Gorosito, Lutz, Gaiotti, Paiva, França, Toledo-Lima, Tolentino, Pinho, Tkach, Fontana, Grande, Santíllan, Caparroz, Roos, Bessa, Nogueira, Moura, Nolasco, Comiche, Kirchgatter, Guimarães, Dispoto, Marini, Weckstein, Batalha-Filho and Collins2019b), we hypothesize that Chilean Elaenia populations that are more distant geographically will harbour a more dissimilar assemblage of haemosporidian parasites during their annual cycle.
Materials and methods
Bird sampling
Our analyses are based on collections of 2,189 blood samples from 244 avian species in Argentina and Brazil, of which records for 1,701 of these were published previously (see Supporting Information Table S1 for the publication source). These samples included 10 bird communities surveyed across 6 South American biomes where Chilean Elaenias spend part of their annual cycle wintering, breeding, and migrating (Fig. 1). All birds were caught using mist nets and, from each individual, approximately 50 μL of blood was collected from the brachial vein and stored either in 95% ethanol or on FTA cards. We prepared 1 or 2 thin blood smears from each of 161 birds captured at 1 stopover site in Serra do Mar State Park (Núcleo Curucutu), Southeastern Brazil, and at 1 breeding site in Esquel, Southern Argentina. Blood smears were air-dried and fixed in absolute methanol after collection in the field. After blood collection, birds were either ringed and released, or euthanized and prepared as museum specimens. Birds were captured in accordance with corresponding permits in Brazil (license issued by Instituto Chico Mendes de Conservação da Biodiversidade – ICMBio numbers: SISBIO 10698, 36538, 59198-5) and Argentina (license issued by Dirección de Fauna y Flora Silvestre del Chubut: DFyFS 86/2016, 35/2017, 27/2018 and Dirección Provincial de Recursos Faunísticos del Neuquén: DET47/2017).
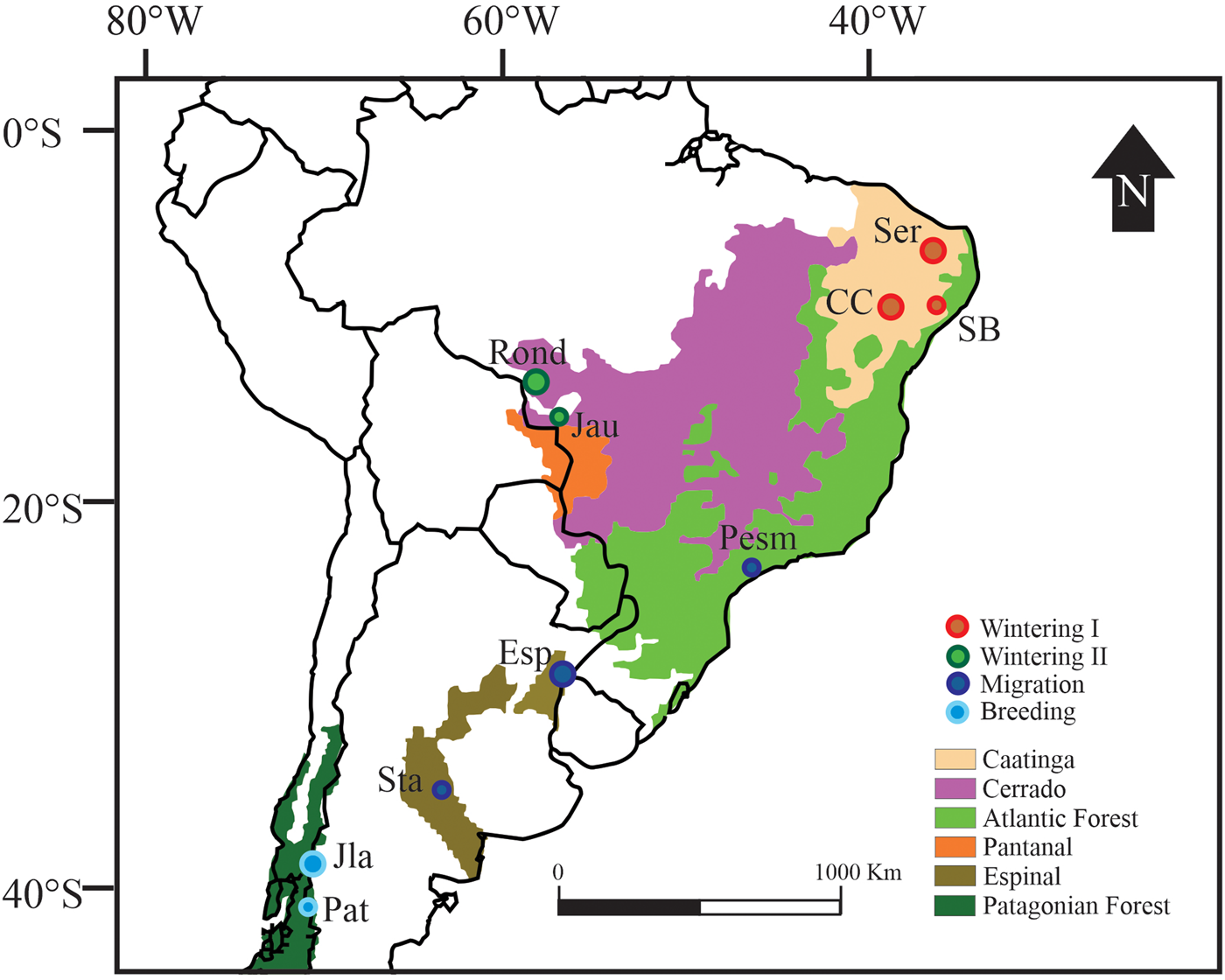
Fig. 1. Sampling areas across the Chilean Elaenia phenological cycle and distributional range. Circle sizes represent parasite prevalence for each avian community according to the phenological cycle. The smallest circle represents a prevalence of 7.0% and the largest circle represents a prevalence of 37.7%. Rectangles depict the biomes where the population was sampled. The names and geographic coordinates of the 10 localities can be found in Supporting Information Table 1.
We selected 4 traits of the sampled host species to assess haemosporidian host functional specificity: diet, foraging strata, body mass, and migratory strategy. We defined the term host functional specificity based on De La Torre et al. (Reference De La Torre, Fecchio, Bell and Campião2022). This index was used previously to assess the lineage specificity for host attributes that affect parasite infection, and consequently can be considered functional traits in host–parasite interaction. The first 3 traits were extracted from Elton Traits 1 (Wilman et al., Reference Wilman, Belmaker, Simpson, de la Rosa, Rivadeneira and Jetz2014), whereas the latter was extracted from Dufour et al. (Reference Dufour, Descamps, Chantepie, Renaud, Guéguen, Shiffers, Thuiller and Lavergne2019). Diet and foraging strata are semiquantitative variables, provided as the proportion for each diet category and the proportion of each stratum that a bird species uses, whereas body mass is a continuous variable. Migratory strategy is a categorical variable with 3 classes: resident, partial migratory, and strictly migratory. Due to the low number of species that are strictly migratory in our dataset, we pooled the 2 classes related to migration and generated a binomial variable with the value of ‘1’ if the species is migratory and ‘0’ if the species is resident. We then calculated the pairwise functional distance between host species using the modified Gower distance (dist.ktab function, provided by ade4 package; Dray and Dufour, Reference Dray and Dufour2007), and generated a hierarchical cluster analysis based on the functional distance dissimilarities. To perform the haemosporidian host phylogenetic specificity, we extracted 1000 phylogenetic trees of the sampled host species from BirdTree Project (Jetz et al., Reference Jetz, Thomas, Joy, Hartmann and Mooers2012). We used the consensus and compute.brlen functions (ape package; Paradis et al., Reference Paradis, Claude and Strimmer2004) to generate a consensus tree with the highest proportion of clade representation, and computed branch lengths (Grafen, Reference Grafen1989; Felsenstein, Reference Felsenstein2004).
Parasite detection and identification
Total DNA was extracted from avian blood using either the Qiagen DNeasy 96 Blood and Tissue kit (Qiagen, Valencia, CA) or Wizard® SV 96 Genomic DNA Purification System (Promega, Madison, WI, USA), following the manufacturers protocols, or using standard phenol–chloroform or ammonium acetate–isopropanol protocols (Sambrook and Russell, Reference Sambrook and Russell2001). For most of the sampled birds (n = 1739) we used the protocols of Hellgren et al. (Reference Hellgren, Waldenström and Bensch2004) to initially screen DNA samples for haemosporidian parasites and then to amplify a 479 bp region of the parasite cytochrome b gene (cyt-b) from infected individuals using nested PCR. More detailed information on laboratory methods, primer names and PCR conditions can be found in Hellgren et al. (Reference Hellgren, Waldenström and Bensch2004). A subset of samples (n = 450) was screened following the protocol of Fallon et al. (Reference Fallon, Ricklefs, Swanson and Bermingham2003) and then the protocol of Hellgren et al. (Reference Hellgren, Waldenström and Bensch2004) as described above was used to amplify the 479 bp region of cyt-b, although this subset of samples was not screened for the genus Leucocytozoon. All PCR amplifications included positive controls with each individual bird being screened only once. Due to the high sensitivity of nested PCR, negative controls were also included in runs to check for possible contamination and false positives, although none were found in any PCR run. PCR products were purified using Exo-SAP-IT (Affymetrix, Santa Clara, CA, USA) and sequenced using BigDye terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA, USA). Sequence identities were verified with a local BLAST against the MalAvi database (Bensch et al., Reference Bensch, Hellgren and Pérez-Tris2009) using BioEdit v.7.2.0 (Hall, Reference Hall1999), and identified lineages were assigned to the taxa Plasmodium, Haemoproteus, Parahaemoproteus, or Leucocytozoon. Parasite lineages were named after the host of origin following a standard protocol (Bensch et al., Reference Bensch, Hellgren and Pérez-Tris2009). All sequences are deposited in MalAvi and GenBank, and accession numbers can be found in the raw dataset (Supporting Information Table S1).
To assure that blood parasites were producing transmissive stages within avian hosts we screened blood smears from 161 birds using traditional microscopy. Blood smears were stained with a 10% Giemsa solution for 1 h and examined for 20–25 min, viewing 100 fields at low magnification (400×) and 100 fields at high magnification (1000×) using an Olympus BX51 light microscope. Parasites were identified morphologically according to Valkiūnas (Reference Valkiūnas2005).
Statistical analysis
We fitted generalized linear mixed models (GLMMs) with binomial error distributions to investigate how the annual cycle, and the diversity and prevalence of blood parasites in the avian community affected the infection probability of haemosporidian parasites in Chilean Elaenias. Models were implemented using the glmer function from the lme4 package (Bates et al., Reference Bates, Mächler, Bolker and Walker2015). We used the ‘bobyqa’ optimizer to increase the number of iterations to achieve convergence of all models. We first investigated whether the fixed effects influenced the probability of infection by blood parasites of any type (i.e., pooled parasites). Then, we investigated the effect of the same explanatory variables independently for each parasite taxon (Leucocytozoon, Parahaemoproteus, and Plasmodium). We used the location where the bird was collected as a random term in all models. We calculated the Shannon–Wiener index per avian community using the function ‘diversity’ from the package vegan (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson, Solymos, Stevens, Szoecs and Wagner2019).
To investigate the association between haemosporidian assemblage composition and the distance between sites we used Mantel tests. We performed these analyses using the whole haemosporidian assemblage, considering lineages only found in Chilean Elaenias. To create the community dissimilarity matrices, we used the Bray–Curtis method employing the function vegdist from the package vegan (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson, Solymos, Stevens, Szoecs and Wagner2019). The geographic distance matrices were created using the function distm from the package geosphere (Hijmans, Reference Hijmans2019). We used the Vincenty (ellipsoid) method to calculate the distance between sites through the function distVincentyEllipsoid. Mantel tests were performed with the function mantel in the vegan package (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson, Solymos, Stevens, Szoecs and Wagner2019).
To investigate the similarity of haemosporidian assemblages among Chilean Elaenias and their phenological cycle, we performed a beta diversity analysis between the parasites found in all sampled Chilean Elaenias and the parasites found in the sampled localities within the Chilean Elaenia's distribution range. The pairwise distances between communities were calculated using the Sorensen index, which we then decomposed into 2 components: turnover and nestedness, based on the beta.pair function (betapart package; Baselga and Orme, Reference Baselga and Orme2012). Turnover represents the parasite lineage replacement among communities regardless of lineage richness, whereas nestedness measures whether a parasite assemblage is a subset of another considering the lineage richness. Sorensen dissimilarity is the total difference in lineage composition, which can be calculated by summing turnover and nestedness (Baselga, Reference Baselga2010). We calculated the standardized effect size (SES) beta diversity of the 3 components, using null models, to identify whether lineage composition is more similar or more different than expected by chance. We used the independent swamp algorithm to randomize lineage composition among parasite assemblages and generated the null models based on 1000 randomizations (Gotelli, Reference Gotelli2000). We then compared the observed values with the null models and considered values below the confidence interval as indicating more similar assemblages than expected by chance, while values above the confidence interval indicated more dissimilar assemblages. As we aim to unravel the association between the haemosporidian compositions of Chilean Elaenias during different stages of their phenological cycle, we selected the pairwise distance values between the Chilean Elaenia lineage composition and the lineage composition from other localities and classified it into 4 categories based on the stage of the phenological cycle for each of these localities: breeding, migration, wintering I, and wintering II. We then, identified the phenological cycle stage in which the Chilean Elaenia's lineage composition was more similar or different than expected by chance. We performed this procedure for each of the 3 haemosporidian genera.
We estimated host specificity for each parasite lineage found in our study sites from 3 perspectives (taxonomic, functional, and phylogenetic). We calculated the taxonomic distinctness (S TD) and host taxon complexity (VarSTD) per lineage (Poulin and Mouillot, Reference Poulin and Mouillot2003). S TD was calculated as S TD = 2ΣΣ wij/s(s − 1), where w = index score between species i and j (e.g., w = 1 for species pairs in the same genus, and w = 4 for species in the same class); and s = the total number of infected species. The variance in S TD represents the level of taxonomic heterogeneity among a group of host species and was calculated as VarS TD = ΣΣ (wij− S TD)2/s(s − 1). The host functional and phylogenetic specificity were calculated using the Net Relatedness Index (NRI) among host species. The NRI is the comparison between the mean pairwise distance (MPD) values and null models, allowing us to identify whether a lineage is more generalist or specialist in relation to host functional distance and host phylogenetic relatedness (Fecchio et al., Reference Fecchio, Wells, Bell, Tkach, Lutz, Weckstein, Clegg and Clark2019a). Therefore, the MDP values among host species of each haemosporidian lineage were calculated from both host phylogenetic and functional distance matrices and compared with a null model and multiplying the result by −1. We generated the null model by randomizing 1000 times the host species name in each distance matrix. As with the SES index, values outside the CI were considered more different than expected by chance, with, values above the CI considered more specialist than expected, whereas values below the CI were more generalist. These indices were not calculated for lineages infecting a single species.
We calculated the unbiased prevalence and its 95% confidence intervals (CI) with Sterne's exact method (Reiczigel et al., Reference Reiczigel, Foldi and Ozsvari2010) using the function epi.prev from the package epiR (Stevenson et al., Reference Stevenson, Nunes, Heuer, Marshall, Sanchez, Thornton, Reiczigel, Robison-Cox, Sebastiani, Solymos, Yoshida, Jones, Pirikahu and Firestone2020). All analyses were conducted using the software R 4.0.2 (R Core Team 2020).
Results
General patterns and factors influencing the probability of parasitism in Chilean Elaenias
A total of 371 Chilean Elaenias were sampled across 10 bird communities over a large latitudinal gradient in South America (≅36 degrees of latitude). Based on molecular screening, haemosporidian infections were observed in 71 of the analysed individuals, corresponding to an overall prevalence of 19.1% (95% CI 15.3–23.6%). The 23 parasite lineages found in Chilean Elaenias belong to Plasmodium (47.8%; n = 11), Leucocytozoon (30.4%; n = 7) and Parahaemoproteus (21.7%; n = 5). As only 9 infections by Haemoproteus were detected out of 2,189 screened birds and mainly constrained to Columbidae, this parasite genus was not considered in the following analysis. Despite being the richest parasite genus in terms of number of lineages, Plasmodium did not exhibit the highest prevalence in Chilean Elaenias. Plasmodium prevalence was 5.7% (95% CI 3.6–8.6%). Most Chilean Elaenias were parasitized by Leucocytozoon with a prevalence of 8.4% (95% CI 5.9–11.7%). Parahaemoproteus had the lowest prevalence, 5.4% (95% CI 3.5–8.2%).
Microscopic examination of 161 blood smears confirmed the presence of trophozoites, meronts, and gametocytes for Plasmodium infection in 5 bird hosts plus 1 mixed infection with Plasmodium and Parahaemoproteus in Serra do Mar, Brazil. The presence of Parahaemoproteus gametocytes was confirmed in 8 infected individuals from the Argentinian Patagonia (breeding site) and Serra do Mar (stopover site). Leucocytozoon infection was not seen in any blood slides from Brazil (n = 139) or Argentina (n = 22). Parasite morphospecies and infective stages for Serra do Mar can be found in Anjos et al. (Reference Anjos, Chagas, Fecchio, Schunck, Costa-Nascimento, Monteiro, Mathias, Bell, Guimarães, Comiche, Valkiūnas and Kirchgatter2021).
Parasite diversity and prevalence in the avian host community, as well as the phases of the Chilean Elaenia annual cycle were not important predictors of overall infection in this host species (Table 1). The predictors also did not affect the probability of Plasmodium and Leucocytozoon infections. However, parasite diversity in the host community was an important predictor of Parahaemoproteus infection in Chilean Elaenias (Z = 2.64; P = 0.008, Table 1). Specifically, the probability of Parahaemoproteus infection decreased with increasing diversity of the parasite assemblage (Fig. 2).

Fig. 2. Effect of haemosporidian parasite lineage diversity on the Parahaemoproteus infection probability in Chilean Elaenias. The grey shading represents the 95% confidence interval.
Table 1. Results of generalized linear mixed models with a binomial error structure to investigate the effects of parasite diversity (Shannon–Wiener diversity index), prevalence, and annual cycle phases (breeding, migrating and wintering) on the probability of infection by blood parasites in Chilean Elaenias
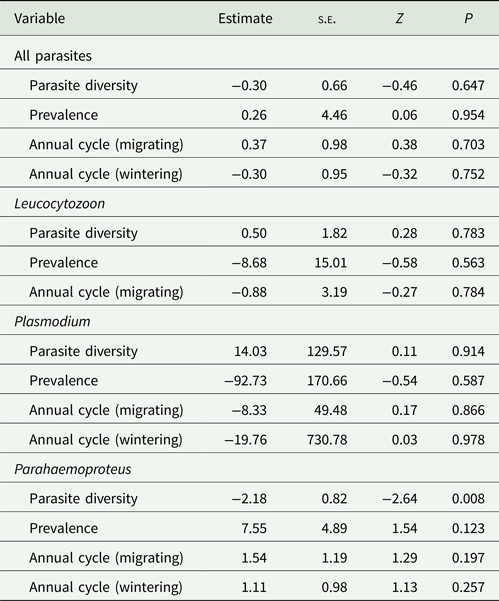
The location ID was used as a random term in the analyses.
Geographic distance and parasite assemblages
We investigated possible geographic structure of parasite assemblages found in Chilean Elaenias and in other hosts across a gradient of ≅ 5200 km. Considering all hosts (except Chilean Elaenias), the analysis revealed that sampling sites which are geographically closer to each other also harbour more similar parasite assemblages (Mantel test, r = 0.44, P = 0.005, 10,000 permutations). These results demonstrate that parasite assemblages of the sympatric host species were significantly structured across the Chilean Elaenia distributional range, but that much similarity still exists between them (Fig. 3). When the analysis was constrained to the parasite assemblages found in Chilean Elaenias only, there was no geographic structure. That is, we found no association between geographic distance and compositional dissimilarity for the parasite assemblages found in Chilean Elaenias (Mantel test, r = 0.11, P = 0.317, 10,000 permutations).

Fig. 3. Association between geographic distance and parasite assemblage dissimilarity for the host sympatric species recorded across the Chilean Elaenia distributional range. The grey shading represents the 95% confidence interval.
Beta diversity of haemosporidians from Chilean Elaenias, and the phenological cycle
Based on the comparison between haemosporidian pairwise beta diversity and its respective null models, we found that Parahaemoproteus lineage composition in Chilean Elaenia was more similar than expected by chance to the lineage composition of the sympatric avian species in 2 localities from wintering II and 1 locality from wintering I (Fig. 4). This similarity is due to low Parahaemoproteus lineage turnover between Chilean Elaenia Parahaemoproteus assemblages and the respective localities, in which lineage replacement was also lower than expected by chance (Fig. 4). Conversely, the nestedness between the Parahaemoproteus lineage composition of Chilean Elaenias and these localities was higher than expected by chance. This means that Parahaemoproteus lineages found in the Chilean Elaenias are a subset from the Parahaemoproteus assemblages located in wintering I, whereas the Parahaemoproteus assemblages from wintering II are subsets of the Parahaemoproteus lineages found in the Chilean Elaenias. Neither Plasmodium nor Leucocytozoon presented significant values of lineage beta diversity (Fig. 4).

Fig. 4. Beta diversity analyses between parasites of Chilean Elaenias from the localities within their phenological cycle. Circles below the confidence interval (dotted lines) indicate more similar composition than expected by chance, whereas circles above the confidence interval indicate more dissimilar assemblages.
Host specificity
In general, parasites showed intermediate taxonomic distinctness, but low host–taxon complexity, indicating that parasite lineages show a consistent host specificity at lower taxonomic levels. Host specificity was similar for Plasmodium lineages S TD (2.99 ± 0.39; mean ± s.d.), Parahaemoproteus lineages (2.66 ± 0.44; mean ± s.d.), and Leucocytozoon lineages (2.46 ± 0.49; mean ± s.d.). Furthermore, there was no difference in taxonomic distinctness and host taxa complexity between the lineages found in Chilean Elaenias and the lineages found in the other sympatric host species (Table 2; P > 0.05). On the other hand, considering lineages only found in Chilean Elaenias, we observed that Plasmodium lineages infected a greater taxonomic range of avian hosts than Parahaemoproteus lineages (P = 0.017; Fig. 5).

Fig. 5. Lineage host specificity of haemosporidian genera found in Chilean Elaenias and other sympatric bird species. Note that lineages above the confidence interval (dotted line) are considered host specialists.
Table 2. Taxonomic distinctness (S TD) and host taxa complexity (VarS TD) for the lineages found in Chilean Elaenias and sympatric host species recorded in the study sites

When analysing lineage specificity considering the functional role of avian host species and their respective evolutionary history, we found that the Chilean Elaenias haemosporidian pool is not composed of specialist nor generalist lineages, with the exception of Parahaemoproteus lineages, in which 2 were phylogenetic specialists and 1 a functional specialist (Fig. 5). In contrast, the 2 Leucocytozoon lineages infecting hosts other than Chilean Elanias are considered functional specialists and 1 lineage is a phylogenetic specialist. We also found 3 phylogenetic specialists and 1 functional specialist Plasmodium lineages infecting sympatric host species (Fig. 5).
Discussion
Our survey of haemosporidians in Chilean Elaenias across South America showed that the prevalence of Plasmodium and Leucocytozoon are similar through the annual cycle of this Neotropical austral migrant, but that the prevalence of Parahaemoproteus is lower in locations with higher diversity of haemosporidian parasite lineages overall. Furthermore, geographic distance explained the dissimilarity of haemosporidian lineages infecting the bird communities during the annual cycle of Chilean Elaenia, even though this migratory host species was infected by a similar composition of lineages across its distributional range. Finally, our study confirms that haemosporidian lineages demonstrate consistent host specificity at a lower avian taxonomic levels, but Plasmodium lineages tend to be phylogenetically specialized in sympatric host species rather than in Chilean Elaenias.
Prevalence of haemosporidians through the annual cycle of Chilean Elaenia
Here we show that Parahaemoproteus was less prevalent in Chilean Elaenia populations occurring in bird communities harbouring a more diverse parasite fauna. This suggests that competition between haemosporidian genera, not deep host evolutionary history, may be a relevant factor explaining parasite persistence in a host population. A recent global synthesis of avian haemosporidian prevalence showed that long-distance migrants are more often infected by Leucocytozoon, but this avian host trait can also influence infection probability in opposing directions across zoogeographical realms for Plasmodium and Parahaemoproteus (Fecchio et al., Reference Fecchio, Clark, Bell, Skeen, Lutz, De La Torre, Vaughan, Tkach, Schunck, Ferreira, Braga, Lugarini, Wamiti, Dispoto, Galen, Kirchgatter, Sagario, Cueto, González-Acuña, Inumaru, Sato, Schumm, Quillfeldt, Pellegrino, Dharmarajan, Gupta, Robin, Ciloglu, Yildirim, Huang, Chapa-Vargas, Álvarez-Medizábal, Santiago-Alarcon, Drovetski, Hellgren, Voelker, Ricklefs, Hackett, Collins, Weckstein and Wells2021b). Avian host susceptibility may be conserved evolutionarily (a trait that can make the host species more competent in coping with infection, see Barrow et al., Reference Barrow, McNew, Mitchell, Galen, Lutz, Skeen, Valqui, Weckstein and Witt2019). We argue that the relationship between a particular avian host species and its haemosporidian parasites changes geographically due to intrinsic properties of a parasite lineage (e.g., competition and colonization) or vector dissimilarity, therefore altering the prevalence of a single avian host species across its distributional range. For example, avian host populations inhabiting fragmented habitats were more often infected by haemosporidian parasites (Pérez-Rodríguez et al., Reference Pérez-Rodríguez, Khimoun, Ollivier, Eraud, Faivre and Garnier2018), presumably due to changes in vector composition in response to recent human-induced landscape changes. The role of geography in structuring avian haemosporidian prevalence across evolutionary independent host populations has also been shown in the Lesser Antilles (Ricklefs et al., Reference Ricklefs, Gray, Latta and Svensson-Coelho2011).
Haemosporidian transmission between wintering and breeding grounds
Biogeographic structure in parasite distribution is expected when host switching is relatively common in host–parasite systems (Weckstein, Reference Weckstein2004), and a recent study has shown that host switching is the main macroevolutionary pattern in avian haemosporidian parasites (Alcala et al., Reference Alcala, Jenkins, Christe and Vuilleumier2017). Across South American biomes, haemosporidian lineage sharing decreases as a function of increased geographic distance between bird communities inhabited by Chilean Elaenias, but the lineage composition within Chilean Elaenia remains similar throughout their annual cycle irrespective of geographic location. This suggests that the Chilean Elaenia plays a negligible role in local parasite transmission along its distributional range in South America. However, haemosporidian lineage sharing is higher in the elaenia's tropical wintering grounds (e.g., Cerrado and Caatinga biomes), and Chilean Elaenias tend to harbour a higher, albeit nested, lineage diversity of Parahaemoproteus during wintering in an arid zone of Northeastern Brazil, the Caatinga biome. Furthermore, localities occupied during the second stage of the wintering cycle of Chilean Elaenia contained nested Parahaemoproteus lineage composition when compared with the Chilean Elaenia parasite assemblage, demonstrating that the movement of this migratory host within its tropical wintering grounds in Brazil may disperse Parahaemoproteus. This implies that Chilean Elaenias contribute with the spread of some lineages across their tropical flyway before the long migration to Patagonian breeding grounds. Possibly, Parahaemoproteus lineage sharing and cross-species transmission between the tropical wintering grounds and the temperate breeding grounds are constrained locally due to the lack of suitable biting midge vectors that enable parasite shifting within sympatric host species or by avian host dissimilarity along this large latitudinal gradient in South America.
Lack of transmission at stopover ground
Due to the phenological cycle of the Chilean Elaenia, this austral migrant inhabits locations with active vectors that can potentially transmit 3 haemosporidian parasite genera year-round. For example, individuals could be infected by Leucocytozoon on their breeding grounds in Patagonia and by Parahaemoproteus and Plasmodium on the wintering grounds in Northeast Brazil. However, the behaviour of this host species during migration across the Atlantic Forest right after the breeding season may prevent parasite transmission at the subtropical stopover sites. Migrating Chilean Elaenias stopover for only a short time during day-light hours for feeding and resting, which might not provide sufficient time to be exposed to local vectors (e.g., crepuscular or nocturnal vector species). This behaviour, based on mist net capture rates in Serra do Mar might prevent individual birds from acquiring local haemosporidian lineages during fall migration (see Cueto et al., Reference Cueto, Sagario and Lopez de Casenave2016 for the same refuelling behaviour during spring migration in Argentina). Moreover, the absence of transmissive stages visualized in blood smears during the stopover in Serra do Mar suggest that Chilean Elaenias migrate carrying a rather low abundance of Leucocytozoon parasites. This low parasitaemia associated with avian host behaviour can impede the transmission of Leucocytozoon at stopover sites. If these individuals infected by Leucocytozoon reach the tropical wintering grounds, the transmission could be also constrained due to the lack of competent blackfly vectors.
Limitations of our results should be acknowledged. First, we did not account for variation in parasite detection across multiple molecular screening of the same individual bird (Ramey et al., Reference Ramey, Schmutz, Reed, Fujita, Scotton, Casler, Fleskes, Konishi, Uchida and Yabsley2015; Rodriguez et al., Reference Rodriguez, Doherty, Piaggio and Huyvaert2021). Second, we did not include in our analyses environment variables to explain turnover between Chilean Elaenias and sympatric host species. Nonetheless, it has been shown that climatic similarity is not important in driving parasite turnover in South America when controlling for the effect of geographic distance and host similarity (Fecchio et al., Reference Fecchio, Bell, Pinheiro, Cueto, Gorosito, Lutz, Gaiotti, Paiva, França, Toledo-Lima, Tolentino, Pinho, Tkach, Fontana, Grande, Santíllan, Caparroz, Roos, Bessa, Nogueira, Moura, Nolasco, Comiche, Kirchgatter, Guimarães, Dispoto, Marini, Weckstein, Batalha-Filho and Collins2019b). Third, we only examined a subset of the blood samples using microscopic examination. This diagnostic method would be imperative to determine if the migratory bird host was harbouring parasite stages capable of completing their cycle in the vector host with potential to be transmitted to other avian host species locally.
Conclusion
Migrant birds connect both avian host communities and parasite assemblages over vast geographic distance and, thus, potentially can spread pathogenic and parasitic organisms during their annual cycle. Indeed, previous studies have shown that migratory birds can disperse haemosporidian parasites between wintering tropical grounds and temperate breeding grounds and influence local transmission in Neotropical and Nearctic regions (Ramey et al., Reference Ramey, Schmutz, Reed, Fujita, Scotton, Casler, Fleskes, Konishi, Uchida and Yabsley2015; Smith and Ramey, Reference Smith and Ramey2015; Ricklefs et al., Reference Ricklefs, Medeiros, Ellis, Svensson-Coelho, Blake, Loiselle, Soares, Fecchio, Outlaw, Marra, Latta, Valkiūnas, Hellgren and Bensch2017; de Angeli Dutra et al., Reference de Angeli Dutra, Fecchio, Braga and Poulin2021a; Reference de Angeli Dutra, Filion, Fecchio, Braga and Poulin2021b). Our study, focusing on an abundant Neotropical austral migrant, points to a different conclusion: because of the lack of generalist lineages infecting Chilean Elaenias and high degree of phylogenetic and functional host specificity of abundant parasite lineages infecting sympatric host species, combined with increased parasite dissimilarity in response to the geographic distance, the contribution of migrant Chilean Elaenias to parasite sharing across their entire distributional range can be negligible. Importantly, Parahaemoproteus lineages dispersed by Chilean Elaenias are shared within wintering tropical grounds, but parasite cross species transmission between tropical and temperate avian communities is constrained, possible due to great dissimilarity in biting midge communities along large geographic and climatic gradients across South America. Finally, based on the lack of transmissive stages of Leucocotyzoon in infected Chilean Elaenias returning from the Patagonian breeding grounds, this long-distance host migrant species does not influence local Leucocytozoon transmission in 1 subtropical stopover site, the Serra do Mar in the Brazilian Atlantic Forest. This negligible role of a long-distance migratory host species in influencing local parasite transmission across its distributional range has been shown previously in a boreal passerine migrant (Pulgarín-R et al., Reference Pulgarín-R, Gómez, Bayly, Bensch, FitzGerald, Starkloff, Kirchman, González-Prieto, Hobson, Ungvari-Martin, Skeen, Castaño and Cadena2019). This study demonstrated that haemosporidian lineage sharing across the range of grey-cheeked thrush (Catharus minimus) may actually represent an abortive infection, thus preventing parasite shifting among sympatric avian host species. Additional screening of Leucocytozoon across Chilean Elanias' migratory flyways in Brazil would be imperative to confirm whether lineages of Leucocytozoon from Patagonian breeding grounds are dispersed by this Neotropical austral migrant and shared among tropical sympatric avian host species.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182022001317.
Data availability
The bird infection data used in this study are available in the raw dataset. The parasite sequences, lineage names and GenBank access numbers are provided in Supporting Information Table S1.
Acknowledgements
We thank all ornithologists and field assistants who helped collect the blood samples used in this study, as well as all collaborators who contributed previously published data. We acknowledge the Curucutu Nucleus team for support in the field. We also thank Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio), Fundacão Florestal de São Paulo (COTEC), CEMAVE/ICMBio and Dirección de Fauna y Flora Silvestre del Chubut that provided permits necessary for collection of tissue samples in Brazil and Argentina.
Author's contributions
AF, FS, GMDLT, JAB, KK, RID and VRC designed the study; AF, MCS, CAG, CL, VQP, JBP, FS and VRC collected data; AF, CCA and CL performed laboratory analyses; JAB and AF organized the data; AF, RID, GMDLT analysed the data and wrote the manuscript; all authors revised the manuscript and contributed with writing.
Financial support
AF was supported by a PNPD scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES (Process number 88887.342366/2019-00). KK is a CNPq research fellow (process number 309396/2021-2).
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
All applicable guidelines from Brazilian and Argentinian institutions for the collecting, care and use of animals were followed.