Introduction
Order Tarphycerida (Early Ordovician–latest Silurian) includes nautiloids with a tightly coiled planispiral shell, a wide siphuncle that changed its position during ontogeny, layered and thick connecting rings, and an aperture possessing a deep, narrow hyponomic sinus (Furnish and Glenister, Reference Furnish and Glenister1964; Dzik, Reference Dzik1984). Tarphycerids inhabited shallow tropical and subtropical epeiric seas (Flower, Reference Flower1957; Westermann, Reference Westermann1998), but occasionally spread pole-ward, into open sea or deeper water settings (Babin and Gutierréz-Marco, Reference Babin and Gutiérrez-Marco1992; Manda, Reference Manda2008a; Kröger et al., Reference Kröger, Servais and Zhang2009). The planispiral shell and deep hyponomic sinus indicate that tarphycerids were capable of active swimming and were, like Nautilus, most probably demersal animals (Dzik, Reference Dzik1984; Westermann, Reference Westermann1998). In some species, the last whorl becomes uncoiled, which indicates a changed mode of life from nektonic towards a demersal habit, with limited swimming ability (Flower, Reference Flower1955). The first tarphycerids appeared in the early Tremadocian, and their diversity suddenly increased, reaching a maximum in early Floian time. Their generic diversity fluctuated only slightly, until a slow diversity decline in the middle Katian and Hirnantian (Kröger and Zhang, Reference Kröger and Zhang2009). The tarphycerida included the vast majority of Ordovician cephalopods possessing coiled shells.
The diversity of tarphycerids after the Late Ordovician extinction was very low throughout the Silurian. Although some species were locally abundant, only three genera/families are known. Ophioceras Barrande, Reference Barrande1865 is the single known representative of the family Ophioceratidae Hyatt, Reference Hyatt1894, and it is the only tarphycerid genus to have originated in the Silurian. It comprises only two long-ranging species, occurring in middle and upper Silurian rocks of the US, England, Bohemia, Poland, Sweden, Estonia (Stridsberg and Turek, Reference Stridsberg and Turek1997), and China (Chen et al., Reference Chen, Liu and Chen1981). Trocholites Conrad, Reference Conrad1838 (Trocholitidae Chapman, Reference Chapman1857) includes more than 20 Ordovician species, but is represented in the early Silurian by only a single species known from the Anglo-Welsh Basin (Holland, Reference Holland2010). Discoceras Barrande, Reference Barrande1867 (= Graftonoceras Foerste, Reference Foerste1925 and Baterboceras Zou, Reference Zou1983; Discoceratidae Dzik, Reference Dzik1984) is a genus with more than 30 Middle and Upper Ordovician species. Including the four species described here and evaluating previously published data, Discoceras includes six Silurian species occurring in Llandovery strata in Bohemia, and Wenlock strata in North America (Foerste, Reference Foerste1925), Australia (Etheridge, Reference Etheridge1904), Inner Mongolia (Zou, Reference Zou1983), Sweden, and Bohemia.
We describe here three new Silurian species of Discoceras from Gotland (Sweden) and Central Bohemia (Czech Republic). Silurian Discoceras retained the tarphycerid shell morphology and habitat of its Ordovician ancestors, but one new species—D. lindstroemi n. sp.—exhibits an unusual coiled shell, resembling that of some heteromorph ammonoids.
Well-preserved specimens of Silurian Discoceras from Gotland have made possible the study of the early growth stages of the shell as well as the reconstruction of the early ontogeny of the genus. This study supports the conclusion that early-hatched tarphycerids possessed minute curved shells, and were planktonic, rather than having the coiled shell and life mode of adults. A planktonic habit for Ordovician tarphycerid juveniles was suggested by Holland (Reference Holland1985). This has been documented by Turek and Manda (Reference Turek and Manda2016) in Silurian Ophioceras, an evolutionarily younger tarphycerid, displaying several evolutionary novelties unknown in other tarphycerids, even those from the Silurian. Thus, to expand the hypothesis of a planktonic habit of juveniles to other tarphycerids, more evidence from evolutionarily basal tarphycerids was needed.
Geological setting and taphonomy
Studied material comes from Silurian rocks of Central Bohemia (Bohemian Massif, Czech Republic) and Gotland (Baltic Shield, Sweden).
The Central Bohemian Paleozoic is a part of the Barrandian area consisting of Proterozoic and Paleozoic rocks (Teplá-Barrandian Unit, Bohemian Massive), which represents the peri-Gondwanan terrain, Perunica (see Torsvik and Cocks, Reference Torsvik and Cocks2013). Silurian rocks are preserved in the Prague Synform, a structure formed during Variscian Orogeny. Silurian rocks form a part of the marine sedimentary succession of the so-called Prague Basin (Ordovician–Middle Devonian). Five lithostratigraphic units were established in the Silurian strata (for summary see Kříž, Reference Kříž1998), of which two yielded Discoceras. The first unit, the Želkovice Formation, is of Rhuddanian and Aeronian age, consists of black laminated graptolitic shales, and has a maximum thickness of ~25 m (Kříž, Reference Kříž1998; Štorch, Reference Štorch2006). The shale was deposited under anoxic conditions in an offshore setting; there is no evidence of near-shore conditions (Štorch, Reference Štorch2006). Benthic fauna includes very rare rhynchonelliform and common linguliform brachiopods that were described from the lowermost and uppermost parts of the formation, respectively (Kříž, Reference Kříž1998). Discoceras occurs in black shales that lack benthic fauna. Graptolites indicate the Demirastrites triangulatus, D. simulans, and D. convolutes biozones (as defined by Štorch, Reference Štorch2006). These graptolitic shales contain rare Discinocaris Woodward, Reference Woodward1866 and Peltocaris Salter, Reference Salter1863 (considered to be cephalopod opercula) (Turek, Reference Turek1978). Despite over 150 years of intensive collecting throughout the formation, only two flattened shells and one fragment of Discoceras are available for study.
The second unit, the Motol Formation, contains an up to 300 m thick sedimentary succession ranging in age from upper Llandovery to uppermost Wenlock (Kříž, Reference Kříž1998). The formation consists of alternating volcano-sedimentary complex sets and shale facies. The single available flattened specimen of Discoceras comes from shale in the lower part of the formation, which has been referred to the Cyrtograptus murchisoni Biozone as defined by Štorch (Reference Štorch1994). Oxygen deficient, offshore graptolite-rich shales dominate the lower part of the formation; the volcanic activity is limited to occasional basalt effusions (Kříž, Reference Kříž1998). Shales and platy muddy limestones with a benthic Niorhinx Community (Havlíček and Štorch, Reference Havlíček and Štorch1990) developed locally in areas of volcanic activity and indicate the presence of low and isolated bottom elevations swept by bottom currents (Kříž, Reference Kříž1998). The community includes rhynchonelliform brachiopods, trilobites, crinoids, gastropods (Havlíček and Štorch, Reference Havlíček and Štorch1990), and cephalopods (straight shelled pelagic forms, the demersal orthocerid Dawsonoceras annulatum [Sowerby, Reference Sowerby1818], the discosorid Phragmoceras munthei Hedström, Reference Hedström1917 [Manda, Reference Manda2008b], and the herein described tarphycerid Discoceras).
The Silurian bedrock of Gotland is a remnant of an extensive low-latitude carbonate platform complex that evolved along the margins of the Baltic Basin and extended from the western parts of the present-day Baltic Sea across the East Baltic and farther southeast to Ukraine (for summary see Calner et al., Reference Calner, Jeppsson and Munnecke2002). The majority of the Discoceras specimens were collected by G. Lindström in the nineteenth century; some of his associated labels indicate his unrealized goal of describing four new species. Precise localities are not specified; rather, the locality is given as the name of the nearest village in the same land registry as the actual locality. Preservational states of the studied specimens, however, facilitate identification of their original horizons and locations (Laufeld, Reference Laufeld1974; Stridsberg, Reference Stridsberg1985). All studied specimens were collected from several sites in the lower part of the Slite beds (units d and g), which correspond with the upper Sheinwoodian to lower Homerian, Kockelella walliseri to Ozarkodina sagitta sagitta conodont biozones and the Monograptus belophorus to Cyrtograptus lundgreni graptolite biozones, respectively (Jeppsson et al., Reference Jeppsson, Viira and Männik1994; Jeppsson and Calner, Reference Jeppsson and Calner2003). Studied specimens were collected from light-colored stromatoporoid boundstone, crinoidal grainstone, and dolomite—facies that form biohermal, biostromal, and shoal areas of a proximal platform characterized by stromatoporoid-coral reef complexes (for summary see Calner et al., Reference Calner, Jeppsson and Munnecke2002; Calner and Jeppsson, Reference Calner and Jeppsson2003). Most of the studied specimens of Discoceras were removed from their rock matrix, and consequently taphonomic information was lost. A few specimens of D. lindstroemi n. sp. were preserved in cephalopod coquina deposited in a reef cavern (Manten, Reference Manten1971). The coquinas consist of small straight cephalopod shells and rounded fragments of stromatoporoids (Fig. 7.1, 7.4), or a diverse assemblage of cephalopods associated with crinoid, stromatoporoid, and coral fragments (Fig. 7.3, 7.5). It is uncertain whether the coquinas are the result of post-mortem accumulations or represent a natural faunal assemblage. The caverns may have served as hatching grounds (evinced by the accumulation of small shells), a refuge, or hunting grounds for large predators. Based on observations of the collections of the Naturhistoriska Riksmuseet in Stockholm, Discoceras was associated with a diverse cephalopod fauna of orthocerids, discosorids, oncocerids, nautilids, and actinocerids, of which only a small part has been described (for summary see Stridsberg, Reference Stridsberg1985, Reference Stridsberg1988a; Stridsberg and Turek Reference Stridsberg and Turek1997). These mostly demersal cephalopods were part of a highly diversified reef and peri-reef fauna including corals, stromatoporoids, bryozoans, crinoids, brachiopods, gastropods, trilobites, and other fauna (for overview see Manten, Reference Manten1971; Laufeld, Reference Laufeld1974; Calner et al., Reference Calner, Jeppsson and Munnecke2002). Several shells of D. lindstroemi n. sp. are infested with microconchid tubes of Annuliconchus sp. (Fig. 6), which are attached to the shell between undulated frills. Apertures of tubes exhibit various orientations, but are usually directed up or down relative to the life orientation of Discoceras. Microconchids attached to both organic and inorganic substrates (Taylor and Vinn, Reference Taylor and Vinn2006). Their occurrence on both sides of the Discoceras shell suggests that in this case microconchids colonized a living animal.
Materials and methods
Morphological characters and measured morphological features are explained in Figure 1. To detail rates of shell expansion, a whorl expansion rate (WER) and revolving index (RI) are used. Whorl expansion rate (WER) is a coiling parameter that is used as defined for gyroconic conchs by Korn and Klug (Reference Korn and Klug2003) as the square of the ratio between maximal shell diameter and maximal shell diameter minus aperture height. WER as defined in regularly coiled ammonoids is not used because the smaller shell diameter is not measurable because the impressed zone in Discoceras is very shallow, irregular, and usually not visible in the material studied. Nevertheless, the values of WER used here are very close to WER used in regularly coiled ammonoids. Whorl width index (WWI) is used as defined by Korn and Klug (Reference Korn and Klug2003) as the ratio of whorl width and whorl height. RI defined by Stridsberg and Turek (Reference Stridsberg and Turek1997) is the proportion of whorl height at any point to whorl height of the previous whorl exactly one turn earlier; revolving index is used because it is also applicable to fragmentary shells and has been used in Silurian tarphycerids (Turek and Manda, Reference Turek and Manda2016).

Figure 1 Schematic drawing explaining shell morphology of Discoceras. bcl: body chamber length; hs: hyponomic sinus; os: ocular sinus; phl: phragmocone chamber length; w/l: width and length of pseudoumbilicus (ps).
Repositories and institutional abbreviations
Tarphycerids from Gotland are housed in the Naturhistoriska Riksmuseet, Stockholm, Sweden (prefix RM Mo). Silurian Discoceras from Bohemia are deposited in the National Museum, Prague, Czech Republic (prefix NM L).
Systematic paleontology
Abbreviations: chamber length (CL), hyponomic sinus width (HW), hyponomic sinus height (HH), internal whorl height (IWH), maximal shell diameter (SD), whorl height (WH), whorl width (WW); minimum (min.), maximum (max.), median (med.), number of measured specimens (N), standard deviation (σ). Three types of sculpture elements are differentiated: growth lines, ridges, and ribs. Ridges are taller than growth lines; contrary to ribs, ridges are never visible on internal molds, while ribs usually form raised zones on the mold. Lateral furrow and pseudoumbilicus, both newly defined terms, are defined below.
Subclass Nautiloidea Agassiz, Reference Agassiz1847
Order Tarphycerida Flower, Reference Flower1950
Family Discoceratidae Dzik, Reference Dzik1984
Genus Discoceras Barrande, Reference Barrande1867
Type species
Clymenia antiquissima Eichwald, Reference Eichwald1842 from the “Lyckholm stage” (Late Ordovician) of Estonia, by original designation.
Diagnosis
Tarphycerid with slightly to moderately depressed evolute shell with variously spaced frills; caecum subcentral, shifted toward ventral side, the siphuncle shifts in second and third chambers toward dorsal side, its final dorsal or subdorsal position is attained in third septum.
Remarks
Previously described Silurian species of Discoceras were assigned to Graftonoceras Foerste, Reference Foerste1925, which differs from the former genus in having a marginodorsal siphuncle in the third and subsequent chambers. In Discoceras, the siphuncle becomes subdorsal when the shell reaches 1.5–2 whorls. Dzik (Reference Dzik1984, p. 46) noted that the relatively slight shift of the siphuncle during late ontogeny is a species-specific feature, and synonymized both genera. Stridsberg and Turek (Reference Stridsberg and Turek1997) reported differences in the location of the siphuncle in the postembryonic growth stage in two different specimens of a single tarphycerid species—Ophioceras rudens Barrande, Reference Barrande1865. Bateroboceras Zou, Reference Zou1983, based on B. oblinquwhorlum Zou, Reference Zou1983, from the middle Silurian of Inner Mongolia, exhibits typical features of Discoceras; therefore, it too is synonymized with this genus.
Although the shell morphology of tarphycerids is well known, a new morphological feature—lateral furrow—has been observed in two species assigned to Discoceras. The furrow appears when the shell attains 0.75–1 whorl. It starts in the ventrolateral or mid-lateral position (Figs. 4.9, 5.1); in the latter case, the furrow shifted abruptly ventrolaterally. The furrow is developed symmetrically on both sides of the shell as a very shallow depression, or sometimes as a light-colored band on the shell (Fig. 5.2). Function of the furrow remains unknown. Pseudoumbilicus is herein introduced for the sickle-shaped cavity between whorls originating due to a brief interval of detached coiling of a shell whorl from preceding whorl (Fig. 1). This cavity corresponds to the secondary umbilical window in early ammonoids (see De Baets et al., Reference De Baets, Landman and Tanabe2015).
Discoceras graftonense (Meek and Worthen, Reference Meek and Worthen1870)
Figures 2.1–2.7, 13.1, 13.2, 13.4, Table 1

Figure 2 Discoceras graftonense (Meek and Worthen, Reference Meek and Worthen1870); (1) NM L 42239, lateral view, Malá Chuchle-Vyskočilka, Wenlock, early Sheinwoodian, Cyrtograptus murchisoni Biozone, NN indicates brachiopod Nyorhinx nyobe (Barrande, Reference Barrande1847) attached on shell surface; (2, 3) RM Mo 59701, ventral and lateral view, Färö, Sandvik, Slite beds, late Sheinwoodian, hi: healed injury; (4) RM Mo 59706, lateral view, Färö, Sandvik, Slite beds, late Sheinwoodian; (5) NM L 42237, lateral view, Koněprusy, Llandovery, Aeronian, Demirastrites simulans Biozone; (6, 7) RM Mo 59696, lateral and ventral view, Lärbro, Slite beds, late Sheinwoodian. Specimens in Fig. 2.2, 2.3, 2.6, and 2.7 were coated by ammonium chloride before photographing. Arrow indicates base of body chamber. Scale bars 10 mm.
Table 1 Dimensions (in millimeters) in D. graftonense (Meek and Worthen). N: specimen RM Mo number; SD: maximal shell diameter; WH: whorl height; WW: whorl width; WWI: whorl width index.

1870 Lituites Graftonensis Reference Meek and WorthenMeek and Worthen, p. 51.
1875 Lituites Graftonensis; Reference Meek and WorthenMeek and Worthen, p. 507, pl. 25, fig. 1
1888 Lituites Graftonensis; Reference NewellNewell, p. 485.
1894 Discoceras graftonense; Reference HyattHyatt, p. 501.
1904 Cyclolituites bowningensis Reference EtheridgeEtheridge, p. 77, pl. 8, figs. 1–6.
1925 Graftonoceras graftonense; Reference FoersteFoerste, p. 59, pl. 12, figs. 2, 3.
1944 Graftonoceras graftonense; Reference Shimer and ShrockShimer and Shrock, p. 543, pl. 222, figs. 4, 5.
1964 Graftonoceras graftonense; Reference Furnish and GlenisterFurnish and Glenister, p. K360, fig. 258/4.
1983 Graftonoceras cf. graftonense; Reference ZouZou, p. 166, pl. 1, figs. 5–7.
1983 Bateroboceras oblinquwhorlum Reference ZouZou, p. 172, pl. 1, figs. 1, 2, 8–15.
1984 Discoceras graftonense; Reference DzikDzik, p. 42, fig. 12.
Holotype
By monotypy, a specimen from the middle Wenlock Port Byron Dolomites of Illinois (Meek and Worthen, Reference Meek and Worthen1870, p. 51; figured by Meek and Worthen, Reference Meek and Worthen1875, pl. 25, fig. 1).
Diagnosis
Discoceras with marginodorsal siphuncle starting with the third phragmocone chamber; growth ridges separating a set of growth lines appeared at about one-fifth to one whorl.
Occurrence
Sweden, Gotland, Silurian, Wenlock, latest Sheinwoodian–early Homerian; Färö, Lansa 1: Slite beds, unit d; Färö, Sandvik: Slite beds; Follingbo, Stora Vede 1: Slite beds, unit g; Larbro: upper Slite beds; Othem, Spillings; Slite beds, unit g; Othem, Samsuguns 1: Slite beds, unit g. Bohemia; Llandovery, Aeronian, Demirastrites triangulatus Biozone, Solopisky locality, Demirastrites simulans Biozone, Koněprusy locality (exact site unknown); Wenlock, Sheinwoodian, Cyrtograptus murchisoni Biozone, Praha, Malá Chuchle-Vyskočilka. USA (Foerste, Reference Foerste1925); Australia (Etheridge, Reference Etheridge1904; Furnish and Glenister, Reference Furnish and Glenister1964); and Inner Mongolia (Zou, Reference Zou1983).
Description
Shell tightly coiled, exogastric, slightly expanding at most with six whorls; WER: min.=1.8, med.=1.99, max=2.48, N=15, σ=0.18 (Fig. 10). RI: min.=0.41, med.=0.5, max.=0.69, N=14, σ=0.06. Umbilical perforation very small, drop shaped, with length ~0.6 mm. Cross section slightly depressed, in later growth stage subcircular or almost subquadrate (WWI: min.=1.02, med. =1.33, max.=1.67, N =11, σ=0.19, Fig. 10), impressed zone shallow. Length of body chamber about two-thirds of whorl. Aperture open, or may be slightly contracted in fully grown specimens with uncoiled adapertural part of the shell. Hyponomic sinus V-shaped (HW/HH: min.=1.54, med.=1.79, max.=2.5, N=6, σ=0.35; Fig. 10). Phragmocone chambers relatively long (WH/CL: min.=1.7, med.=2.71, max.=4.3, N=36, σ=0.69; Fig. 10). First six chambers longer than those succeeding; first chamber cup-like (CL=2.7 mm, WH=2.9 mm), length of following chambers measured as abscises along the ventral side (Fig. 1): 3.7 mm (second), 3.6 mm (third to sixth), and 3.1 mm (seventh and following). Depth of septa during shell growth increases from about one-third of chamber length (first to fifth chamber) to about one-half of phragmocone chamber length, and later becomes slightly shorter. Suture oblique, with shallow lateral lobe, ventrally straight. Caecum central, with diameter 0.5 mm, thick-walled; in second phragmocone chamber siphuncle shifted dorsally and in third chamber reached dorsal side. Diameter of siphuncle in apical shell increased to reach constant relative thickness one-fifth of IWH in third chamber. Septal necks hemichoanitic. Connecting rings relatively thick, tubular or slightly vaulted. Sculpture consists of growth lines and transversal growth ridges. Early shell with fine regularly arranged growth lines, densely packed and appear when shell reached one-fifth to one-third of whorl. In following growth stages, growth lines run obliquely to shell axis and form deepening V-shaped hyponomic sinus; middle part of hyponomic sinus rounded. Growth lines in later shell growth stages irregularly arranged with one or two more distinct growth lines between ridges. Growth ridges distinct laterally as well as ventrally, parallel with growth lines; internal mold usually smooth. Crests of growth ridges sharp. Lateral furrow, observed in a single specimen, is very shallow, ~2 mm wide and accentuated by distinct lighter-colored band on shell. Maximum shell thickness 2 mm.
Three flattened specimens of D. graftonense preserved in shale are known from Bohemia. Specimen NM L 42237 (Fig. 2.5) is a damaged, moderately expanding shell (SD=56 mm, three and one-quarter whorls); adapertural part of the shell missing. Shell tightly coiled, impressed zone very shallow, umbilical perforation small. Sculpture consists of prominent growth ridges and fine growth lines. Growth ridges, discernible from the beginning of the second whorl, appear as curved narrow elevations running obliquely to the shell axis. They are densely spaced, moderately vaulted to the aperture laterally, indicating a deep hyponomic sinus ventrally. Growth lines are prominent, regularly arranged, and parallel with growth ridges; 7–10 lines between crests of adjacent growth ridges. The second specimen of D. graftonense (NM L 4223) is a flattened internal mold of a body chamber (Fig. 2.1). The third specimen (NM L 46556; not figured) is a flattened fragment of the ventral side showing ribs forming a deep hyponomic sinus.
Materials
17 specimens from Gotland: RM Mo 59467, 59505, 59696, 59698, 59701, 59706, 59709, 59712, 59714, 59718, 59775, 59782, 59801, 59803, 59806, 59839, 155975; three specimens from Bohemia: NM L 42237, L 42239, L 46556.
Remarks
Specimens from Gotland and Bohemia are very similar in the shape of the shell, hyponomic sinus, course of growth lines, position and thickness of siphuncle, sutures, and length of phragmocone chambers to D. graftonense from Ohio and Illinois. Two type specimens figured by Foerste (Reference Foerste1925, pl. 12, figs. 2, 3) fall within the variability of WER and WWI described herein (Fig. 10). Four endemic species diverged from widely distributed D. graftonense (shell morphology of first whorl in all these species and principal conch parameter [WER] are very similar with D. graftonense): D. ortoni (Meek, Reference Meek1873) from Ohio differs in having a shallow hyponomic sinus (HW/HH=2.7–3, which is a higher value than those in D. graftonense from Gotland); D. amissus (Barrande, Reference Barrande1865); D. stridsbergi n. sp., which has distinct ribs; and D. lindstroemi n. sp., which has undulated frills, a less-depressed cross section, and a heteromorphic shell.
Discoceras stridsbergi new species

Figure 3 Discoceras stridsbergi n. sp. from Slite Beds of Gotland. (1) RM Mo 59719, lateral view, Färö, Sandvik, late Sheinwoodian; (2) RM Mo 59948, ventral view to adapertural region, Storugns, early Homerian (hi: healed injury, ca: contracted aperture); (3) RM Mo 59836, lateral view, Follingbo, Stora Vede 1, early Homerian; (4) RM Mo 59786, ventral view, Follingbo, Stora Vede 1, early Homerian; (5) RM Mo 59457, lateral view, Follingbo, Stora Vede 1, early Homerian; (6) RM Mo 59463, lateral view, Färö, Sandvik, late Sheinwoodian; (7) RM Mo 59452, cross section Follingbo, Stora Vede 1, early Homerian (gr: growth ridges); (8) RM Mo 59777, lateral view, Follingbo, Stora Vede 1, early Homerian. Specimens in Figure 3.5 and 3.8 were coated with ammonium chloride before photographing. Arrows in Figure 3.3, 3.5, and 3.8 indicate base of body chamber. Scale bars 10 mm.
Table 2 Dimensions (in millimeters) in D. stridsbergi n. sp. For abbreviations see Table 1.

Type specimens
Holotype, an almost complete shell RM 59777 (Fig. 3.8), from Stora Vede (Gotland), Slite Beds, early Homerian, Wenlock, Silurian. Paratypes: RM 59452 (Fig. 3.7), 59463 (Fig. 3.6), 59457 (Fig. 3.5), 59719 (Fig. 3.1), 59786 (Fig. 3.4), 59836 (Fig. 3.3), and 59948 (Fig. 3.2).
Diagnosis
Discoceras with moderately expanding shell and recurrent sharp ribs around the shell, running obliquely to shell axis laterally and forming prominent and deep hyponomic sinus ventrally.
Occurrence
Stora Vede 1, Larbo, Storugns, and Follingbo, Slite Beds, early Homerian, Wenlock.
Description
Evolute, exogastric shell with at most four whorls (WER: min.=1.7, med.=2.02, max.=2.66, N=24, σ=0.20; Fig. 10). RI: min.=0.43, med.=0.48, max.=0.73, N=16, σ=0.08. Cross section of early whorls strongly depressed, later moderately depressed to subcircular (WWI: min.=1.03, med.=1.37, max.=1.68, N=26, σ=0.16; Fig. 10). Length of body chamber slightly less than one-half of whorl. Aperture open or slightly contracted. Hyponomic sinus V-shaped (HW/HH: min.=1.79, med.=1.9, max.=2.4, N=9, σ=0.21; Fig. 10). Phragmocone chambers relatively long (WH/CL: min.=2.11, med.=2.73, max.=3.78, N=37, σ=0.44; Fig. 10). Septa slightly vaulted, suture oblique with shallow lateral lobes. Siphuncle relatively thin (one-ninth of IWH). Distinct growth lines running obliquely to shell axis are laterally straight or slightly undulate. Ventral lobe (= hyponomic sinus) deep, V-shaped, with rounded apex. Slightly indicated ribs in form of narrow ridges appear at beginning of second whorl. Their course is parallel with growth lines, and they are well developed at the end of the second whorl. Height of ribs increases with shell growth, but only on the last whorl do the ribs become visible on the internal mold as a faintly developed raised zone. Distance between ribs equal to phragmocone chamber length; their density is 6–8 per quarter whorl. Ribs asymmetric in cross section, adapertural part of ribs much steeper than adapical part. Maximum shell thickness 1.2 mm.
Etymology
Specific name stridsbergi after Sven Stridsberg (Lund University, Sweden), who has made significant contributions to our knowledge of Silurian cephalopods.
Materials
28 specimens, including types (Table 2).
Remarks
Discoceras stridsbergi n. sp. most probably diverged from D. graftonense because both species have a similar early shell morphology. Shell shape in both species is similar, but D. stridsbergi n. sp. differs in having a shorter body chamber, sharp ribs around the shell, and a wider hyponomic sinus. Ribs evolved from growth ridges in early whorls, ridges here are much lower and symmetrical in cross section.
Discoceras lindstroemi new species
Figures 4–7, 12, 13.3, 13.5, Table 3

Figure 4 Discoceras lindstroemi n. sp. from Slite beds, late Sheinwoodian, Gotland; ps: pseudoumbilicus. (1) RM Mo 59420, lateral view, Othem, Spillings: Slite beds; (2) RM Mo 59442, lateral view, counterpart, Färö; (3, 5), RM Mo 59432, lateral and ventral view, Lännaberget; (4) RM Mo 590265, cross section, Othem, Samsuguns 1; (6, 14) RM Mo 59427, ventral and lateral view, ps: pseudoumbilicus, Othem, Samsuguns 1; (7, 9, 12) RM Mo 59511, (7) lateral view, (9) detail of early shell showing lateral furrow (lf) and a healed injury (hi), (12) detail of sculpture, Othem, Samsugun; (8) RM Mo 157592, lateral view, polished specimen with undulated frills (uf), Martebo; (10) RM Mo 509506, cross section, Othem, Spillings; (11) RM 181926 ventral view to adapertural region, Farö; (13) RM Mo 59887, lateral view to adapertural region, ocular sinus slightly indicated, Othem, Samsuguns 1; (15) RM Mo 59416, lateral view, protruding last whorl with contracted aperture, Othem, Samsuguns 1, os: ocular sinus, hs: hyponomic sinus. All specimens except Figure 4.8 were coated with ammonium chloride before photographing. Scale bars 10 mm.
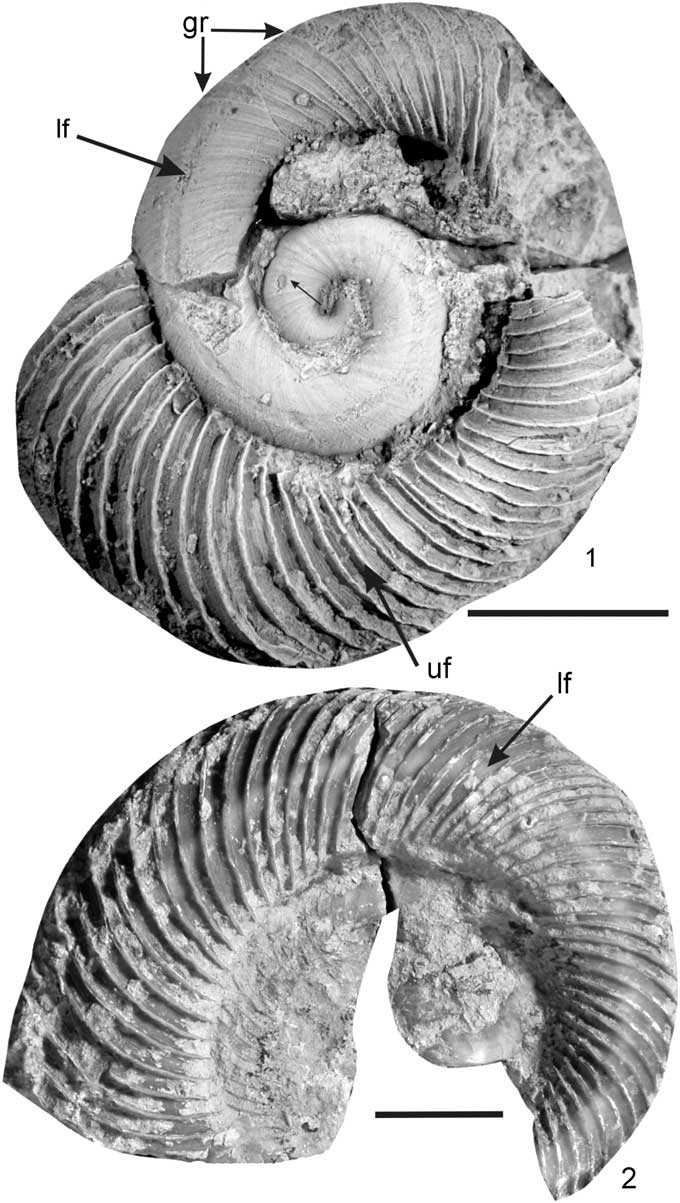
Figure 5 Lateral furrow (lf) in Discoceras lindstroemi n. sp., Othem, Klints, Slite beds, late Sheinwoodian. (1) RM Mo 59422, lateral view (gr: growth ridges, uf: undulated frill); (2) RM Mo 59419, lateral view. Specimen in Figure 5.1 was coated with ammonium chloride before photographing. Scale bars 10 mm.

Figure 6 Epibionts in Discoceras lindstroemi n. sp., Othem, Klints, Slite beds, late Sheinwoodian. (1, 2, 4, 5) RM Mo 59505, right and left lateral (1, 5), ventral (2) views and detail of microconchid tubes in lateral shell side (4); (3) RM Mo 162186, ventral view to adapertural region. Arrows indicated aperture orientation of tubes. All specimens were coated by ammonium chloride before photographing. Scale bars 5 mm (1–3, 5) and 1 mm (4).

Figure 7 Discoceras lindstroemi n. sp. preserved in reef cephalopod coquinas, late Sheinwoodian, Slite Beds, Gotland; (1, 4) RM Mo 40265, Othem, Spillings; (2) Polished slab with cross section of D. lindstroemi, RM Mo 59882, Othem, Spillings; (3) Two aggregated specimens, one from anterior and one from posterior view, RM Mo 59891, Othem, Samsuguns 1; (5) Lechritrochoceras sp. and two fragments of D. lindstroemi, RM Mo 59891, Othem, Samsuguns 1. Specimens in Figure 7.1 and 7.3–7.5 were coated by ammonium chloride before photographing. Scale bars 10 mm.
Table 3 Dimensions (in millimeters) and aperture characteristic of selected specimens of D. lindstroemi n. sp. SD1: shell diameter at beginning pseudoumbilicus; SD2: shell diameter at termination of pseudoumbilicus; PL: pseudoumbilicus length; PW: pseudoumbilicus width; LPS: length of protruding shell (ND: not developed); A: Aperture (O: open, C: contracted); for other abbreviations, see Table 1.

Type specimens
Holotype, a complete shell RM Mo 59511 (Fig. 4.7) from Othem, Kvarnberget 1, Gotland, Slite Beds, late Sheinwoodian, Wenlock, Silurian. Paratypes: RM Mo 40265 (Figs. 4.4, 7.4), 59420 (Figs. 4.1, 12.3), 59432 (Fig. 4.3), 59427 (Fig. 4.6, 4.14), 59442 (Fig. 4.2), 59505 (Fig. 6.1), 157592 (Fig. 4.8), 59506 (Fig. 4.10), 59887 (Fig. 4.13), 59416 (Fig. 4.15), 59419 (Fig. 5.2), 59884 (Fig. 13.3), 59422 (Fig. 5.1), 162186 (Fig. 6.3), and 181926 (Fig. 2.11) from Slite Beds, late Sheinwoodian, Wenlock.
Diagnosis
Discoceras with heteromorph shell, pseudoumbilicus and undulate growth frills.
Occurrence
Silurian, Wenlock, latest Sheinwoodian–early Homerian; Slite beds; Gotland; Färö, Lannaberget 1; Färö, Lansa 1; Follingbo, Stora Vede 1; Othem, Spillings; Othem, Samsuguns 1; Slite, Kvarnberget 1.
Description
Evolute, exogastric, slightly depressed shell with a maximum of three whorls (WER: min.=1.83, med.=1.98, max.=2.46, N=19, σ=0.20; Fig. 10). RI: min.=0.33, med.=0.47, max.=0.6, N=16, σ=0.07. Shell heteromorphic: early shell tightly coiled (0.5–1.5 whorl), following part of the shell (about one-half of whorl, Table 3) coiled in loose spire, followed by shell tightly coiled; adapertural part of shell in adult specimens straight. Indication of an additional enrolling, observed in a single specimen (Fig. 13.3), appeared at end of second whorl. The shell before the end of the second whorl is here tightly coiled, in the following one-quarter whorl, a very narrow window between the external shell walls of two neighboring whorls appears. Only distal ends of growth ridges oriented adapically are here in contact with the last whorl (body chamber). Umbilical perforation very small. Cross section slightly depressed, impressed zone shallow (WWI: min.=0.73, med.=0.96, max.=1.18, N=17, σ=0.10; Fig. 10). Length of body chamber equal to two-thirds of whorl. Aperture open, in fully grown shells with protruding adapertural part of last whorl, aperture sometimes slightly contracted.
Hyponomic sinus V-shaped (HW/HH: min.=1.6, med.=2.14, max.=3.17, N=11, σ=0.5; Fig. 10). Ocular sinus well developed, situated close to dorsal side (Fig. 4.15). Phragmocone chambers relatively long (WH/CL: min.=1.7, med.=2.71, max.=4.43, N=36, σ=0.69; Fig. 10). In early shell (Fig. 12.2), first five chambers are longer than succeeding chambers. Initial chamber cup-like, with maximum length 1 mm, dorsoventral distance in first septum is 2.9 mm, length of following chambers measured on ventral side ~2.9 mm (second to fourth), following chambers are shorter, length ~1.5 mm (Fig. 13.5). Depth of septa during shell growth increases from about one-third of chamber length (second to fourth chamber) to about one-half of phragmocone chamber length in following chambers. Suture forms oblique angle with shallow lateral lobe, but is straight ventrally.
Caecum subcentral, slightly expanded within first chamber (diameter 0.5 mm). In second and third chamber, siphuncle shifted toward dorsal side and diameter markedly increased. In the third chamber, siphuncle reaches dorsal side and diameter is about one-sixth of whorl height. Septal necks hemichoanitic; connecting rings relatively thick, tubular or slightly vaulted. Episeptal cameral deposits slightly developed close to siphuncle. First half of whorl smooth or with fine irregular growth lines; growth ridges, accompanied by small constriction of shell, are developed in some specimens at beginning of pseudoumbilicus (WH=5.2–7.2 mm; Fig. 12.3); irregularly situated growth ridges appeared between WH 4–7.4 mm, they pass rapidly into regularly distributed growth ridges and finally into regular undulated frills near end of pseudoumbilicus (Figs. 4, 5.1). Height of frills continually increases up to 3 mm with shell growth (Figs. 4.8, 4.12, 7.2). Distance of frills increases from 1 mm to 2.5 mm. In fully grown specimens, the frills at the aperture are densely packed, and a shallow ocular sinus appeared (Fig. 4.13). Frills are intercalated with irregularly distributed growth lines. Transverse sculptural elements are oblique to the shell axis, with shallow lateral saddles. Maximum shell thickness is 3 mm.
In four specimens, lateral furrows are developed symmetrically in both sides of the shell. The furrows form shallow depressions or light-colored bands on shell; width of furrows equals one-quarter of whorl height. Furrows appear on shell reaching three-quarters of a whorl (Figs. 4.9, 5). They start in the mid-lateral part of the shell, and rapidly shift toward the venter to attain a ventrolateral position. The furrows fade away with the appearance of distinct growth ridges or continue as a light-colored band.
The sculpture in a single incomplete counterpart from Färö (Figs. 4.2, 12.1) differs from the sculpture of specimens from Othem. Distinct widely spaced growth ridges with one or two inserted fine growth lines appear in one-half of the first whorl, where the ventral side of the shell lost contact with the following whorl. Subsequently, growth ridges become laterally slightly vaulted to the aperture, and subordinate growth lines are more distinct. A prominent repair is located dorsolaterally at the beginning of the second whorl.
Etymology
Specific name, lindstroemi, after Gustav Lindström, who has made significant contributions to the understanding of the Silurian fauna of Gotland.
Materials
In addition to the types, these nine specimens: RM Mo 59418, 59425, 59426, 59428, 59443, 59499, 59504, 158752, 162186.
Remarks
Discoceras lindstroemi n. sp. exhibits considerable variability in the shape of the hyponomic sinus. In comparison with coeval species, the cross section of the shell is less depressed (Fig. 10). In addition, the protruding final whorls in D. lindstroemi n. sp. have a contracted aperture with well-developed ocular sinuses. Similar mature modification is known in the contemporaneous tarphycerid Ophioceras Barrande, Reference Barrande1865 and Ordovician lituitids (Furnish and Glenister, Reference Furnish and Glenister1964).
Discoceras sp. indet.

Figure 8 Discoceras sp. indet., RM Mo 59972, lateral view, Gotland, locality unknown. Scale bar 10 mm.
Description
Incomplete specimen preserved as a weathered internal mold in gray dolomite. Specimen comprises at least two and one-half whorls, adapertural part of the shell strongly ventrally damaged. Shell evolute, gradually expanding, exogastric, RI is 0.68. Complete reconstructed shell reached four whorls (SD=90 mm). First half of second whorl coiled in loose spire, followed by tightly coiled whorls. Cross section subcircular, impressed zone not developed. Prominent regularly arranged ribs, no substantial changes in density of ribs during growth (10 ribs per quarter whorl). Hyponomic sinus deep and wide.
Occurrence
Middle Wenlock of Gotland, Sweden.
Material
A single specimen RM Mo 59972.
Remarks
In mode of coiling, Discoceras sp. indet. resembles D. lindstroemi n. sp.; nevertheless, straightening of the final whorl is not documented. Ribbing in Discoceras sp. indet. is similar to that in D. stridsbergi n. sp. In comparison with later species, the shell is more gradually expanding (WER is not measurable due to preservation, but RI is higher than in D. lindstroemi n. sp.), ribs on internal molds are more pronounced and more densely spaced, and cross section is sub-circular. Because only one specimen of Discoceras sp. indet. was available for study, it is left in open nomenclature.
Discoceras amissus (Barrande, Reference Barrande1865)

Figure 9 Discoceras amissus (Barrande, Reference Barrande1865), NM L 9107), holotype, lateral view, specimen flattened in shale, Bohemia, Beroun-Králův Dvůr, Ovčinec, Llandovery, Aeronian, Demirastrites convolutus Biozone. Scale bar 10 mm.
1865 Lituites (Ophioceras) amissus Reference BarrandeBarrande, pl. 45, figs. 26, 27.
1867 Ophidioceras (Lituites) amissus; Reference BarrandeBarrande, p. 182.
Holotype
Holotype by monotypy, NM L 9107, illustrated by Barrande (Reference Barrande1865) on pl. 45, figs. 26, 27 from Králův Dvůr, Ovčinec, Bohemia; Landovery, Aeronian, Demirastrites convolutus Biozone.
Diagnosis
Discoceras with combination of distinct growth lines and prominent ribs, symmetrical in cross section.
Description
Fragment of shell consists of one and three-quarter whorls, SD=30 mm, WER=2.07. Adapertural part of shell missing. Shell evolute, gradually expanding, exogastric, cross section unknown, due to flattening in shale. First half of first whorl smooth, almost equally developed growth lines appeared on second half of first whorl, forming deep and wide hyponomic sinus. Near beginning of second whorl, narrow crests appear, passing gradually into prominent wide ribs running obliquely to shell axis. Laterally, ribs slightly vaulted adaperturally; ventrally, they pass into the deep hyponomic sinus. Top parts of ribs rounded. Prominent growth lines inserted between ribs. On last quarter of shell, nine ribs are visible. Growth lines regularly arranged, parallel with ribs, 5–11 in number between crests of adjacent ribs.
Material
Holotype only.
Remarks
Discoceras amissus resembles D. stridsbergi n. sp., differing in having fewer more distinct ribs that fade out towards the adapertural end of the shell; ribs are symmetrically rounded in cross section.
Distribution of Discoceras
More than 30 species of the widely distributed genus Discoceras have been described from Middle and Upper Ordovician strata. Discoceras has been reported from Australia (Teichert and Glenister, Reference Teichert and Glenister1954); Canada (Whiteaves, Reference Whiteaves1897); Denmark (Rasmussen and Surlyk, Reference Rasmussen and Surlyk2012); Estonia (Balashov, Reference Balashov1953; Stumbur Reference Stumbur1962); Kazakhstan (Barskov, Reference Barskov1972); northwest China (Lai and Wang, Reference Lai and Wang1986; Lai, Reference Lai1987); Norway (Strand, Reference Strand1934; Sweet, Reference Sweet1958); Sweden (Kröger et al., Reference Kröger, Ebbestad, Högström and Frisk2011; Kröger, Reference Kröger2013); Tibet (Lai, Reference Lai1982); and Wisconsin (Whitfield, Reference Whitfield1882; Hyatt, Reference Hyatt1894). Discoceras has also been found in erratic boulders of Baltoscandian origin (Lossen, Reference Lossen1860; Roemer, Reference Roemer1861; Remelé, Reference Remelé1890; Hyatt, Reference Hyatt1894; Dzik, Reference Dzik1984). Discoceras is globally dispersed in a belt between 20°N and 50°S when the Ordovician reconstruction of Torsvik and Cocks (Reference Torsvik and Cocks2013) is used. It is known from the Baltica, North China, NE Gondwana, Kazakhstania, Laurentia, and Tibet paleocontinents. Discoceras inhabited carbonate platforms, in shallows as well as in their deeper parts up to a depth of ~100 m. Some species are also known from off-shore shales deposited under dysoxic/anoxic conditions (Kröger et al., Reference Kröger, Servais and Zhang2009, Reference Kröger, Ebbestad, Högström and Frisk2011; Kröger and Ebbestad, Reference Kröger and Ebbestad2014).
Contrary to the worldwide dispersion of tarphycerids during the Ordovician, Discoceras and Trocholites (the latter represented by a single species in the Llandovery of Great Britain; Holland, Reference Holland2010) are the only tarphycerid genera known to have survived the late-Ordovician extinction event. This event was accompanied by a marked decrease in nautiloid diversity (Frey et al., Reference Frey, Beresi, Evans, King and Percival2004; Kröger and Zhang, Reference Kröger and Zhang2009). Discoceras graftonense is a widely distributed species, ranging from the Aeronian (Llandovery) to early Homerian (Wenlock). Other Silurian species of Discoceras were endemic, with very limited distribution even in one basin (Table 4). The widespread geographic distribution of D. graftonense and the origin of four species of Discoceras in the latest Sheinwoodian and early Homerian represented the last weak diversification and dispersion event of tarphycerids, terminated by the mid-Homerian extinction (Mulde and Lundgreni events; Calner, Reference Calner2008). Only a single tarphycerid, Ophioceras Barrande, Reference Barrande1865, survived this extinction, including two long-ranging species; the youngest of these became extinct just below Silurian-Devonian boundary (Stridsberg and Turek, Reference Stridsberg and Turek1997; Turek and Manda, Reference Turek and Manda2016). No tarphycerid species originated following the middle Homerian; there is no evidence of a splitting lineage or a reason to split a lineage into a series of successive chronospecies. Ophioceras co-occurs with Discoceras in Gotland, North America, and North China. While Discoceras retained shell morphology similar to Ordovician tarphycerids, the shell of Ophioceras acquired a thin siphuncle and a higher expansion rate of the shell (Stridsberg and Turek, Reference Stridsberg and Turek1997: revolving index 1.3–1.7; cf. RI of Silurian Discoceras 0.33–0.73).
Table 4 Distribution of Discoceras species in the Silurian. PG indicates peri-Gondwana.

The low diversity and extinction of tarphycerids in the Silurian, together with their relatively thick marginal siphuncle, is consistent with a macroevolutionary trend toward reduction of siphuncle diameter and thinning of connecting rings in Paleozoic cephalopods (Kröger, Reference Kröger2003). It is also consistent with an exceptionally low adaptive pressure toward planispirally coiled shells during the Silurian and the tendency towards a coiled shell with a central siphuncle (Kröger, Reference Kröger2005).
In the Wenlock of the Baltic Basin, Discoceras inhabited a shallow water environment close to reefs and its shells also occur in reef caverns. Discoceras also inhabited shallow water platforms in proximity to reefs in the Midwestern craton in Laurentia (Shaver, Reference Shaver1991) and the North China plate (Li et al., Reference Li, Rong, Dong, Yang, Su and Wang1983). A single occurrence was documented from deeper water shales with a pioneer benthic community in the Prague Basin (Turek, Reference Turek1983). The geographic distribution of Discoceras in the Silurian, in contrast to the wide distribution of Ordovician tarphycerid genera, is restricted to the proximal areas of low-latitude platforms and occasionally more distal areas of black shale sedimentation between 20°N and 30°S (Table 4, Fig. 11).
The occurrence of Discoceras in Llandovery off-shore black graptolitic shales of the Prague Basin (Perunica microplate, peri-Gondwana), which were deposited under anoxic near-bottom conditions (Štorch, Reference Štorch2006) well removed from a carbonate platform, resembles Ordovician occurrences in black graptolite shales of Baltoscandia (Kröger et al., Reference Kröger, Servais and Zhang2009; Rasmussen and Surlyk, Reference Rasmussen and Surlyk2012). The earlier Silurian anoxic episode restricted almost all fauna except graptolites in peri-Gondwanan basins. Nautiloid immigration from low latitudes into peri-Gondwanan basins with isolated elevated submarine regions is linked with a decline in early Silurian anoxia, and activation of currents beginning in the middle Llandovery to Wenlock (Stridsberg, Reference Stridsberg1988b; Manda, Reference Manda2008b; Histon, Reference Histon2012; Evans et al., Reference Evans, Ghobadipour, Popov and Jahangir2015; Fig. 11).
Pioneer nautiloid immigrants were forms with a coiled shell. Aeronian Discoceras is the earliest known stray immigrant into the Prague Basin; later, in the latest Llandovery, the discosorid Phragmoceras Broderip, 1839 in Murchison (Reference Murchison1839) appeared. Permanent nautiloid populations and continuous faunal exchange in the Prague Basin appeared in the late Wenlock (Manda, Reference Manda2008b). The occurrence of Discoceras in black off-shore shales in association with graptolites indicates its migration potential and swimming ability in surface currents across an open sea. Dispersion potential also could have been enhanced for possible planktotrophic juveniles transported by ocean currents. This also explains the dispersion of D. graftonense in distant continents (e.g., Laurentia and the Northeast China Plate). Empty shells could have been transported together with immigrants via current (Hamada et al., Reference Hamada, Tanabe and Hayasaka1980). However, even the fine details of the shell sculpture are well preserved, suggesting that drift time and distance of empty shells were probably relatively short. The lack of bioerosion exclude their long-running drift between continents, which is consistent with rather limited post-mortem transport suggested for early Paleozoic nautiloid assemblages (Flower, Reference Flower1957; Hewitt and Watkins, Reference Hewitt and Watkins1980; Frey, Reference Frey1989).
Embryonic development and hatching time in Tarphycerida
The tarphycerid early shell is planispiral and tightly coiled with a very small umbilical perforation (Furnish and Glenister, Reference Furnish and Glenister1964; Dzik, Reference Dzik1984). Its apex is blunt, the first chamber is curved and cup-like, and its length varies between 0.8–3.5 mm (Stumbur, Reference Stumbur1959; Shimansky and Zhuravleva, Reference Shimansky and Zhuravleva1961). The nepionic constriction characterizing the end of the embryonic phase in later nautilids is not present, but sculpture usually shows some change in growth line spacing. However, the sculpture development in embryonic and juvenile growth stages of tarphycerid shells is still poorly known. Consequently, the internal structures of the phragmocone have been used for determination of hatching time. This is a methodological approach inferred from extant Nautilus (Schindewolf, Reference Schindewolf1934; Stumbur, Reference Stumbur1959).
A change in septal spacing between the seventh and eight chambers in Nautilus coincides with its emergence from an egg and formation of the nepionic constriction (Naef, 1921–Reference Naef1923; Arnold et al., Reference Arnold, Landman and Mutvei1987; Ward, Reference Ward1987; Tajika et al., Reference Tajika, Morimoto, Wani, Naglik and Klug2015). Both reflect stress following the emergence of the hatched animal from the egg capsule (Arnold et al., Reference Arnold, Landman and Mutvei1987, Reference Arnold, Landman and Mutvei2010). Although the change in septal spacing is often linked with hatching in Nautilus, the intraspecific variability in the chamber length pattern is high (Stenzel, Reference Stenzel1964). Except in Ophioceras (Turek and Manda, Reference Turek and Manda2016), the variability of septal spacing in early ontogeny has not been studied in early Paleozoic nautiloids. A change in septal spacing in the first whorl in Ordovician tarphycerids was linked with hatching by Schindewolf (Reference Schindewolf1934). Stumbur (Reference Stumbur1959, Reference Stumbur1960) studied the early ontogeny in four genera representing the main evolutionary lineages of Ordovician tarphycerids. According to him, the hatching in tarphycerids is manifested by a sudden change in septal spacing—the first five to seven phragmocone chambers are longer than those following, in which length decreases gradually or abruptly. Following this hypothesis, the shell of early-hatched tarphycerids would have reached 1.25–1.5 whorls (WH=4–8 mm, SD=10–20 mm) and the phragmocone would consist of 5–7 chambers. Consequently, the hatching size in Tarphycerida would be close to that in post-Triassic nautilids (Wani et al., Reference Wani, Kurihara and Ayyasami2011). Stumbur (Reference Stumbur1959) supported his conclusion with two species of Ordovician Discoceras from Norway, illustrated by Sweet (Reference Sweet1958). However, in these species, the change in septal spacing occurs between the eight and ninth chambers. Sweet (Reference Sweet1958) illustrated a specimen of Estonioceras proteus demissa Holm, Reference Holm1885 in which the first two chambers are long and the third chamber markedly shortened; length of the following chambers gradually decreased, and after the ninth chamber increased again. Similarly, in Eurystomites amplectens Ruedemann, Reference Ruedemann1906 (pl. 18, fig. 5) and Tarphyceras multicameratum Ruedemann, Reference Ruedemann1906 (pl. 19, fig. 3), the first two chambers are longer than the following ones, which have almost equal lengths. In Estonioceras imperfectum (Quenstedt, Reference Quenstedt1845), the first chamber is long, the second chamber is shorter, the third to eighth chambers are almost the same length as the second; the ninth and following chambers are, again, markedly shorter (Stumbur, Reference Stumbur1959; fig. 1a). Stumbur (Reference Stumbur1959) considered this development atypical, and assumed that the change in length between the seventh and eighth chambers indicates hatching. However, Shimansky and Zhuravleva (Reference Shimansky and Zhuravleva1961, p. 78) described another tarphycerid, Trocholites sp., with a high first chamber, a much shorter second chamber, followed by chambers with gradually increasing lengths.
Differing interpretations of the significance of changes in septal spacing during early development were addressed by Shimansky and Zhuravleva (Reference Shimansky and Zhuravleva1961, p. 84). They suggested two early ontogenetic paths in tarphycerids: (1) the shell in the egg capsule possessed one or two chambers and a body chamber (e.g., Estonioceras imperfectum and Trocholites sp.), in which case, the hatched animal differed from an adult in having a small curved shell; and (2) the embryonic shell consisted of five to six chambers and a body chamber (all other tarphycerids), and early-hatched animals possessing coiled shells that did not differ from adults. Nevertheless, such differing hatching size reflecting remarkable differences in the early development of hatchlings seems unlikely in such closely related taxa.
Turek and Manda (Reference Turek and Manda2016) demonstrated that septal spacing in Silurian Ophioceras is not coupled with hatching; hatching in this case undoubtedly preceded the change in septal spacing, and juveniles had small curved shells with only one phragmocone chamber. However, Ophioceras is evolutionarily the youngest tarphycerid with some evolutionary novelties (e.g., a single ventral retractor muscle scar), and it is uncertain whether this type of embryonic development is applicable to older tarphycerid taxa.
Hatching indication in the Silurian Discoceras
The apex of D. graftonense is very similar in shape to that of Ordovician species of Discoceras. The earliest part of the shell is smooth; growth lines have been observed starting at about three-eighths of the first whorl and at whorl height 7–9 mm (SD=16–22 mm) growth lines become raised. Length of the first chamber is ~2.7 mm, and the second to sixth phragmocone chambers are about one-third longer than following chambers (Fig. 13.4). Change in septal spacing occurs at a whorl height of 3 mm, when the shell attained one whorl (SD=8.8 mm). Following Schindewolf´s (Reference Schindewolf1934) and Stumbur´s (Reference Stumbur1959) approach to linking hatching with change in septal spacing, the shell of hatched D. graftonense would have reached one and one-half of a whorl and consisted of six phragmocone chambers plus a body chamber about half a whorl in length (WH=5.2 mm, SD=14 mm). A change in sculpture appeared immediately after the supposed indication of hatching. However, in specimen RM Mo 59803 (Fig 13.1), the difference in chamber length is not obvious, because chamber lengths increase gradually.
The early shell of D. lindstroemi n. sp. is similar in shape and caecum position to D. graftonense, but differs in having much less pronounced growth lines, and a change in septal spacing occurs between the fifth and sixth chambers (WH=2.6 mm, shell reached first whorl; Figs. 12.2, 13.5). If this change really indicates hatching time (Stumbur, Reference Stumbur1959), then the whorl height would have reached 5 mm and shell diameter 12 mm.
A unique morphological feature—a deep groove situated at three-quarters of the first whorl (WH=5 mm)—preceded the change in septal spacing (Fig. 4.9). The character of the groove and its adapical widening on the ventral side of the shell indicate serious mechanical damage caused by a predator. The injury originated after hatching and thus preceded the change in septal spacing (i.e., the shell of the hatched individual did not reach three-quarters of a whorl). Close to three-eighths of the first whorl, a change in shell coiling occurs, and growth lines are enhanced.
It is highly probable that the change in septal spacing did not coincide with hatching in Discoceras, as well as in Tarphycerida in general (see Turek and Manda, Reference Turek and Manda2016). According to Stumbur (Reference Stumbur1959), the growth sculpture appeared in tarphycerids after hatching. However, in Ophioceras, fine growth lines have been observed on the first chamber. In the Ordovician species Discoceras vasalemmense Balashov, Reference Balashov1953, growth lines are discernible in about a half of the first whorl; they are regularly arranged, and in the adjacent juvenile part of the shell no striking change in their spacing has been observed. In Silurian Discoceras, growth lines appear at about three-eighths to one-half of the first whorl. Also at close to three-eighths of the first whorl, a slightly changed course of the shell spire and slight shell expansion has been observed. Taken together, there is as yet no clear evidence indicating hatching time in Discoceras. More likely, the hatchling possessed a curved shell (one-half or greater than three-quarters of a whorl) with two/three phragmocone chambers. Early hatched specimens thus differed in life from adults; the large volume of the first phragmocone chamber and the undeveloped hyponomic sinus suggest a macroplanktic habit. This is consistent with the early development of the evolutionarily youngest tarphycerid, Ophioceras (Turek and Manda, Reference Turek and Manda2016). Longer chambers in the first whorl may be linked with accelerated growth before the shell reached one whorl, resulting in a stable shell orientation and higher rigidity of the shell.
Heteromorph shell in Discoceras
Silurian Discoceras shows shell morphology and habitat derived directly from Ordovician species. However, D. lindstroemi n. sp. (Figs. 4, 5) possesses a heteromorphic shell and elaborate sculpture. The early shell of D. lindstroemi n. sp. is tightly coiled, but subsequently, during growth, the second whorl becomes loosely coiled and then reverts again to being tightly coiled (a slight indication of a second decoiling may once again appear; Fig. 13.3). In adult specimens, the final growth stage of the shell is straight. Changes in shell coiling resulted in a change in both the aperture orientation of the animal and the hydrodynamic properties of the shell (Chamberlain, Reference Chamberlain1976, Reference Chamberlain1981; Naglik et al., Reference Naglik, Tajika, Chamberlain and Klug2015). Growth stages with tightly coiled shells were nektonic, with the aperture oriented forward as in other tarphycerids (e.g., Flower, Reference Flower1957; Westermann, Reference Westermann1998). In the growth stage with a loosely coiled shell, and in the adult stage possessing a protruding adapertural part of the shell, the aperture was oriented obliquely downward. Shells of the openly coiled stage exhibit longer phragmocone chambers, while whorl height slightly decreases. Apertures of fully grown specimens are constricted, with deepened ocular sinuses, suggesting well-developed eyes. Similarly, the deep hyponomic sinus suggests a well-developed hyponome.
A premature part of the shell, either loosely coiled or straight, is known in coiled Tarphycerida (Furnish and Glenister, Reference Furnish and Glenister1964), Nautilida (Hyatt, Reference Hyatt1894; Turek, Reference Turek1975), and Oncocerida (Manda and Turek, Reference Manda and Turek2011). A similar shell shape is unknown in post-Triassic nautilids, probably due to increasing competitive pressure among demersal predators (Klug et al., Reference Klug, Kröger, Kiessling, Mullins, Servais, Frýda, Korn and Turner2009). A final uncoiled growth stage is frequently coupled with the development of a contracted aperture; coiled nautiloids with an aperture oriented forward in life position always have an open aperture. The constricted aperture in nautiloids with an aperture oriented obliquely toward the seafloor probably had a protective function against predators (Flower, Reference Flower1957), and provided an improved protection of the mantle margin against parasite infestation, which occurred rather frequently in the late Silurian Ophioceras (Turek and Manda, Reference Turek and Manda2016). Growth of the uncoiled part of the shell coupled with the change in biological orientation could be beneficial to individuals on reaching a critical size. The length of uncoiled parts of the shells is highly variable; it is lower in more robust specimens and higher in specimens with slender shells. As in recent Nautilus (Collins and Ward, Reference Collins and Ward1987), an increase in volume of the body chamber during adolescence probably corresponded to an increase in the size of the gonads (Stridsberg and Turek, Reference Stridsberg and Turek1997; Manda and Turek, Reference Manda and Turek2011; Turek and Manda, Reference Turek and Manda2016). Changes in shell coiling occurred at least twice during the ontogeny of D. lindstroemi n. sp., but variability in the length and timing of the first decoiling remained high. A similar mode of coiling is indicated in Late Ordovician Aphetoceras farnsworthi (Billings, Reference Billings1861) and A. attenuatum (Hyatt, Reference Hyatt1894) (Ulrich et al., Reference Ulrich, Foerste, Miller and Furnish1942, pl. 1, fig. 2, pl. 4, fig. 7). It is also seen in other estonioceratids (e.g., Vasalemmoceras Stumbur, Reference Stumbur1962) and some lituitids (B. Kröger, personal communication, 2016). A poorly developed pseudoumbilicus also occurs in the Silurian uranoceratid Boionautilus sternbergi (Barrande, Reference Barrande1865) (Turek, Reference Turek2008, fig. 3d). Repeated significant decoiling of the shell resulted in a change in the living position of the animal, similar to that postulated for heteromorph ammonoids (Naglik et al., Reference Naglik, Tajika, Chamberlain and Klug2015; Kakabadze, Reference Kakabadze2016). The aperture in both cases reached a lower position in comparison with that in a tightly coiled shell (Fig. 14). Decoiling during the late juvenile and adolescent stages of D. lindstroemi n. sp. and other nautiloids might have enhanced the contact of the animal with the bottom (Stridsberg and Turek, Reference Stridsberg and Turek1997; Turek and Manda, Reference Turek and Manda2016). Any advantage to this first decoiling is somewhat problematic. Decoiling of the shell could significantly alter the drag coefficient. Such a decoiled shell would have had a higher relative drag in comparison with a tightly coiled shell. The highly elaborate sculpture in D. lindstroemi n. sp. probably also negatively influenced the hydrodynamic properties of the shell (Chamberlain, Reference Chamberlain1976, Reference Chamberlain1981). The appearance of a heteromorph shell in D. lindstroemi n. sp. was probably an unsuccessful random evolutionary event among stratigraphically younger tarphycerids.
Conclusions
The first tarphycerids appeared in the early Tremadocian, then their diversity suddenly increased, reaching a maximum in the early Floian. Their generic diversity slowly declined in the late Upper Ordovician (Kröger and Zhang, Reference Kröger and Zhang2009). Starting at the Late Ordovician extinction, diversity of tarphycerids was low throughout the Silurian, prior to their extinction just below Silurian-Devonian boundary. Three genera of tarphycerids are known from the Silurian, two of which survived the Late Ordovician extinction: Discoceras Barrande, Reference Barrande1867 and Trocholites Conrad, Reference Conrad1838, from which Ophioceras Barrande, Reference Barrande1865 probably diverged. Including the four species described here and evaluating previously published data, Discoceras comprises six Silurian species occurring in Llandovery (peri-Gondwanan Perunica) and Wenlock strata (Baltica, Laurentia, NE Gondwana, Northeast China Plate, peri-Gondwanan Perunica). The widespread geographic distribution of D. graftonense and the origin of four endemic species of Discoceras in the middle Wenlock represented the last weak diversification and dispersion event of tarphycerids, terminated by the mid-Homerian extinction. A single tarphycerid genus, Ophioceras Barrande, Reference Barrande1865, which includes two long-ranging species, survived this extinction.
The geographic distribution of Discoceras in the Silurian is restricted to proximal areas of low-latitude platforms, and occasionally more distal areas of black shale sedimentation. The occurrence of Discoceras in off-shore shales and the dispersion of D. graftonense in distant continents indicate its migration potential and swimming ability in surface currents across an open sea.
Schindewolf (Reference Schindewolf1934) was the first to link a change in septal spacing in the first whorl of Ordovician tarphycerids with their hatching phase. In this concept, hatching in tarphycerids is manifested by a sudden decrease in phragmocone chamber volume in phragmocone chambers six through eight. As a consequence, the shell of early-hatched tarphycerids would have reached slightly more than one whorl, and a shell diameter of 10–20 mm. Nevertheless, there is as yet no clear evidence indicating hatching time in Discoceras. A repaired injury in a Silurian Discoceras that took place after hatching indicates that the shell of the hatched individual did not reach three-quarters of a whorl, and thus hatching preceded the change in septal spacing. Moreover, this change in spacing is not present in every specimen studied. Growth lines appear in Silurian Discoceras at about three-eighths to one-half of the first whorl. Their appearance on the shell surface coincides with a slightly changed course of the shell spire, and a slight shell expansion. More likely, the hatchling possessed only a curved shell with two or three phragmocone chambers. Early hatched specimens thus differed in habit from adults (demersal swimmers). The large volume of the first phragmocone chamber and the undeveloped hyponomic sinus in juveniles suggest a macroplanktic habit, which is consistent with early development of the evolutionarily youngest tarphycerid Ophioceras (Turek and Manda, Reference Turek and Manda2016).
Silurian Discoceras retained the morphology and habitats of its Ordovician ancestors. Nevertheless, the early shell of D. lindstroemi n. sp. is tightly coiled, but subsequently, during growth, the second whorl becomes loosely coiled and then reverts again to tight coiling; furthermore, a slight indication of a second decoiling may once again appear. A heteromorphic planispiral shell with coiling that changed during ontogeny resulted in changing aperture orientation and maneuverability in life. The appearance of a heteromorphic shell in D. lindstroemi n. sp. was probably an unsuccessful random evolutionary event among Silurian tarphycerids. Simultaneously, it was the stratigraphically first appearance of a well-elaborated heteromorph shell in externally shelled cephalopods in the fossil record.

Figure 10 Boxes (minimum, maximum, median, and first and second quartile) showing WER, WWI, HW/HH ratio and WH/CL ratio in Silurian Discoceras from Gotland; g: D. graftonense, s: D. stridsbergi, l: D. lindstroemi. Gray cross indicates value in holotype of G. graftonense (data from Foerste, Reference Foerste1925).

Figure 11 Paleogeographic map (after Torsvik and Cocks, Reference Torsvik and Cocks2013) showing distribution of tarphycerids and current system (after Wilde et al., Reference Wilde, Berry and Quinby1991) in the late Wenlock. Alternative paleographic position of Central Bohemia (2), microcontinent Perunica (1), respectively, after Cocks and Torsvik (Reference Cocks and Torsvik2002); this position corresponds better with the dispersion pattern of Discoceras, which never occurs in cool water areas influenced by subpolar current (Silurian tarphycerids are unknown in such areas of peri-Gondwana including Austria, France, Germany, Spain, etc.). A indicates Australia.

Figure 12 Early shell of Discoceras lindstroemi n. sp.; arrows indicate appearance of growth lines, cc indicates a change in shell coiling. (1) RM Mo 59442, lateral view, counterpart, Färö, Slite beds, late Sheinwoodian. Growth ridges regularly spaced, visible from first half of whorl. Minor shell damage seen dorsolaterally at one and one-half whorls; (2, 3) RM Mo 59420, lateral view, Othem, Samsugun, Slite beds, late Sheinwoodian; (4) RM Mo 59422, lateral view, specimen with well-developed lateral furrow, Othem, Samsugun, Slite beds, late Sheinwoodian. Specimens in Figure 12.1, 12.3, and 12.4 coated with ammonium chloride before photographing. Scale bars 5 mm.

Figure 13 Inner structure in median section of shell in Discoceras from Silte beds (cd: cameral deposits, ps: pseudoumbilicus, id: indication of the second decoiling). (1, 2, 4) D. graftonense (Meek and Worthen, Reference Meek and Worthen1870); (1) RM Mo 59803 Follingbo, Stora Vede 1, early Homerian; (2, 4) RM Mo 155975, Othem, Samsuguns 1, late Sheinwoodian, note rapidly increasing volume of siphuncle in third chamber and marked shortening of seventh chamber; (3, 5) D. lindstroemi n. sp. RM Mo 59884, white arrows indicate relicts of septa. Scale bars 5 mm.

Figure 14 Schematic drawing of changing biological orientation in D. lindstroemi n. sp. throughout ontogeny.
Acknowledgments
Strategic Research Plan of the Czech Geological Survey project no. 339900, Czech Grant Agency 14124S (ŠM), and Ministry of Culture project 2018/06, National Museum, 00023272 (VT) supported the research. The authors thank J. Bergström, C. Franzen, and J. Hagström (Rickmuseet Stockholm, Sweden) and S. Stridsberg (University Lund, Sweden) for access to collections and their kind assistance during our stay in their institutions; P. Daneš (Prague) and R. Parsley (Tulane University, New Orleans) for reading the manuscript and for improving the English; and J. Wagner (Prague) for assistance in locating some publications. Reviewer’s comments by J. Dzik, D. Evans, B. Kröger, and R.H. Mapes were important for the final form of the manuscript as well as comments by associate editor D.M. Work.




















