Introduction
Helminth parasites are infectious agents belonging to a diverse group of the phylum Nematoda (roundworms) and the phylum Platyhelminthes (flatworms). Among the nematodes is the group of the soil-transmitted helminths (STH), also known as geohelminths, classified as parasites which are infective agents, including embryonated eggs or larval stages are transmitted to the host by direct contact with the soil through either skin penetration or oral ingestion (Lustigman et al., Reference Lustigman, Prichard, Gazzinelli, Grant, Boatin, McCarthy and Basáñez2012). The STH, Ascaris lumbricoides and Ascaris suum, Trichuris trichiura and both Necator americanus and Ancylostoma duodenale are the most important etiological agents of the most common intestinal parasitic diseases of developing countries, being part of the neglected tropical diseases (NTD), such as ascariasis, trichuriasis and hookworm infections, respectively (Lustigman et al., Reference Lustigman, Prichard, Gazzinelli, Grant, Boatin, McCarthy and Basáñez2012). Among them, the most common and prevalent NTD is the human ascariasis caused by A. lumbricoides or A. suum, by which recent studies estimate that ~819 million people are infected worldwide (Pullan et al., Reference Pullan, Smith, Jasrasaria and Brooker2014). This high prevalence is associated with poverty and precarious health conditions, mainly in tropical and subtropical areas of developing countries such as sub-Saharan Africa, Southeast Asia and South America (Bethony et al., Reference Bethony, Brooker, Albonico, Geiger, Loukas, Diemert and Hotez2006; WHO, 2015, 2019).
Human ascariasis is transmitted through the faecal–oral route. Infection occurs by ingestion of water or food contaminated with embryonated eggs containing the fully developed L3 larval stages. The eggs hatch in the intestine, and the L3 larvae that pass through the intestinal wall and migrate along the liver and heart, up to the lungs. In the lung tissue followed by the airways passage, the larvae are expectorated and then swallowed, passing through the gastrointestinal tract until they arrive at the small intestine, where they mature into adult worms, which after mating, females release millions of fertilized eggs with the faeces, contaminating the environment (Douvres et al., Reference Douvres, Tromba and Malakatis1969; WHO, 2011, 2019; CDC, 2019; Conterno et al., Reference Conterno, Turchi, Corrêa and Monteiro de Barros Almeida2020).
Clinically, ascariasis can be divided into 2 distinct phases in the human host because of its complex biological life cycle. The initial phase, known as larval or acute ascariasis, is caused by hepato-tracheal migration of the larval forms of the parasite in the first weeks of infection, characterized by a profound inflammatory response in the affected organs, mostly in the lungs, leading to diffuse lung disease as a consequence of the tissue damage provoked by the migrating larval stages (Weatherhead et al., Reference Weatherhead, Gazzinelli-Guimaraes, Knight, Fujiwara, Hotez, Bottazzi and Corry2020). When the migrating larval stages complete their quest for program development with the maturation into adult worms in the intestine, the second phase of human ascariasis initiates, which is characterized by a chronic and long-term infection (Crompton, Reference Crompton1985).
Although the chronic infection in most cases is associated with light to moderate burden, with nonspecific symptoms, human ascariasis is considered a worldwide public health problem due to the clinical complications observed in individuals with a high parasitic burden. The severe form of the disease is associated with abdominal distension, nausea, diarrhoea and can be fatal due to intestinal obstruction by adult worms (Chan, Reference Chan1997). Morbidity and mortality increase with the intensity of the disease (de Silva et al., Reference de Silva, Chan and Bundy1997). Moreover, in moderate infections, ascariasis is correlated with nutritional deficit, growth retardation and cognitive deficit (Dold and Holland, Reference Dold and Holland2011). Considering the limitation of data to quantify the complications of ascariasis, the estimated number of deaths worldwide in 2017 due to human ascariasis was 3206 (Vos et al., 2017), causing a global burden of 0.861 million years of disability-adjusted life years (DALYs) in 2017 (Kyu et al., 2018).
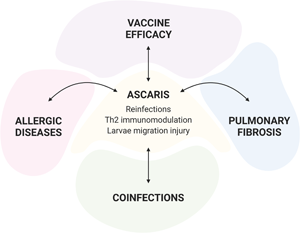
Moreover, due to its high prevalence and wide geographic distribution, a high rate of co-morbidities and co-infections associated with ascariasis is expected. In this review, we will provide insights about the immune response of Ascaris infection in the context of reinfection and co-morbidities such as lung fibrosis, allergic diseases, and co-infections.
Reinfections
Developing strategies to control the spread of Ascaris is a major challenge. The development of an integrated control strategy, consisting of preventive chemotherapy (PC), combined with health education and environmental sanitation is needed to interrupt transmission of STH (Jia et al., Reference Jia, Melville, Utzinger, King and Zhou2012). Even with the existence of highly effective drugs (Keiser and Utzinger, Reference Keiser and Utzinger2008; Jia et al., Reference Jia, Melville, Utzinger, King and Zhou2012; Moser et al., Reference Moser, Schindler and Keiser2017), the constant presence of infective parasite eggs in the environment guarantees reinfection months after treatment. Jia et al. (Reference Jia, Melville, Utzinger, King and Zhou2012), in a meta-analysis study, showed that the prevalence of ascariasis tended to regress to the pretreatment levels 12 months post-treatment. On top of that, after decades of scientific discussion, currently, it has been a consensus that A. suum, the etiological agent of the swine ascariasis, with a massive cosmopolitan distribution among pigs, are also capable to infect human, causing human ascariasis. Although zoonotic infection is a rare event, A. suum infection represents a risk for farmers and farming areas worldwide, creating another obstacle for the control and elimination programs (Nejsum et al., Reference Nejsum, Parker, Frydenberg, Roepstorff, Boes, Haque, Astrup, Prag and Skov Sørensen2005; Thamsborg et al., Reference Thamsborg, Nejsum and Mejer2013; Alves et al., Reference Alves, da S, Conceição and Leles2016).
As an initiative to eliminate morbidities caused by STH infections, especially in children, World Health Organization (WHO) launched in 2012 a strategic plan aimed to increase the coverage of PC from 15 to 75% of school-age children and preschoolers (WHO, 2011). With the impact of increased PC coverage, in 2015 STH control programs prevented the loss of >500 000 DALYs (Kassebaum et al., 2016). Therefore, the existence of a gap between PC and the prevention of reinfections, makes reinfection an extremely important phenomenon for ascariasis.
Despite its epidemiological importance, what is known about acute ascariasis was described based on experimental models, due to the difficulty of an early diagnosis in humans. Thus, in recent decades, a special focus has been given to the long-term ascariasis, with the assessment of immunological aspects of chronically infected individuals from endemic areas (McSharry et al., Reference McSharry, Xia, Holland and Kennedy1999; Cooper et al., Reference Cooper, Chico, Sandoval, Espinel, Guevara, Kennedy, Urban, Griffin and Nutman2000, Reference Cooper, Chico, Sandoval and Nutman2004; Geiger et al., Reference Geiger, Massara, Bethony, Soboslay, Carvalho and Corrêa-Oliveira2002; Jackson et al., Reference Jackson, Turner, Rentoul, Faulkner, Behnke, Hoyle, Grencis, Else, Kamgno, Boussinesq and Bradley2004). In this way, there are many gaps in the understanding of how initial infection factors (such as larval migration) may influence the development of the immune response and the induction of resistance/susceptibility to infection. Therefore, the understanding of the immunobiological aspects of larval ascariasis in primary infection and reinfection, makes it possible to understand the type of initial immune response necessary for infection control.
The characterization of the mechanisms developed by the parasite to evade the host's immune response contribute to the basic scientific knowledge necessary for the development of more effective immunoprophylactic strategies to interrupt the parasite's transmission cycle before it establishes chronicity in the host.
In this sense, the use of an animal model for the study of larval ascariasis has been shown to be efficient, especially in understanding the mechanisms of the immune response and pathophysiology after multiple exposures (Fig. 1). Initially, with the use of pigs as an experimental model, it was demonstrated that repeated infections with A. suum would generate resistance to new infections. This finding was evidenced by the reduction in the number of larvae found at intestinal, hepatic and pulmonary levels after reinfection, and with the reduction in the number of milk-spots in the liver in necropsy (Urban et al., Reference Urban, Alizadeh and Romanowski1988; Eriksen et al., Reference Eriksen, Nansen, Roepstorff, Lind and Nilsson1992; Nejsum et al., Reference Nejsum, Thamsborg, Petersen, Kringel, Fredholm and Roepstorff2009b). Afterwards, Eriksen et al. (Reference Eriksen, Nansen, Roepstorff, Lind and Nilsson1992) in an elegant paper demonstrated that the protective immune response was dose dependent, with the highest first inoculum leading to a lower final burden after reinfection. In addition, it was verified by analyzing the sizes of the worms in the small intestine, that the adult worms established themselves mainly from the first doses of inoculated eggs, giving rise to a patent infection, while the newly inoculated larvae were less successful (Eriksen et al., Reference Eriksen, Nansen, Roepstorff, Lind and Nilsson1992; Mejer and Roepstorff, Reference Mejer and Roepstorff2006; Nejsum et al., Reference Nejsum, Thamsborg, Petersen, Kringel, Fredholm and Roepstorff2009b).

Fig. 1. Ascaris reinfection. Figure compiles the data obtained from the different experimental model for 1 or multiple infections with A. suum. In the lungs, repeated infection leads to higher lung inflammation and cellularity with increase number and activation status of neutrophils, eosinophils, lymphocytes and macrophages. Nevertheless, reinfected animals display lower larvae burden, less haemorrhage and pulmonary impairment. Necropsy also evidenced a reduction in the number of milk spots in the liver from reinfected animals. In the intestine, reinfected animals show an increase in number and activation status of eosinophils, mastocytes and goblet cells with higher expression of IL-13 that leads to increased contractility and mucus secretion. All those factors culminate with lower L4/young adult's larvae. The prolonged infection has been shown to induce the generation of specific antibodies to the parasite, with a predominance of IgG1 and IgA, while recent primary infection generates polyreactive IgG1, IgG3 and IgE. The cytokine response also differs from single to reinfection models. With a predominance of a Th2/Th17 response in the latter compared to Th1/Th2 phenotype in primo-infected animals.
The protective phenotype in the reinfection of A. suum had been also previously demonstrated by Urban and collaborators in pigs inoculated orally with UV-irradiated eggs, leading to protection against infection (Urban and Tromba, Reference Urban and Tromba1984). Such findings also corroborate with studies that demonstrated that previous exposure to other helminths contributed to the reduction of the parasitic burden, of Strongyloides ratti (Dawkins and Grove, Reference Dawkins and Grove1982), of Neodiplostomum seoulensis (Yu et al., Reference Yu, Hong, Chai and Lee1995), Clonorchis sinensis (Sohn et al., Reference Sohn, Zhang, Choi and Hong2006), S. stercoralis (Rotman et al., Reference Rotman, Yutanawiboonchai, Brigandi, Leon, Gleich, Nolan, Schad and Abraham1996) and Trichuris suis (Nejsum et al., Reference Nejsum, Thamsborg, Petersen, Kringel, Fredholm and Roepstorff2009a).
The immune pathways responsible for controlling the parasitic burden of Ascaris are not fully understood. Prolonged helminth infections have been shown to induce the generation of specific antibodies to the parasite, with a predominance of IgG1 and IgA; on the other hand, recent primary infections generate polyreactive IgG and IgE antibodies (McCoy et al., Reference McCoy, Stoel, Stettler, Merky, Fink, Senn, Schaer, Massacand, Odermatt, Oettgen, Zinkernagel, Bos, Hengartner, Macpherson and Harris2008). And, although it is not clear, the passive transfer of immune serum, or IgG, showed that the humoral immune response plays an important role in resistance to Ascaris, contributing to the control of the parasitic burden in animals challenged after the transfer (Khoury et al., Reference Khoury, Stromberg and Soulsby1977; Gazzinelli-Guimarães et al., Reference Gazzinelli-Guimarães, Gazzinelli-Guimarães, Nogueira, Oliveira, Barbosa, Amorim, Cardoso, Kraemer, Caliari, Akamatsu, Ho, Jones, Weatherhead, Bottazzi, Hotez, Zhan, Bartholomeu, Russo, Bueno and Fujiwara2018). Interestingly, it was demonstrated in vitro that circulating eosinophils degranulated in direct contact with larvae, in the presence of serum from reinfected animals, which means that specific antibodies and complement components can contribute to protection via eosinophils (Masure et al., Reference Masure, Vlaminck, Wang, Chiers, Van den Broeck, Vercruysse and Geldhof2013). In addition to IgG, animals infected with A. suum have high levels of specific IgA, which contributes to the control of the weight and size of parasites in the intestinal lumen (Marbella and Gaafar, Reference Marbella and Gaafar1989; Kringel et al., Reference Kringel, Thamsborg, Petersen, Göring, Skallerup and Nejsum2015).
Several studies suggested that the immune response triggered in the mucosal tissue contribute to control Ascaris larvae migration; with an increase in the number and activation status of eosinophils in lung tissue as well as in the intestine, suggesting an important role of eosinophils in reducing Ascaris burden (Masure et al., Reference Masure, Vlaminck, Wang, Chiers, Van den Broeck, Vercruysse and Geldhof2013; Nogueira et al., Reference Nogueira, Gazzinelli-Guimarães, Barbosa, Resende, Silva, de Oliveira, Amorim, Oliveira, Mattos, Kraemer, Caliari, Gaze, Bueno, Russo and Fujiwara2016; Gazzinelli-Guimaraes et al., Reference Gazzinelli-Guimaraes, De Queiroz Prado, Ricciardi, Bonne-Année, Sciurba, Karmele, Fujiwara and Nutman2019). Masure et al. (Reference Masure, Vlaminck, Wang, Chiers, Van den Broeck, Vercruysse and Geldhof2013), demonstrated that recruitment of eosinophils to the caecum of reinfected animals was further supported by increased levels of interleukin-5 (IL-5), interleukin-13 (IL-13), C-C motif chemokine ligand 11 (CCL11) and eosinophil peroxidase transcripts in the cecal mucosa of reinfected swine. In the same work, using a porcine model demonstrated that the reduction in the number of larvae in reinfected animals was associated with eosinophilia, mastocytosis and hyperplasia of goblet cells in the cecum (Masure et al., Reference Masure, Vlaminck, Wang, Chiers, Van den Broeck, Vercruysse and Geldhof2013).
Recently, using the murine model, it has been demonstrated that A. suum reinfected mice showed an important and significant reduction in parasitic burden at the pulmonary level (Nogueira et al., Reference Nogueira, Gazzinelli-Guimarães, Barbosa, Resende, Silva, de Oliveira, Amorim, Oliveira, Mattos, Kraemer, Caliari, Gaze, Bueno, Russo and Fujiwara2016), however, reinfected mice have greater tissue damage. After multiple exposures to A. suum, Nogueira et al. (Reference Nogueira, Gazzinelli-Guimarães, Barbosa, Resende, Silva, de Oliveira, Amorim, Oliveira, Mattos, Kraemer, Caliari, Gaze, Bueno, Russo and Fujiwara2016) observed a significant increase in lung tissue and airways cellularity, characterized by an increase in lymphocytes and macrophages, in addition to a marked eosinophilic and neutrophilic inflammation of the lungs. In addition, it was observed that induction of a mixed Th2/Th17 systemic response defined by elevated levels of IL-4, IL-5, IL-10, IL-6, tumor necrosis factor (TNF) and IL-17A compared to single-infected mice. Similar to what was observed in Toxocara canis infection (Resende et al., Reference Resende, Gazzinelli-Guimarães, Barbosa, Oliveira, Nogueira, Gazzinelli-Guimarães, Gonçalves, Amorim, Oliveira, Caliari, Rachid, Volpato, Bueno, Geiger and Fujiwara2015).
The presence of eosinophils can also be implicated in tissue repair and remodelling due to the extensive mechanical injury and haemorrhage associated with larvae migration (Isobe et al., Reference Isobe, Kato and Arita2012). It was also highlighted that reinfection induced a larger area of lung injury when compared to primary infection, also suggesting that multiple exposures can lead to repeated tissue injury and chronic inflammation (Nogueira et al., Reference Nogueira, Gazzinelli-Guimarães, Barbosa, Resende, Silva, de Oliveira, Amorim, Oliveira, Mattos, Kraemer, Caliari, Gaze, Bueno, Russo and Fujiwara2016). The contribution of the inflammatory response in the control of parasitic burden has been evidenced in models of susceptibility and resistance to single infection by A. suum (Lewis et al., Reference Lewis, Behnke, Cassidy, Stafford, Murray and Holland2007; Dold et al., Reference Dold, Cassidy, Stafford, Behnke and Holland2010). Hepatic inflammation has been associated with infection control seen in animals resistant to A. suum infection (Dold et al., Reference Dold, Cassidy, Stafford, Behnke and Holland2010). Pulmonary inflammation, on the other hand, was considered important for the control of A. suum infection, but directly associated with tissue repair induced by larval migration (Lewis et al., Reference Lewis, Behnke, Cassidy, Stafford, Murray and Holland2007).
In general, previous studies have shown that the mechanism of protection against helminth reinfection is mediated by an eosinophil dominated-type 2 immune response, and susceptibility is associated with the Th1 immunity (Gause et al., Reference Gause, Urban and Stadecker2003; Hayes et al., Reference Hayes, Bancroft and Grencis2007). During experimental Ascaris reinfection, elevated levels of IL-4 and IL-10 in serum (Nogueira et al., Reference Nogueira, Gazzinelli-Guimarães, Barbosa, Resende, Silva, de Oliveira, Amorim, Oliveira, Mattos, Kraemer, Caliari, Gaze, Bueno, Russo and Fujiwara2016) was also observed. The combination of these 2 cytokines was crucial for the control of the damage caused by Nippostrongylus braziliensis larvae migration through the host's organs (Chen et al., Reference Chen, Liu, Wu, Rozo, Bowdridge, Millman, Van Rooijen, Urban, Wynn and Gause2012). However, the findings by Nogueira et al. (Reference Nogueira, Gazzinelli-Guimarães, Barbosa, Resende, Silva, de Oliveira, Amorim, Oliveira, Mattos, Kraemer, Caliari, Gaze, Bueno, Russo and Fujiwara2016), demonstrated that the protection driven by reinfection was associated with a mixed Th2/Th17 response pattern (). In fact, the increase in IL-17A levels after multiple exposures to A. suum may reflect intense and chronic inflammation, analogous to the pulmonary fibrosis model (Wilson et al., Reference Wilson, Madala, Ramalingam, Gochuico, Rosas, Cheever and Wynn2010).
The pulmonary physiological changes observed in A. suum infected mice, such as loss of lung volume, airway flow and elasticity, were observed due to intense parenchymal injury, haemorrhage and edema (Nogueira et al., Reference Nogueira, Gazzinelli-Guimarães, Barbosa, Resende, Silva, de Oliveira, Amorim, Oliveira, Mattos, Kraemer, Caliari, Gaze, Bueno, Russo and Fujiwara2016). After multiple exposures, the persistence of physiological modulation and chronic lung parenchymal injury, associated with intense eosinophilia, were consistent with human ascariasis associated with Loeffler syndrome/eosinophilic pneumonitis (Nutman, Reference Nutman2007; Kunst et al., Reference Kunst, Mack, Kon, Banerjee, Chiodini and Grant2011). Such findings demonstrate that collagen deposition and fibrogenesis is a cumulative effect of larval ascariasis, and together with the persistence of eosinophils in the tissue, those factors can lead to restrictive lung disease. It is worth mentioning, acute injury to the lung parenchyma caused by the migration of the larvae, detected mainly in simple infections, and tissue remodelling by fibrogenesis, detected in prolonged simple and multiple infections, can lead to increased pulmonary resistance, and decrease complacence, accentuated in single infected animals (Nogueira, et al., Reference Nogueira, Gazzinelli-Guimarães, Barbosa, Resende, Silva, de Oliveira, Amorim, Oliveira, Mattos, Kraemer, Caliari, Gaze, Bueno, Russo and Fujiwara2016). The fibrogenesis caused due to tissue healing induced by the Th2/Th17 immune response and already proven in the larval ascariasis model (Oliveira et al., Reference Oliveira, Hemanoel da Paixão Matias, Kraemer, Clara Gazzinelli-Guimarães, Vieira Santos, Cássia Oliveira Amorim, Silva Nogueira, Simões Freitas, Vidigal Caliari, Castanheira Bartholomeu, Lacerda Bueno, Castro Russo and Toshio Fujiwara2019).
Ascaris comorbidities
Pulmonary fibrosis
Pulmonary fibrosis is a chronic and progressive lung condition caused by excessive collagen deposition in the lung parenchyma that can severely disrupt lung function and contribute to the development of lethal fibrotic pathology (Wilson and Wynn, Reference Wilson and Wynn2009; Gause et al., Reference Gause, Wynn and Allen2013). After acute injury or infection, resolution of inflammation is important to restore normal tissue architecture. However, the healing process can become pathogenic when important checkpoints are missed and chronic inflammation can result in scar tissue formation (Wynn, Reference Wynn2004; Wilson and Wynn, Reference Wilson and Wynn2009).
Cytokines such as ransforming growth factor beta 1 (TGF-β1), IL-1β, IL-8 and IL-17A have already been recognized and is well characterized as important mediators in the development process of pulmonary fibrosis, inducing fibroblast proliferation and consequent type I collagen deposition (Russo et al., Reference Russo, Guabiraba, Garcia, Barcelos, Roffê, Souza, Amaral, Cisalpino, Cassali, Doni, Bertini and Teixeira2009, Reference Russo, Alessandri, Garcia, Cordeiro, Pinho, Cassali, Proudfoot and Teixeira2011; Wilson et al., Reference Wilson, Madala, Ramalingam, Gochuico, Rosas, Cheever and Wynn2010). In addition, contributing to collagen deposition, type 2 responses are linked to fibrogenic processes of tissue regeneration and repair following injury. Several studies have shown the involvement of cytokines IL-4, IL-5 and IL-13 in the resolution of inflammation, with the participation of macrophages, eosinophils, mast cells, basophils, T helper type 2 cells and type 2 innate lymphoid cells. In response to persistent chronic insults and injury, the wound healing process can become pathogenic (Van Dyken and Locksley, Reference Van Dyken and Locksley2013; Guo et al., Reference Guo, Huang, Chen, Hu-Li, Urban and Paul2015; Minutti et al., Reference Minutti, Knipper, Allen and Zaiss2017; Gieseck et al., Reference Gieseck, Wilson and Wynn2018). The participation of these cells in type 2 fibrosis defines whether type 2 response results in a beneficial tissue repair or fibrogenic process with associated pathology. ILC2 and Th2 cells promote fibrosis contributing to the local secretion of type 2 cytokines such as IL-4, IL-5 and IL-13, which support cell recruitment and activation. Eosinophils can be important promoters of inflammatory and tissue damage, releasing type 2 cytokines and TGFβ1, a potent profibrotic eosinophil secretory cytokine that stimulates fibroblasts to promote the synthesis and direct deposition of many extracellular matrix proteins (Raghow, Reference Raghow1991; Rosenberg et al., Reference Rosenberg, Dyer and Foster2013; Aceves, Reference Aceves2014). Alternatively, activated macrophages are substantial to regulate the initiation, maintenance, and resolution of inflammation, contributing to repair the following injury by clearance of matrix and cell debris along with the production of cytokines, growth and angiogenic factors that promote fibroproliferation and angiogenesis (Leibovich and Ross, Reference Leibovich and Ross1976; Martin and Leibovich, Reference Martin and Leibovich2005; Gieseck et al., Reference Gieseck, Wilson and Wynn2018).
Chronic helminth infections are considered potent inducers of type 2 immunity, which is the main protective immune response against helminth parasites, important for worm expulsion and to regulate tissue repair that are frequently related to fibroproliferative response during chronic stages of disease (Van Dyken and Locksley, Reference Van Dyken and Locksley2013; Gieseck et al., Reference Gieseck, Wilson and Wynn2018). Studies have shown that IL-13 is crucial for lung and liver fibrosis induction in schistosomiasis. An IL-13 inhibitor was able to block the development of liver fibrosis in a murine model of schistosomiasis (Chiaramonte et al., Reference Chiaramonte, Donaldson, Cheever and Wynn1999). In another study, IL-13 receptor α1 (IL-13Rα1) – deficient mice infected with Schistosoma mansoni showed an increased survival rate due to fibrosis suppression (Ramalingam et al., Reference Ramalingam, Pesce, Sheikh, Cheever, Mentink-Kane, Wilson, Stevens, Valenzuela, Murphy, Yancopoulos, Urban, Donnelly and Wynn2008).
Experimental infections by Ascaris have contributed to the understanding of the elements involved in the immune response, inflammatory process, and pathogenesis of ascariasis, especially in the acute phase. However, there are few studies investigating the relationship of Ascaris sp. infection with the process of developing fibrosis in affected organs after larval migration. Studies reported by our group, as previously mentioned, demonstrate in a murine experimental model that in the larval migration phase, there is a polarization of the immune response in ascariasis, with a mixed profile of Th2/Th17 cytokines (Gazzinelli-Guimarães et al., Reference Gazzinelli-Guimarães, Gazzinelli-Guimarães, Silva, Mati, de Dhom-Lemos, Barbosa, Passos, Gaze, Carneiro, Bartholomeu, Bueno and Fujiwara2013, Reference Gazzinelli-Guimarães, Gazzinelli-Guimarães, Nogueira, Oliveira, Barbosa, Amorim, Cardoso, Kraemer, Caliari, Akamatsu, Ho, Jones, Weatherhead, Bottazzi, Hotez, Zhan, Bartholomeu, Russo, Bueno and Fujiwara2018; Nogueira et al., Reference Nogueira, Gazzinelli-Guimarães, Barbosa, Resende, Silva, de Oliveira, Amorim, Oliveira, Mattos, Kraemer, Caliari, Gaze, Bueno, Russo and Fujiwara2016; Oliveira et al., Reference Oliveira, Hemanoel da Paixão Matias, Kraemer, Clara Gazzinelli-Guimarães, Vieira Santos, Cássia Oliveira Amorim, Silva Nogueira, Simões Freitas, Vidigal Caliari, Castanheira Bartholomeu, Lacerda Bueno, Castro Russo and Toshio Fujiwara2019). Notably, most of the cytokines present in Ascaris infection are known to contribute effectively to the production and deposition of collagen (IL-17A and TGF-β1) (Leask and Abraham, Reference Leask and Abraham2004; Wilson et al., Reference Wilson, Madala, Ramalingam, Gochuico, Rosas, Cheever and Wynn2010; Gieseck et al., Reference Gieseck, Wilson and Wynn2018) or to have a fibrogenic potential (IL-4, IL-6, IL-13 and IL-33), contributing indirectly for the fibrosis development (Gharaee-Kermani et al., Reference Gharaee-Kermani, Nozaki, Hatano and Phan2001; Saito et al., Reference Saito, Tasaka, Inoue, Miyamoto, Nakano, Ogawa, Yamada, Shiraishi, Hasegawa, Fujishima, Takano and Ishizaka2008; Wilson et al., Reference Wilson, Madala, Ramalingam, Gochuico, Rosas, Cheever and Wynn2010; Gause et al., Reference Gause, Wynn and Allen2013; Li et al., Reference Li, Guabiraba, Besnard, Komai-Koma, Jabir, Zhang, Graham, Kurowska-Stolarska, Liew, McSharry and Xu2014; Gieseck et al., Reference Gieseck, Wilson and Wynn2018).
Indeed in A. suum infection, it was reported collagen deposition around the lower airways, blood vessels and in the alveolar wall as a result of injuries caused by larval migration, followed by tissue remodelling (Nogueira et al., Reference Nogueira, Gazzinelli-Guimarães, Barbosa, Resende, Silva, de Oliveira, Amorim, Oliveira, Mattos, Kraemer, Caliari, Gaze, Bueno, Russo and Fujiwara2016; Gazzinelli-Guimarães et al., Reference Gazzinelli-Guimarães, Gazzinelli-Guimarães, Nogueira, Oliveira, Barbosa, Amorim, Cardoso, Kraemer, Caliari, Akamatsu, Ho, Jones, Weatherhead, Bottazzi, Hotez, Zhan, Bartholomeu, Russo, Bueno and Fujiwara2018; Oliveira et al., Reference Oliveira, Hemanoel da Paixão Matias, Kraemer, Clara Gazzinelli-Guimarães, Vieira Santos, Cássia Oliveira Amorim, Silva Nogueira, Simões Freitas, Vidigal Caliari, Castanheira Bartholomeu, Lacerda Bueno, Castro Russo and Toshio Fujiwara2019). These findings suggest that ascariasis could predispose or contribute to the worsening of the progressive development of pulmonary fibrosis.
Recently, using the bleomycin experimental model of pulmonary fibrosis (Benítez, Reference Benítez2006; Walters and Kleeberger, Reference Walters and Kleeberger2008; Liu et al., Reference Liu, De Los Santos and Phan2017) and Ascariasis (Gazzinelli-Guimarães et al., Reference Gazzinelli-Guimarães, Gazzinelli-Guimarães, Silva, Mati, de Dhom-Lemos, Barbosa, Passos, Gaze, Carneiro, Bartholomeu, Bueno and Fujiwara2013), our group shed light on the comorbidity environment generated by these 2 pathologies. In this study, it was observed that the co-existence of lung fibrosis and Ascaris infection led to the exacerbation of lung damage, evidenced by a loss of pulmonary physiological parameters that were related to the increase in exudative phenomena and haemorrhage induced by larval migration (Oliveira et al., Reference Oliveira, Hemanoel da Paixão Matias, Kraemer, Clara Gazzinelli-Guimarães, Vieira Santos, Cássia Oliveira Amorim, Silva Nogueira, Simões Freitas, Vidigal Caliari, Castanheira Bartholomeu, Lacerda Bueno, Castro Russo and Toshio Fujiwara2019). Although the comorbidity directed the immune response to a profibrogenic profile with increased cytokines IL-1, IL-4, IL-6, IL-13, and IL-33, the study did not observe collagen deposition alteration or change in the levels of IL-17A and TGF-β1 expression. In addition, the previous fibrosis in the pulmonary parenchyma, around airways, blood vessels and thickening of alveolar septa, did not impair the larval migration, which was carried out in preserved areas of the lung parenchyma (Fig. 2). (Oliveira et al., Reference Oliveira, Hemanoel da Paixão Matias, Kraemer, Clara Gazzinelli-Guimarães, Vieira Santos, Cássia Oliveira Amorim, Silva Nogueira, Simões Freitas, Vidigal Caliari, Castanheira Bartholomeu, Lacerda Bueno, Castro Russo and Toshio Fujiwara2019). There is a clear need for further studies addressing comorbidities produced by helminth infections and pulmonary fibrosis, considering different association times to elucidate and a better understanding of immunopathological changes, which directly affect the progression of the diseases involved.

Fig. 2. Pulmonary fibrosis. The association of previous pulmonary fibrosis and A. suum infection increases lung damage, characterized by an increase in exudative phenomena and haemorrhage induced by larval migration. The presence of A. suum in pulmonary parenchyma that already present fibrosis increased the expression of IL-1, IL-4, IL-6, IL-13, IL-17A, IL-33 and TGF-β1 cytokines. Despite the increase in the expression of cytokines with a profibrogenic profile during comorbidity, this did not contribute to the worsening of lung fibrosis. Larval migration is not impaired by pulmonary fibrosis. The success of the loss cycle in comorbidity occurs due to A. suum larvae migration non-fibrous areas in the lung parenchyma.
Pulmonary allergic inflammation
The comorbidities associated with Ascariasis and other helminth infections can be triggered by 2 important features of their parasites' biologies, including the progressive larval development in the host – characterized by a transient larval migration through organs and tissues – and their molecular and structural similarities with other pathogens or agents, including common environmental allergens. Over the years, many studies have examined the interface between allergic diseases and helminth infections (Van Den Biggelaar et al., Reference Van Den Biggelaar, Lopuhaa, Van Ree, Van Der Zee, Jans, Hoek, Migombet, Borrmann, Luckner, Kremsner and and Yazdanbakhsh2001; Daniłowicz-Luebert et al., Reference Daniłowicz-Luebert, O'Regan, Steinfelder and Hartmann2011; Gazzinelli-Guimarães et al., Reference Gazzinelli-Guimarães, Bonne-Année, Fujiwara, Santiago and Nutman2016). These largely immunoepidemiologic studies associate chronic helminth infections with the modulation of allergic responses through the induction of IL-10, expansion of regulatory T cells, and blockade of IgG4 antibodies (Van Den Biggelaar et al., Reference Van Den Biggelaar, Van Ree, Rodrigues, Lell, Deelder, Kremsner and Yazdanbakhsh2000; Satoguina et al., Reference Satoguina, Weyand, Larbi and Hoerauf2005; Yazdanbakhsh and Wahyuni, Reference Yazdanbakhsh and Wahyuni2005). On the other hand, there are studies in humans and in experimental models which demonstrate that helminth infections are associated with increased allergenicity. Notably, the allergy-like reactions and syndromes seen in helminth-infected patients (e.g. urticaria in Strongyloides infection (Zubrinich et al., Reference Zubrinich, Puy, O'Hehir and Hew2019); angioedema and tropical pulmonary eosinophilia in filarial infections (Van Dellen, Reference Van Dellen1985; Ottesen and Nutman, Reference Ottesen and Nutman1992; Rakita et al., Reference Rakita, White and Kielhofner1993); atopic dermatitis and asthma-like syndrome in ascariasis (Caraballo et al., Reference Caraballo, Acevedo and Buendía2015; Qualizza et al., Reference Qualizza, Losappio and Furci2018); and swimmer's itch in schistosomiasis (Kolářová et al., Reference Kolářová, Skirnisson and Horák1999)) have been associated with the acute stages of the infections. These allergic-type inflammatory responses are normally a consequence of the helminth-driven, dominant type-2 immune response orchestrated by the host as an attempt to kill or expel these early stages of parasites (Cruz et al., Reference Cruz, Cooper, Figueiredo, Alcantara-Neves, Rodrigues and Barreto2017). Notably, A. lumbricoides infection has been implicated in inducing asthma and wheezing (Leonardi-Bee et al., Reference Leonardi-Bee, Pritchard and Britton2006), while other murine studies have indicated that the pre-sensitization to Ascaris antigens triggers mite-specific IgE responses upon subsequent mite antigen inhalation (Suzuki et al., Reference Suzuki, Hara, Ichikawa, Kamijo, Nakazawa, Hatanaka, Akiyama, Ogawa, Okumura and Takai2016).
Ascaris parasites have transient life cycles in which migration through the lung tissue is a necessary step for development in the host. The migrating lung-stage larvae lead to diffuse lung infiltrates (Gazzinelli-Guimarães et al., Reference Gazzinelli-Guimarães, Gazzinelli-Guimarães, Silva, Mati, de Dhom-Lemos, Barbosa, Passos, Gaze, Carneiro, Bartholomeu, Bueno and Fujiwara2013; Weatherhead et al., Reference Weatherhead, Gazzinelli-Guimaraes, Knight, Fujiwara, Hotez, Bottazzi and Corry2020) and eosinophilic pneumonia, termed Löeffler's Syndrome (Gelpi and Mustafa, Reference Gelpi and Mustafa1968).Gazzinelli-Guimaraes et al. (Reference Gazzinelli-Guimaraes, De Queiroz Prado, Ricciardi, Bonne-Année, Sciurba, Karmele, Fujiwara and Nutman2019) demonstrated by using a controlled model of multiple timepoints of early Ascaris infection that in primary exposure to Ascaris, the L3-stage larvae migrate to the lung parenchyma in their quest to reach the airways, leading to a marked influx of neutrophils and associated levels of IL-6, and penetrate into the alveolar spaces, causing bleeding and mechanical damage in the organ. These migrating Ascaris larvae, while growing in size towards the L4 stage of development (Douvres et al., Reference Douvres, Tromba and Malakatis1969), induce a tissue inflammatory type 2 immune response in the lungs characterized by increased IL-5 levels followed by the production of IL-4 and IL-13, culminating in the differentiation of M2 macrophages and eosinophilia in the tissue. Very similar to a severe allergic airway disease, this diffuse lung inflammation induced by Ascaris infection is characterized by an eosinophil-dominated type-2 immune response and manifests clinically as coughing, wheezing and in severe cases, respiratory failure (Aleksandra et al., Reference Aleksandra, Barbara, Natalia, Danuta, Renata and Ewa2016). Notwithstanding, Weatherhead et al. (Reference Weatherhead, Porter, Coffey, Haydel, Versteeg, Zhan, Guimarães, Fujiwara, Jaramillo, Bottazzi, Hotez, Corry and Beaumiera2018) using a murine model, demonstrated that Ascaris larval migration in the lung tissue induces significant pulmonary damage, including airway hyperresponsiveness and type 2 inflammatory lung pathology resembling an extreme form of allergic airway disease. These authors and others, given similar clinical features, support the idea that ascariasis may be an important cause of allergic airway disease in regions of high endemicity. Moreover, immunoepidemiologic studies suggest that children with Ascaris-induced allergic airway disease have increased cross-reactivity to bystander antigens such as house dust mites (HDM) (Acevedo and Caraballo, Reference Acevedo and Caraballo2011).
Many studies have shown that helminth infection promotes type 2-associated immune polarization that converges into IgE antibody production (Jarrett and Bazin, Reference Jarrett and Bazin1974; Urban, Reference Urban1982). As a result of the structural similarities of helminth and common allergen B-cell epitopes, helminth antigens eventually drive allergen-specific cross-reactive IgE antibodies (Sereda et al., Reference Sereda, Hartmann and Lucius2008; Acevedo et al., Reference Acevedo, Sánchez, Erler, Mercado, Briza, Kennedy, Fernandez, Gutierrez, Chua, Cheong, Jiménez, Puerta and Caraballo2009; da Costa Santiago and Nutman, Reference da Costa Santiago and Nutman2016). Indeed, it has been shown that the allergic sensitization with environmental common allergens, including HDM, drives cross-reactive immune responses to homologous helminth proteins (Santiago et al., Reference Santiago, Bennuru, Boyd, Eberhard and Nutman2011; Da Costa Santiago et al., Reference da Costa Santiago, Ribeiro-Gomes, Bennuru and Nutman2015). Several molecules from common aeroallergens, including those from the dust mites Dermatophagoides pteronyssinus (Der p 1, Der p 8 and Der p 10) have been characterized as having significant IgE cross-reactivity with helminth proteins from a variety of parasites including Ascaris (Santos et al., Reference Santos, Rocha, Oliver, Ferriani, Lima, Palma, Sales, Aalberse, Chapman and Arruda2008; Acevedo et al., Reference Acevedo, Sánchez, Erler, Mercado, Briza, Kennedy, Fernandez, Gutierrez, Chua, Cheong, Jiménez, Puerta and Caraballo2009; Santiago et al., Reference Santiago, Bennuru, Boyd, Eberhard and Nutman2011). A recent study investigating the interface between Ascaris infection and pulmonary allergic inflammation induced by HDM identified the Ascaris larval antigens recognized by HDM-specific antibodies as Ascaris tropomyosin and enolase based on high sequence and structural similarity to HDM homologs (Gazzinelli-Guimarães et al., Reference Gazzinelli-Guimarães, Bennuru, de Queiroz Prado, Ricciardi, Sciurba, Kupritz, Moser, Kamenyeva and Nutman2021). In this study, it was shown that HDM-triggered IgE cross-reactive antibodies were functional as they mediated hypersensitivity responses in skin testing. Moreover, helminth tropomyosin was capable of inducing a severe type-2-associated pulmonary inflammation following sensitization with the homologous HDM tropomyosin (Der p 10), indicating a potential mechanism to understand how helminth infections induce or exacerbate allergic inflammation.
The immunobiology of Ascaris co-infections
Individuals are continuously exposed to multiple pathogens with co-infections being extremely likely during life. Nevertheless, there is still poor knowledge about how combinations of co-infections may impact our immune response and can influence disease outcomes. Generally, in infections that affect humans, the prevalence of parasites in each region depends not only on the climate or the presence of susceptible hosts but also on the social, political and economic conditions of the population that can favour the spread and perpetuation of diseases. That explains how certain regions tend to be more affected by parasites (Murray et al., Reference Murray, Preston, Allen, Zambrana-Torrelio, Hosseini and Daszak2015; Osakunor et al., Reference Osakunor, Sengeh and Mutapi2018).
Therefore, there are several studies that aim to evaluate the mechanisms of interaction among different pathogens and their implication in the outcome of diseases (Salgame et al., Reference Salgame, Yap and Gause2013). However, it is worth mentioning the importance of helminth infections, in the context of coinfections, since helminth infections are present in all tropical regions of the planet, and may influence the course, diagnosis, and treatment of various infections by different pathogens. As mentioned before, among the NTDs commonly encountered, ascariasis is by far the most prevalent (Bethony et al., Reference Bethony, Brooker, Albonico, Geiger, Loukas, Diemert and Hotez2006; Brooker, Reference Brooker2010).
Since helminths stimulate Th2 type of response, many researchers have focused on the investigation of the relationship between helminths and pathogens that require a Th1 immune response, such as Mycobacterium tuberculosis (MTB). Helminth infections appear to indirectly affect the diagnosis of tuberculosis by modulating the immune response of MTB antigens (Babu and Nutman, Reference Babu and Nutman2016). In South Africa, children with IgE antibodies against Ascaris are less likely to mount a positive response to the tuberculin skin test (Gebreegziabiher et al., Reference Gebreegziabiher, Desta, Howe and Abebe2014).
Patients with tuberculosis and infected with S. mansoni had lower sputum bacterial loads (Mhimbira et al., Reference Mhimbira, Hella, Said, Kamwela, Sasamalo, Maroa, Chiryamkubi, Mhalu, Schindler, Reither, Knopp, Utzinger, Gagneux and Fenner2017). In agreement with this, Mycobacterium bovis bacterial loads are also decreased in cattle co-infected with Fasciola hepatica (Garza-Cuartero et al., Reference Garza-Cuartero, O'Sullivan, Blanco, McNair, Welsh, Flynn, Williams, Diggle, Cassidy and Mulcahy2016). A study with Brazilian patients showed that coinfection with Ascaris and MTB does not alter the clinical evolution of pulmonary tuberculosis, though it may influence the severity of pulmonary lesions. The coinfection also did not influence the Th1, Th2 and Th17 responses or the percentage of innate and adaptative cell subpopulations (Santos et al., Reference Santos, Bührer-Sékula, Melo, Cordeiro-Santos, Pimentel, Gomes-Silva, Costa, Saraceni, Da-Cruz and Lacerda2019). In a recent article, the exposure to A. lumbricoides proteins induced an enhanced capacity to control MTB growth in human monocyte-derived macrophages but the minimal effect in human PBMCs (Togarsimalemath et al., Reference Togarsimalemath, Pushpamithran, Schön, Stendahl and Blomgran2020).
Another well-studied interaction is the co-infection between Ascaris and Plasmodium. Literature suggests that there is a biological association between Plasmodium and helminths when they coexist in a host (Mwangi et al., Reference Mwangi, Bethony and Brooker2006; Nacher, Reference Nacher2011; Degarege and Erko, Reference Degarege and Erko2016). Studies as early as 1978 indicated that anti-helminth treatment of ascariasis in a high-transmission area was followed by an increase in symptomatic malaria (Murray et al., Reference Murray, Murray, Murray and Murray1978; Spiegel et al., Reference Spiegel, Tall, Raphenon, Trape and Druilhe2003). Conversely, other studies showed a beneficial effect in co-infections between helminths and Plasmodium (Tshikuka et al., Reference Tshikuka, Scott, Gray-Donald and Kalumba1996; Nacher et al., Reference Nacher, Singhasivanon, Yimsamran, Manibunyong, Thanyavanich, Wuthisen and Looareesuwan2002; Lyke et al., Reference Lyke, Dabo, Sangare, Arama, Daou, Diarra, Plowe, Doumbo and Sztein2006). Ascaris infection has been associated with protection from cerebral malaria in Thailand (Nacher et al., Reference Nacher, Gay, Singhasivanon, Krudsood, Treeprasertsuk, Mazier, Vouldoukis and Looareesuwan2000) and with the occurrence of severe malaria attack in a case-control study in Senegal (Le Hesran et al., Reference Le Hesran, Akiana, Ndiaye, Dia, Senghor and Konate2004). It was suggested that helminth infection might reduce the number and frequency of mature schizonts and therefore reducing severe malaria, although the mechanisms are yet not understood (Nacher et al., Reference Nacher, Singhasivanon, Silachamroon, Treeprasertsuk, Vannaphan, Traore, Gay and Looareesuwan2001). A randomized controlled trial was performed in Madagascar. The study showed a negative interaction between Ascaris and Plasmodium in children older than 5 years of age, with an increase in Plasmodium density after anti-helminthic treatment (Brutus et al., Reference Brutus, Watier, Briand, Hanitrasoamampionona, Razanatsoarilala and Cot2006). Of note, although Ascaris and Plasmodium were the predominant parasites, the presence of other helminths such as S. mansoni or hookworms may have interfered in the results observed.
Another possible consequence of the immune alterations driven by helminth infection is based on the impact of vaccines. The protective efficacy of BCG against pulmonary tuberculosis presents great variation around the globe, ranging from 80% in the United Kingdom to 0–50% in countries where helminths are often endemic (Roy et al., Reference Roy, Eisenhut, Harris, Rodrigues, Sridhar, Habermann, Snell, Mangtani, Adetifa, Lalvani and Abubakar2014). The lower cellular immune response against MTB antigens in individuals co-infected with helminths, together with the skew to a Th2 phenotype may explain, at least in parts, the effect of helminth infection on BCG efficacy (Elias et al., Reference Elias, Wolday, Akuffo, Petros, Bronner and Britton2001, Reference Elias, Britton, Aseffa, Engers and Akuffo2008). A similar effect could play a role in a malaria vaccine, by affecting the induction of an efficient Th1 immune response (Hartgers and Yazdanbakhsh, Reference Hartgers and Yazdanbakhsh2006). Recently, using a model of Litomosoides sigmodontis-infected mice it was demonstrated that concurrent helminth infection reduces the efficacy of vaccination against seasonal influenza and H1N1 influenza A virus. Importantly, the impaired response was also observed after helminth clearance. The suppression of vaccination efficacy was mediated by sustained IL-10 levels and abrogated by IL-10 receptor blockade (Hartmann et al., Reference Hartmann, Brunn, Stetter, Gagliani, Muscate, Stanelle-Bertram, Gabriel and Breloer2019). In a similar way, A. suum infection negatively affected the protection after Mycoplasma hyopneumoniae in pigs. Infected animals display a higher percentage of lung pathology after challenge and lower sero-conversion after vaccination. The effect was mediated by a skewed Th2 response induced by A. suum infection (Steenhard et al., Reference Steenhard, Jungersen, Kokotovic, Beshah, Dawson, Urban, Roepstorff and Thamsborg2009).
In addition to MTB and malaria, it has been hypothesized that helminth infections may increase susceptibility to the human immunodeficiency virus (HIV). Infection with Ascaris, S. mansoni and Trichuris were linked to increased frequencies of activated HLA-DR + T cells and a potential higher risk of HIV-1 transmission in patients from Tanzania (Chachage et al., Reference Chachage, Podola, Clowes, Nsojo, Bauer, Mgaya, Kowour, Froeschl, Maboko, Hoelscher, Saathoff and Geldmacher2014). HIV + patients with high Ascaris IgE display high viral loads and lower CD4 + counts compared with the HIV negative group in South Africa (Mkhize-Kwitshana et al., Reference Mkhize-Kwitshana, Taylor, Jooste, Mabaso and Walzl2011). Furthermore, a randomized, double blind, placebo-controlled trial conducted in Kenya showed that treatment of A. lumbricoides with albendazole in HIV co-infected adults resulted in significantly increased CD4 counts and may potentially reduce plasma HIV-1 RNA viral load (Walson et al., Reference Walson, Otieno, Mbuchi, Richardson, Lohman-Payne, MacHaria, Overbaugh, Berkley, Sanders, Chung and John-Stewart2008). Similar results were obtained in Ascaris/HIV co-infected patients from Southern Ethiopia (Abossie and Petros, Reference Abossie and Petros2015). Albendazole treatment in co-infected patients was associated with decrease IL-10 plasma levels (Blish et al., Reference Blish, Sangaré, Herrin, Richardson, John-Stewart and Walson2010). In another study, egg excretion and/or Ascaris specific IgE was associated with lower proliferative capacity and reduced Th1 cytokines in HIV-1 + patients (Mkhize-Kwitshana et al., Reference Mkhize-Kwitshana, Mabaso and Walzl2014). Although promising, more studies are needed to better clarify the importance of deworming in HIV progression. Our group has demonstrated that concomitant infection with Ascaris and Vaccinia virus (VACV) in experimental model induces a reduction in interferon gamma (INFγ) produced by CD4 + T cells and a robust pulmonary inflammation that were associated with increased morbidity/mortality in the coinfected compared to single infected mice (Gazzinelli-Guimarães et al., Reference Gazzinelli-Guimarães, de Freitas, Gazzinelli-Guimarães, Coelho, Barbosa, Nogueira, Amorim, de Dhom-Lemos, Oliveira, da Silveira, da Fonseca, Bueno and Fujiwara2017).
To better understand how Ascaris and other helminth infection may influence the course of a concurrent viral infection, and the mechanisms of immunoregulation present during co-infection is an important field that needs further investigation. Indeed, it is possible that larvae migration, as well as, immune modulation during Ascaris infection may influence the clinical outcome of COVID-19. Indeed, very recently papers have attempted to argue if helminth SARS CoV-2 coinfection would be beneficial or detrimental to the host. Bradbury et al. (Reference Bradbury, Piedrafita, Greenhill and Mahanty2020) suggested that immune modulation by helminths could reduce the resistance to SARS CoV-2 infection. Nonetheless, Hays et al. (Reference Hays, Pierce, Giacomin, Loukas, Bourke and McDermott2020) argue that the Th2 immunomodulation observed during helminth infection may have a mitigating effect. Indeed, Fonte et al. (Reference Fonte, Acosta, Sarmiento, Ginori, García and Norazmi2020) point out that the great variability in COVID-19 lethality rate between countries and regions might be related to helminth prevalence, particularly the low COVID-19 lethality in Sub-Saharan Africa. It is important to note that, as described earlier, the immune modulation driven by helminth infection could also interfere with the efficacy of a SARS CoV-2 vaccine. In agreement with others, we do believe that it is key to investigate the influence of helminth co-infection on COVID-19 outcome and vaccine efficacy.
Conclusion
Animals and humans are continuously exposed to different pathogens. A better understanding of how different pathogens interact in the same host, as well as how chronic infection may impact the response to injuries and immunological challenges is crucial. Increasing data indicate that Ascaris infection consequences are beyond ascariasis and may influence the clinical outcome of a variety of conditions. Conflicting results and conclusions from different studies illustrate that there are still many unknown factors involved, and this is a promising field with a complexity of factors.
Acknowledgements
Figures were made using biorender.com.
Author contribution
LM, RTF and LLB conceptualized, wrote, reviewed, and edited the manuscript and figures. DSN, PHGG, FMSO, LK, ACGG, FVS wrote, reviewed, and edited the manuscript and figures.
Financial support
RTF and LLB are research fellows supported by CNPq (Brazilian National Council for Scientific and Technological Development). DSN, FMSO, LK and LM are CAPES fellows, ACGG is CNPq fellow and FVS is Fapemig fellow. This study was supported by the Division of Intramural Research, NIH
Conflicts of interest
Authors declare no conflict of interest.
Ethical standards
Not applicable.