Introduction
Recently, it has become evident that interactions between invasive plants and soil microbes can facilitate plant invasions of nature reserves and range-land agroecosystems, support the persistence of these invasions, and reduce prospects for restoration of more desirable plant communitiesReference Caesar1–Reference Reinhart and Callaway3. However, relatively little is known about interactions between field-crop weeds and soil microbesReference Vatovec, Jordan and Huerd4. Current trends in management of field-crop agroecosystems are promoting practices that tend to stabilize the soil environment, which will likely increase the importance of weed–soil microbe interactions. These practices include cover cropping and conservation tillage. Cover crops have been shown to increase diversity and abundance of arbuscular-mycorrhizal fungi (AMF)Reference Gosling, Hodge, Goodlass and Bending5, affect soil-borne pathogensReference Abawi and Widmer6, Reference Manici, Caputo and Babini7 and increase microbial antagonists to soil-borne pathogensReference Wiggins and Kinkel8. Conservation tillage methods reduce physical disturbance of the within-soil hyphal network formed by AMFReference Gosling, Hodge, Goodlass and Bending5, Reference McGonigle and Miller9, Reference Jansa, Mozafar, Kuhn, Anken, Ruh, Sanders and Frossard10 and thereby promote plant interactions with AMF. If current trends in field-crop agroecosystems are intensifying plant–soil microbe interactions in these systems, what might be the effects on field-crop weeds?
Previous work on weed–soil microbe interactions in field-crop agroecosystems has emphasized weed interactions with microbial antagonists, chiefly deleterious root-associated bacteriaReference Kremer11–Reference Kremer and Li13, AMFReference Jordan, Zhang and Huerd14 and bacterial and fungal predators of weed seedsReference Chee-Sanford, Williams, Davis and Sims15. These investigations have suggested that ‘weed-suppressive’ soils might be developed; such a soil would harbor antagonistic microbiota capable of reducing weed pressure to an agronomically-significant extentReference Quimby, King and Grey16–Reference Davis, Anderson, Hallett and Renner18. Weed suppression might be exerted by microbes typically viewed as pathogensReference Kremer and Li13, but strong antagonistic effects can be also be directly exerted by AMF on non-host plant species, including certain important field-crop weedsReference Francis and Read19–Reference Gange, Lindsay and Ellis25. The mechanistic basis of such antagonistic effects has not been established, but may involve induction of necrosis in seedlings of non-host species19.
In addition to reducing weed abundance through ‘weed suppression’, interactions with soil microbiota may affect diversity and distribution of biomass in weed communities. It is now thought that the predominant effect of plant–soil microbe interactions is to increase plant community diversityReference Bever26–Reference Hart, Reader and Klironomos28. At least two mechanisms can lead to this outcome: negative plant–soil feedback processes can limit abundance of potentially dominant species3, and increased microbial diversity and abundance can support plant diversity by providing essential mutualists to increasing numbers of plant speciesReference van der Heijden, Boller, Weimken and Sanders29–Reference Golubski32. Microbial effects on weed community diversity are of interest because such diversity may significantly affect a variety of important properties of field-crop agroecosystems, including capacities for soil nutrient cycling and biodiversity conservationReference Wardle, Yeates, Nicholson, Bonner and Watson33–Reference Murphy, Clements, Belaoussoff, Kevan and Swanton37.
However, very little is known about interactions of field-crop weeds with soil microbiota under field conditions. In the study reported below, we addressed this knowledge gap by manipulation of certain soil microbiota in experimental weed communities established in corn and soybean crops, applying a soil fungicide to reduce diversity and abundance of soil fungi. We used a fungicide, benomyl, which has been used extensively for this purpose in other ecosystems. Benomyl has been repeatedly shown to alter soil fungal communities, to thereby affect plant population and community processes, and to primarily affect AMFReference Hartnett and Wilson38. Moreover, it has been shown to have only modest artifactual effects on nutrient cycling and non-fungal soil biotaReference Chen, Edwards and Subler39, Reference Martikainen, Haimi and Ahtiainen40, and, overall, to have fewer drawbacks than other methods for manipulating soil fungal communities in large-scale field experimentsReference Hartnett and Wilson38. From current understanding of AMF–plant interactions, we hypothesized that suppression of this group of soil fungi would change the composition of experimental weed communities in corn and soybean crops, reduce performance of AMF-host weeds relative to non-host species, and reduce diversity and evenness in these weed communities.
Materials and Methods
Field experiments
All field experiments were carried out at the University of Minnesota Experiment Station (Rosemount, MN, 44° 41′N, 93° 04′W, well-drained Waukegan silt loam). The experimental field was planted with a cover crop (winter rye (Secale cereale L.), hairy vetch (Vicia villosa Roth), field pea (Pisum sativum L.)) in autumn 1999; then, in spring 2000, cover-crop regrowth was suppressed with glyphosate applications and rototilling. Permanent plots (2 m×3 m) were marked and benomyl fungicide (Benlate SP; E.I. du Pont de Nemours and Co., Wilmington, DE) applications were begun on 25 May 2000. Fungicide was applied as a soil drench at 2.5 g a.i.m−2; control plots received equal amounts of water. Fungicide applications were made at biweekly intervals until 17 July 2000. Plots were planted with a no-till drill on 7 May 2001; these same plots were rototilled by hand tiller and planted with a no-till drill 17 May 2002. Biweekly benomyl applications to plots receiving fungicide resumed immediately after tillage. In 2002, benomyl rate was increased to 3.75 g a.i.m−2, because the previous rate (2.5 g a.i.m−2) had weak effects on AMF infection rates in 2001 (see Table 3). Soybean (Glycine max (L.) Merr. ‘Surge’) and corn (Zea mays L. sethoxydim resistant) were planted on 7 June 2000, 29 May 2001 and 17 June 2002; soybean was sown in rows (20 cm spacing) with a conservation tillage drill, while corn was sown with a standard drill in 76 cm rows.
Due to logistical constraints on crop planting operations, we conducted two separate experiments in adjacent sections of the experimental field. One experiment had the crop sequence soybean/corn/soybean in 2000, 2001 and 2002, respectively, while the other experiment followed the sequence corn/soybean/corn in the same years. In each experiment, fungicide or control treatments were applied to plots in a randomized block design with 10 replicates; plots received those same treatments in 2000–2002. No nutrients were applied to plots, as recommended by soil tests (based on 20 5 cm by 15 cm soil core samples collected systematically across the experimental site). At the start of the experiment, soils contained 4.3% organic matter, 33 μg g−1 available P (Bray 1 test), 67 μg g−1 potassium, 3.3 μg g−1 NO3-N, and had a pH of 7.2. In 2000 and 2001, certain grass weeds (not part of experimental weed communities) were highly abundant in experimental plots; in accordance with standard agronomic practice, these weeds were suppressed by a selective grass herbicide application (Poast, BASF, Floram Park, NJ) on 22 July 2000 and 28 June 2001 to all plots at a standard rate (2.34 l ha−1). In 2002, grass weeds were less abundant and no herbicide was applied.
Weed seeds were sown in the entire area of each plot immediately after crop planting. In 2000, laboratory germination percentages were used to determine seeding densities (Table 1) sufficient to achieve an expected seedling density of 125 m−2, evenly divided between AMF-host and non-host species. In 2001 and 2002, sowing densities were increased to an expected seedling density of 175 seedlings m−2, again evenly divided into AMF-host and non-host species (Table 1). Heavy rains occurred after weed planting in 2001 and emergence of most species was very limited; accordingly, all species were replanted at the same seeding rate on 26 June 2001. After planting, a 0.5 m2 subplot was marked within each plot, and all seedlings of sown experimental weed species within this subplot were marked. In each plot, surviving weeds were counted and aboveground biomass harvested when most weed species were beginning to flower (15 August 2000, 21 August 2001, 28 August 2002). Biomass was oven-dried and weighed from 3 to 7 days at 60°C.
Table l. Seeding rates (seeds m−2) and composition of experimental weed communities.

In 2000 and 2002, aboveground biomass of each crop was estimated at the time of weed biomass harvest by clipping a sample of crop plants at soil level. In soybean plots, soybean plants within a section (30 cm) of row were harvested; in corn plots, three plants were harvested. Crop biomass was oven-dried and weighed. In 2001, crop biomass was harvested on 19 September 2001.
AMF colonization assays
In 2000 and 2001, root samples of the strong AMF host Ambrosia artimisiifolia L. (AMBEL, see Table 3) were collected by plot in late summer to assess fungicide effects on AMF colonization levels. In 2002, in addition to A. artimisiifolia, root samples of soybean and several additional weed species (Abutilon theophrasti Medik. (ABUTH), Sida spinosa L. (SIDSP), and Solanum nigrum L. (SOLNI)) were collected at the time of weed biomass harvest to assess mycorrhizal colonization. In all years, plant roots were held on ice after collection, then cleaned and frozen. Roots of each species were subsampled and stained with aniline blueReference Grace and Stribley41. Roots were examined microscopically (200×) to assess mycorrhizal root colonization (hyphae, arbuscules, and vesicles) using the magnified intersection methodReference McGonigle, Miller, Evans, Fairchild and Swan42.
Disease assays
Soil from the Rosemount Experiment Station was collected and stored in a cooler for 3 months. At time of planting, soil was evenly mixed 1:3:3 soil:sterile sand:vermiculite (approx. 14% inoculum). This mix was placed in clean pots (3.8×18 cm). Seeds of five weed species (Abutilon theophrasti, Amaranthus retroflexus L., Ambrosia artemisiifolia, Chenopodium album L. and S. nigrum) were surface-sterilized (5% sodium hypochlorite) for 5 min and rinsed well, planted, and covered with additional vermiculite. Pots were arranged in a randomized complete block with 5 species×2 treatments×10 replicates=100 pots. Germinating seedlings were thinned to 1 seedling pot−1. At 10 and 24 days after planting, benomyl was applied to half of the pots at a standard rate of 2.5 g a.i.m−2; water was applied to the remaining (control) pots. All pots were watered daily for 5–6 weeks. After 6 weeks, plants were harvested for biomass and roots were scored for disease incidence/severity by an experienced observer. Roots that were blemish-free and not discolored were classified as non-diseased; roots classified as diseased showed discoloration and lesions consistent with microbial infections by common soil-borne pathogens (Fusarium, Phytophthora, Pythium, etc.).
Statistical analyses
Separate analyses were carried out for each year of the study (2000, 2001 and 2002), because of substantial differences among weed communities establishing in each year, despite nearly uniform seedling treatments (Table 1). Multi-response permutation procedure (MRPP) was used to test fungicide effects on weed community composition; MRPP provides a robust non-parametric alternative to MANOVAReference Pellicane and Mielke43. ANOVA was used to test fungicide effects on weed community attributes (total density, biomass and diversity). Mixed-model ANOVA was used to test differential effects of fungicide treatment on AMF-host and non-host weed species; species within each category was considered as a random factor. All ANOVA analyses were done with SAS44; MRPP was done with Pcord version 4 (http://home.centurytel.net/~mjm/pcordwin.htm; visited 4 August 2006). Crop biomass production was used as a covariate in most ANOVA analyses, to focus analysis on direct effects of AMF on weeds, rather than effects mediated through crop growth.
Results
Weed community attributes
Fungicide applications had little effect in the first year of application (2000, data not shown), but significantly affected weed density and biomass during the second (2001, Fig. 1) and third (2002, Fig. 2) experimental years. In the second year, fungicide increased total weed density by 34% in corn (P=0.039, ANOVA), but only slightly in soybean; total weed biomass was not significantly increased in either crop. Total weed density in the third year was moderately increased by fungicide in both crops but only the soybean effect approached significance (P=0.056, ANOVA). Fungicide increased total weed biomass in corn by 49%, but had little effect in soybean.

Figure 1. Fungicide effects on total density and biomass production of experimental weed communities in corn and soybean crops in 2001, with standard errors.

Figure 2. Fungicide effects on total density and biomass production of experimental weed communities in corn and soybean crops in 2002, with standard errors.
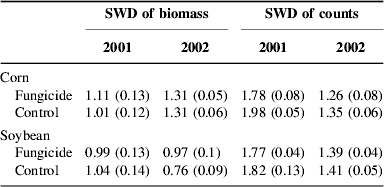
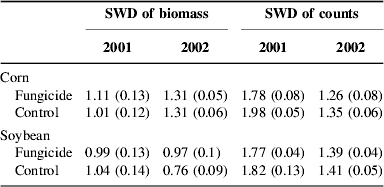
Fungicide also affected weed community composition in the second (Fig. 3) and third years (Fig. 4). The distribution of density among weed species was altered by fungicide in corn in the second year (Fig. 3a; P=0.013, MRPP), and a similar but marginally non-significant effect occurred in soybean in the third year (Fig. 4b; P=0.10, MRPP). The distribution of biomass among species was affected by fungicide in the corn crop in the third year (Fig. 4c; P=0.014, MRPP). In both years, fungicide effects on both density and biomass production were manifest as modest responses (Figs 3 and 4) in most of the 11 and 6 weed species analyzed in second and third years, respectively. Diversity (Shannon–Weiner)Reference Greig-Smith45 values were calculated for weed communities developing in the second and third years. Calculations were based on the same weed species in all plots (11 and 6 in second and third years, respectively) and therefore treatment effects on diversity indicate effects on the evenness of the distribution of density or biomass among weed species. Fungicide did not significantly affect weed community diversity (Table 2), except in corn in 2001, where fungicide caused the distribution of weed density to become more uneven (P=0.02, ANOVA).

Figure 3. Fungicide effects on weed density (a and b) and biomass production (c and d) by weed species in experimental weed communities in corn and soybean crops in 2001, on natural-logarithm scales, with standard errors. Weed species: Abutilon theophrasti (ABUTH), Ambrosia artimisiifolia (AMBEL), Hibiscus trionum (HIBTR), Plantago lanceolata (PLALA), Sida spinosa (SIDSP), Solanum nigrum (SOLNI), Amaranthus retroflexus (AMARE), Brassica nigra (BRSNI), Chenopodium album (CHEAL), Polygonum lapathifolium (POLLA), and Portulaca oleracea (POROL).

Figure 4. Fungicide effects on weed density (a and b) and biomass production (c and d) by weed species in experimental weed communities in corn and soybean crops in 2002, on natural-logarithm scales, with standard errors. Weed species: Ambrosia artimisiifolia (AMBEL), Sida spinosa (SIDSP), Solanum nigrum (SOLNI), Amaranthus retroflexus (AMARE), Chenopodium album (CHEAL), and Galium aparine (GALAP).
Table 2. Shannon–Weiner diversity (SWD) means, with standard error of mean in parentheses, for 2001 and 2002 weed communities; all means based on 10 plots.
Relative performance of AMF-host and non-host species
In the second year, fungicide application did not significantly affect relative performance of the two classes of weeds (Fig. 5). In the third year, strong effects were evident in soybean (Fig. 6), where fungicide significantly reduced the performance of AMF-host species relative to non-host species, affecting both density and biomass (P=0.004 for density and P=0.01 for biomass, fungicide by species type interactions, ANOVA). AMF-host species were less dense than non-hosts in both treatments, and fungicide increased the differential: AMF-host density was 80% of non-host density without fungicide, but only 41% with fungicide (Fig. 6a; note log scale). Fungicide also increased the proportional performance of non-host species with respect to biomass production (Fig. 6b). Fungicide reduced relative performance of AMF-host species in the corn crop in the third year as well, but only the effect on density approached significance (Fig. 6a, P=0.08, ANOVA). The observed increases in the relative performance of non-hosts resulted from responses of A. retroflexus and C. album; note that fungicide caused substantial increases in density of A. retroflexus and C. album in both second and third years (Figs 3a, 4a and 4b). Most non-hosts were unresponsive to fungicide; for example, the non-host group in the second year included Brassica kaber (DC.) Wheeler, Polygonum lapathifolium L. and Portulaca oleracea L., and neither density nor biomass of these species was affected by fungicide. Therefore, the promotion of the relative performance of non-hosts by fungicide was species-specific, and did not reflect a general response by the non-host species group.

Figure 5. Fungicide effects on the performance of AMF-host and non-host weed species with respect to density (a) and biomass production (b) in corn and soybean crops in 2001, on natural-logarithm scales, with standard errors.

Figure 6. Fungicide effects on the performance of AMF-host and non-host weed species with respect to density (a) and biomass production (b) in corn and soybean crops in 2002, on natural-logarithm scales, with standard errors.
Fungicide effects on AMF colonization
To indicate the effect of benomyl applications on mycorrhiza formation in AMF-host weed species, AMF colonization rate (Table 3) was monitored in A. artemisiifolia in each year of the study. Fungicide reduced colonization in all cases, but reductions were moderate and largely non-significant, approaching or reaching significance in 3 of 6 cases. In 2002, colonization was assessed in three additional weed species (Psida spinosa, Solanum nigra, A. theophrasti) and soybean. Generally, colonization was only modestly reduced in weed species, but was sharply reduced in soybean (P<0.01, ANOVA). Evidently, fungicide applications did have strong effects on some plant species, but in general only modest reductions in colonization were observed.
Table 3. Fungicide effects on AMF colonization levels in roots of selected weed species and soybean, given as percentage of AMF presence observed in 100–140 views/root sample; n is number of examined root samples, with standard error of mean in parentheses. Weed species: Abutilon theophrasti (ABUTH), Ambrosia artimisiifolia (AMBEL), Sida spinosa (SIDSP), and Solanum nigrum (SOLNI).

Fungicide effects on disease symptoms
In four of the five species examined, the percentage of sampled root length showing visible lesions and discoloration was not affected by fungicide treatment; in velvetleaf, the fungicide reduced infected root length by 40% (P=0.02, t-test; data not shown). These results must be interpreted with some caution, because this study was conducted in a glasshouse and did not assess cumulative effects of multi-year fungicide applications. However, these results suggest that fungicide application did not have strong effects on pathogen infection for most weed species examined in this study, supporting the inference that fungicide effects result principally from effects on AMFReference Hartnett and Wilson38.
Discussion
We found that weed density, biomass, community composition and the relative performance of AMF-host and non-host weed species were repeatedly responsive to experimental manipulations of soil fungi, with agronomically significant effects evident after the second and third seasons of fungicide applications. In the second year, fungicide applications were associated with increases in weed density (34% in the corn crop) that significantly increase the risk of substantial immediate yield loss and persistent increases in future weed pressure. In the third year, agronomically significant effects included substantial increases in total weed density (49% in corn) and increases in the relative performance of two non-host weeds (A. retroflexus and C. album) that are often highly problematic in annual field crop production. These results emerged despite the relatively low power of our experimental design, and suggest that the influence of soil fungi on population and community ecology of field-crop weeds can be large, given that our fungicide application rates apparently caused only moderate reductions in AMF symbiosis with AMF-host weed species (as indicated by colonization levels).
Our findings and results from previous experimental use of benomyl in a variety of plant communitiesReference Hartnett and Wilson38, Reference Hartnett and Wilson46–Reference Dhillion and Gardsjord48, suggest that suppression of interactions with mycorrhizal fungi was the primary effect of our applications of benomyl fungicide. We note, however, that our observation of weak fungicide effects on disease symptoms does not exclude the possibility that other pathogenic or saprophytic fungi were functionally important to our observed results. With this caveat, our results strengthen a growing base of evidence that AMF can have important influences on the composition, diversity and agroecological functioning of weed communities in field crop agroecosystems. In glasshouse experiments, AMF symbiosis in field crop weeds has been shown to increase growth, seed production and seed qualityReference Koide, Li, Lewis and Irby49–Reference Heppell, Shumway and Koide54, and substantial variation among field-crop weed species in AMF colonization and subsequent growth responses have been demonstratedReference Vatovec, Jordan and Huerd4. Given the wide range of biomass responses observed among field-crop weed species, it is possible that AMF could have a substantial effect on the dynamics of weed communities containing these species, particularly in agroecosystems that minimize soil disturbance and mechanical weed control for soil and water conservation purposes. In such agroecosystems, AMF are likely to be more diverse and abundant, and other factors known to affect weed community dynamics (e.g., selective tillage) are likely to be less influential.
We note that fungicide effects often differed between corn and soybean crops, although our design did not permit evaluation of the statistical significance of observed differences. At the close row spacing used in our experiments, soybean crops produced denser and more closed canopies than corn crops. Our observation that weed interactions with soil fungi were apparently modulated by crop species is consistent with previous studies. Mycorrhizal responsiveness among a set of 14 field-crop weeds was generally less positive under reduced light and temperature levelsReference Vatovec, Jordan and Huerd4, a result consistent with the hypothesis that AMF may typically provide lower net benefits to AMF hosts when photosynthesis is restrictedReference Johnson, Grahan and Smith22. Thus, although our observations of crop effects on weed–fungi relations must be interpreted cautiously, these results add to evidence suggesting the different sub-canopy environments created by different crop species (or by different planting densities for a single crop) may significantly modulate the effects of soil fungi on weed population and community dynamics. Indeed, AMF interactions with sub-canopy weed plants may be a very important facet of weed–AMF interactions, since small, sub-canopy weed plants can produce considerable seedReference Forcella, Colbach and Kegode55 and are probably highly important to population persistence in many weed species
In the third year, we observed a significant increase in relative performance of non-host species when soil fungi were suppressed by fungicide, although this effect was strongly significant only in the soybean crop. A number of mechanisms may underlie such effectsReference Allison, Rajaniemi, Goldberg and Zak56, including direct antagonistic effects of AMF or other fungi on non-host weed species and indirect effects acting via increased interference with non-host growth by AMF-host species, as a result of interactions with AMF or other fungi. Our findings are consistent with a direct effect. AMF or other fungi may have improved crop growth and thus suppressed weed growth, but we controlled statistically for such effects. Soil fungi might have increased interference effects of AMF-host weeds on non-host species, but in the third year, AMF-host-biomass responses to fungicide were small (except for Solanum, for which biomass production was sharply increased by fungicide) and therefore presumably interfered with non-host species less strongly in the absence of fungicide. We note that our results must be interpreted cautiously as the 2002 analysis addressed a relatively limited number of weeds (six species), due to limited weed establishment in that year. Therefore, our results may reflect idiosyncratic responses of these species to soil fungi rather than agroecological differences between AMF-host and non-host weed species. With these caveats, our findings provide results from a field-crop agroecosystem consistent with a substantial number of independent previous studies that have observed powerful direct antagonistic (i.e., pathogenic) effects of AMF on a number of non-host species, some of which are important agronomic weedsReference Francis and Read19,22–Reference Gange, Lindsay and Ellis25. For example, relative growth rate and survivorship of C. album was reduced by 42 and 33%, respectively, when grown with AMFReference Francis and Read19. Many problematic agricultural weeds belong to families that are typically non-hostsReference Francis and Read19, Reference Hamel57. Therefore, these observations of antagonistic effects of AMF on non-host species raise the possibility that AMF could provide a broad-spectrum biocontrol measure against non-host weed species. It is also possible that general increase in the abundance of AMF-host weeds would occur in AMF-enhanced cropping systems.
As we have noted in the present paper, a substantial number of independent studies (cited herein) have observed quite powerful antagonisms of this sort in a variety of plant communities.
Experimental suppression of soil fungi also caused certain changes in weed community composition that were not evidently related to AMF antagonism to non-host species and consequent changes in relative performance of AMF-host and non-host weed species. In the third year, per individual biomass production by the AMF-host species Solanum was sharply increased by fungicide in the corn crop, and more weakly so in the soybean crop. Since fungicide caused AMF colonization to decline by ca. 25% (albeit non-significant, Table 3), this response may reflect a negative growth response to AMF symbiosisReference Johnson, Grahan and Smith58, Reference Klironomos59, although its magnitude is larger than most such responses observed to date, or the pathogenic effect of non-AMF fungi. Similarly, in the second year, fungicide significantly affected the distribution of density among weed species in the corn crop but without any indication of differential responses among AMF-host and non-host species. Among 11 weed species, responses were (at most) modest in all but two species; these were both non-hosts (Brassica and Amaranthus) but showed opposite responses to fungicide.
Our field experiment shows that soil fungi can decrease weed density, biomass and the relative abundance and growth of both AMF-host and non-host weed species. If such effects occur widely in field-crop agroecosystems, there are a number of implications for weed management. First, antagonistic fungal effects (such as that suffered by Solanum in the third year) might serve as one of the ‘small hammers’ by which agroecological processes limit weed abundance and interference with crop productionReference Liebman, Gallandt and Jackson60. Such interactions may be of considerable significance in the context of integrated weed management systems. Typically, only a few weed species are well-enough adapted to the management factors operant in any particular agroecosystem to become highly problematic in that system. Antagonistic fungal effects on these species could be very useful in their management.
Moreover, in addition to potential value to integrated weed management, interactions between weeds and soil fungi may affect weed diversity. Theory and limited empirical evidence suggest that if management factors serve to intensify weed interactions with soil fungi, the diversity of weed communities may be increasedReference Bever26, Reference Reynolds, Packer, Bever and Clay27, Reference Gange, Brown and Sinclair61, although we found only limited evidence of this outcome. If negative effects on crop yield or otherwise are acceptably small, increasing weed diversity may provide a useful option for restoring useful microbial biodiversity in field-crop agroecosystems, given mounting evidence that diverse weed communities provide certain agroecological ‘services’Reference Jordan, Vatovec and Inderjit36, Reference Murphy, Clements, Belaoussoff, Kevan and Swanton37, Reference Albrecht62 that are relevant to both production and conservation in agroecosystems. For example, AMF-host weeds may benefit crop production by maintaining diversity and abundance of agronomically beneficial AMF taxa; removal of AMF-host weeds from agroecosystems can cause changes in diversity, abundance and functioning of AMF, and can reduce beneficial AMF effects on crop growthReference Feldmann and Boyle63, Reference Kabir and Koide64. Diverse weed communities may provide a range of other services, including increased interference among weed species that may reduce herbicide resistance riskReference Murphy, Clements, Belaoussoff, Kevan and Swanton37, and maintenance of valued wildlife species and predatory and pollinating arthropod species in certain agroecosystemsReference Marshall, Brown, Boatman, Lutman, Squire and Ward34, Reference Jordan, Vatovec and Inderjit36.
Taken together, indications of the significance of weed–soil fungi interactions for weed management and ecological services from weeds, and our finding that these interactions can be strong under field conditions, should encourage further investigations of weed–fungi interactions in field-crop agroecosystems. Such work is particularly warranted in cropping systems where conservation tillage and cover cropping techniques have been practiced for some time. Weed–fungal microbe interactions are likely to be generally stronger in such situations; if these interactions have real agronomic importance, it will likely be in such agroecosystems.
Acknowledgements
We are grateful to several colleagues for pre-submission reviews of the manuscript, and to a terrific group of undergraduate field assistants (too numerous to list). Gratitude is also expressed to the editors of Renewable Agriculture and Food systems and to the reviewers of our paper. In particular, Dr Gupta Vadakattu (CSIRO, Australia) was willing to have his review comments, and our responses to them, published as an addendum to our manuscript (doi:10.1017/S1742170508002305). Publication of this exchange enriches and deepens our work, promoting critical dialogue on the strengths and limitations of our methods, findings and inferences, and is part of an ongoing RAFS experiment with new formats for scientific publication. Funding was provided by a grant from the US Department of Agriculture.