Introduction
Once known as the picolinic carboxylic acids, the pyridine carboxylic acid (PCA) herbicide family of synthetic auxins has been utilized in many facets of weed management since their discovery in the 1960s (Gantz and Laning Reference Gantz and Laning1963; Hamaker et al. Reference Hamaker, Johnston, Martin and Redeman1963; Shaner Reference Shaner2014). This family of herbicides are predominately utilized in non-cropland areas such as pastures, rangeland, natural areas, right-of-ways, and industrial sites due to their selectivity, low use rates, and soil persistence (Akanda et al. Reference Akanda, Mullahey, Dowler and Shilling1997; Arnold and Farmer Reference Arnold and Farmer1979; Ferrell et al. Reference Ferrell, Mullahey, Langeland and Kline2006; Herr et al. Reference Herr, Stroube and Ray1966; Shaner Reference Shaner2014). However, some PCA herbicides provide selective weed control and are registered for use in select crop species such as canola (Brassica napus L.), sugarbeet (Beta vulgaris L.), field corn (Zea mays L.), rice (Oryza sativa L.), and small grains (O’Sullivan and Kossatz Reference O’Sullivan and Kossatz1982; Shaner Reference Shaner2014).
Several PCA herbicides have similar molecular structures characterized by a pyridine ring, chlorine functional groups, and a carboxyl group (Figure 1). Despite their similarities, differential activity has been often documented among PCA herbicides and plant species. For example, blackberry (Rubus spp.) is more tolerant to picloram and clopyralid than to triclopyr or fluroxypyr in combination with triclopyr (Ferrell et al. Reference Ferrell, Sellers, MacDonald and Kline2009; Harrington et al. Reference Harrington, Rader-Dixon and Taylor2003). Bovey and Mayeux (Reference Bovey and Mayeux1980) investigated the efficacy of clopyralid and triclopyr for the control of honey mesquite (Prosopis glandulosa Torr. var. glandulosa) and concluded that honey mesquite was more sensitive to clopyralid than triclopyr. Aminopyralid was more effective for control of tropical soda apple (Solanum viarum Dunal) than triclopyr, picloram, or clopyralid plus 2,4-D (Ferrell et al. Reference Ferrell, Mullahey, Langeland and Kline2006). Huisache [Acacia farnesiana (L.) Willd.] is more sensitive to picloram than triclopyr, and cotton (Gossypium hirsutum L.) is more sensitive to triclopyr than clopyralid or picloram (Bovey et al. Reference Bovey, Ketchersid and Merkle1979; Jacoby et al. Reference Jacoby, Meadors and Clark1990). Conversely, PCA herbicides have demonstrated similar activity on certain species. For example, mile-a-minute (Mikania micrantha Kunth) responded similarly to aminopyralid, fluroxypyr, and triclopyr under greenhouse conditions (Sellers et al. Reference Sellers, Lancaster and Langeland2014).
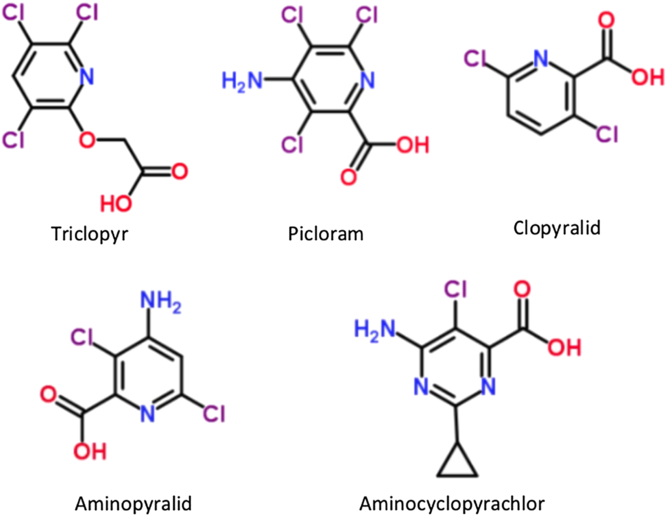
Figure 1. Molecular structures of triclopyr, picloram, clopyralid, aminopyralid, and aminocyclopyrachlor.a
Distinct from PCA herbicides, aminocyclopyrachlor (AMCP) is synthetic auxin belonging to the pyrimidine carboxylic acid herbicide family that is labeled for use in non-crop areas (Armel et al. Reference Armel, Klingeman, Flanagan, Breeden and Halcomb2009; Claus et al. Reference Claus, Turner, Armel and Holliday2008; Jenks Reference Jenks2010; Turner et al. Reference Turner, Claus, Hidalgo, Holliday and Armel2009). Although AMCP largely does not exhibit activity on monocots, herbicidal activity has been reported in some grass species such as winter wheat (Triticum aestivum L.), cogongrass [Imperata cylindrica (L.) P. Beauv.], and select warm-season turfgrass species (Edwards Reference Edwards2008; Enloe et al. Reference Enloe, Belcher, Loewenstein, Aulakh and van Santen2012; Flessner et al. Reference Flessner, McCurdy and McElroy2011a, Reference Flessner, McElroy and Wehtje2011b; Kniss and Lyon Reference Kniss and Lyon2011; Lewis et al. Reference Lewis, Hoyle, Fisher, Yelverton and Richardson2011; Vassios et al. Reference Vassios, Douglass, Bridges, Lindenmayer and Nissen2009). AMCP has a similar structure to that of PCA herbicides but it differs by having an additional nitrogen atom in its ring structure (Figure 1). Because AMCP has not been available for as long as the PCA herbicides, little is known of their relative activities on target weed species. However, crop sensitivity studies have suggested that AMCP may exhibit similar activity to some PCA herbicides. For example, Solomon and Bradley (Reference Solomon and Bradley2014) reported that AMCP induced similar yield reductions in soybean [Glycine max (L.) Merr] as the PCA herbicides clopyralid and picloram under field conditions, although yield reductions differed from those caused by aminopyralid, fluroxypyr, and triclopyr.
Slight functional group changes in PCA herbicide molecules have been often shown to result in large changes in herbicidal activity and soil persistence (Bovey et al. Reference Bovey, Ketchersid and Merkle1979; Fast et al. Reference Fast, Ferrell, MacDonald, Krutz and Kline2010; Ferrell et al. Reference Ferrell, Mullahey, Langeland and Kline2006; Gorrell et al. Reference Gorrell, Bingham and Foy1988; Hall and Vanden Born Reference Hall and Vanden Born1988; Jacoby et al. Reference Jacoby, Meadors and Clark1990; Marple et al. Reference Marple, Al-Khatib, Shoup, Peterson and Claassen2007; Orfanedes et al. Reference Orfanedes, Wax and Liebl1993; Solomon and Bradley Reference Solomon and Bradley2014). The mechanism for differential activity among PCA herbicides has been extensively studied, although little work has occurred to evaluate the relative activity of AMCP. Most research has found little to no differences in herbicide absorption, translocation, or metabolism between the PCA herbicides; therefore, many authors conclude differential sensitivity of the target site among species as the proposed mechanism of reduced activity (Bovey and Mayeux Reference Bovey and Mayeux1980; Bukun et al. Reference Bukun, Gaines, Nissen, Westra, Brunk, Shaner, Sleugh and Peterson2009; Gorrell et al. Reference Gorrell, Bingham and Foy1988; Hall and Vanden Born Reference Hall and Vanden Born1988; Orfanedes et al. Reference Orfanedes, Wax and Liebl1993).
Although structure-activity relationships have been described for some herbicide families such as imidazolinone, triketone, and diphenylethers, structure-activity relationships and relative activity using dose-response techniques have not been described for PCA herbicides or AMCP (Ladner Reference Ladner1990; Lee et al. Reference Lee, Knudsen, Michaely, Chin, Nguyen, Carter, Cromartie, Lake, Shribbs and Fraser1998; Nandihalli et al. Reference Nandihalli, Duke and Duke1992). Therefore, the objective of this study was to investigate the differential activity among four PCA herbicides and AMCP using a whole plant, dose-response bioassay in canola, squash, and okra. Due to previously reported differential responses to PCA herbicides, we hypothesized that the relative activity of AMCP compared to PCA herbicides will be species dependent.
Materials and Methods
Plant Material and Growing Conditions
Greenhouse dose-response experiments were conducted on canola (‘5525 CL’, Brett Young, Winnipeg, MB), okra (‘Clemson spineless’, Burpee, Warminster, PA), and squash (‘Early Summer Crookneck’, Burpee) in 2016 at the University of Florida in Gainesville, FL. Plant species were chosen as representatives of the Brassicaceae, Malvaceae, and Cucurbitaceae families due to a modicum of innate tolerance. Species of Solanaceae and Asteraceae were not included in experiments due to the high level of sensitivity observed in a pilot study and in previous literature (Flessner et al. Reference Flessner, McElroy, Cardoso and Martins2012; Tomkins and Grant Reference Tomkins and Grant1974). Crop species within the Brassicaceae, Malvaceae, and Cucurbitaceae families were used instead of weeds to ensure uniform germination and growth. Seeds from each species were planted into square plastic pots (8 by 8 cm) uniformly filled with commercial potting media (Fafard Mixes for Professional Use, Conrad Fafard, Agawan, MA) amended with slow-release fertilizer (Osmocote 14-14-14 Smart-Release Plant Food, Scotts-Sierra Horticultural Products, Marysville, OH). Shortly after emergence, experimental units were thinned to 1 plant pot−1. Plants were grown in a greenhouse maintained at 30 C daytime and 24 C night-time (±3 C) temperatures. Supplemental lighting was utilized to maintain a 14-h photoperiod and plants were sprinkler irrigated as needed.
Experimental Design and Herbicide Applications
Separate experiments were conducted for each species. A completely randomized design with four replications was utilized for all experiments and each was repeated twice. Treatments were arranged as a factorial consisting of five herbicides and eight doses. Aminocyclopyrachlor (Method 50SG®, Bayer CropScience, Research Triangle Park, NC), aminopyralid (4-amino-3,6-dichloro-2-pyridinecarboxylic acid; Milestone®, Corteva Agriscience, Wilmington, DE, 240 g ae L−1), picloram (4-amino-3,5,6-trichloro-2-pyridinecarboxylic acid; Tordon 22K®, Corteva Agriscience, 240 g ae L−1), and clopyralid (3,6-dichloro-2-pyridinecarboxylic acid; Stinger®, Corteva Agriscience, 360 g ae L−1) were applied at doses of 0, 0.25, 1, 4, 16, 64, 256, and 512 g ae ha−1. Based on previous research by Dias et al. (Reference Dias, Banu, Sperry, Enloe, Ferrell and Sellers2017), triclopyr ([(3,5,6-trichloro-2-pyridinyl) oxy] acetic acid; Remedy Ultra®, Corteva Agriscience, 480 g ae L−1) was applied at a different rate titration (0, 1, 4, 16, 64, 300, 650, and 1,300 g ae ha−1) to ensure a full range of responses were exhibited. Herbicides were applied once plants reached the 2- to 3-leaf growth stage, approximately 20 d after planting (DAP). Applications were made using a compressed air-pressurized moving single-nozzle spray chamber (Generation II Spray Booth, Devries Manufacturing Corp., Hollandale, MN) equipped with a Teejet 8002 EVS spray nozzle (TeeJet® Technologies, Spraying Systems Co., Wheaton, IL) calibrated to deliver 187 L ha−1 at 172 kPa. All treatments and the nontreated control (NTC) included a nonionic surfactant (Activator 90, Loveland Products Inc., Greeley, CO) at 0.25% v/v−1. Herbicide treatment sequences started with the lowest dose and ended at the highest dose to prevent contamination of herbicide residue to treatments of lower doses. Additionally, the entire application system was thoroughly cleaned between active ingredients with three rinses of ammonia and water to prevent contamination of previously applied active ingredients. Plants were returned to the greenhouse and maintained as previously described after herbicides dried on the leaf surfaces.
Data Collection and Statistical Analysis
Aboveground biomass was harvested 21 d after treatment (DAT) then dried at 65 C in a force-aired dryer for 5 d. Data were subjected to ANOVA to test significance of main effects and interactions (α = 0.05). Normality, independence of errors, and homogeneity were examined, and all data were square root–transformed to meet ANOVA assumptions; however, nontransformed means are presented for clarity. The effective dose needed to reduce biomass by 50% (ED50) for each herbicide was derived from a three-parameter log-logistic regression model using the ED function under the drc package in R (version 0.98.1091, RStudio Inc, Boston, MA):
 $$Y = d/1 + \exp [b(\log x - \log e)]$$
$$Y = d/1 + \exp [b(\log x - \log e)]$$Where Y is the response variable (aboveground biomass expressed as a percent of the NTC), x is herbicide dose (g ae ha−1), b is the relative slope at the inflection point, d is the upper limit of the curve, and e is the inflection point (ED50) of the fitted line (Knezevic et al. Reference Knezevic, Streibig and Ritz2007). Model selection was based on Akaike’s information criterion (AIC) in the qpcR package of R (Ritz and Spiess Reference Ritz and Spiess2008; Spiess and Neumeyer Reference Spiess and Neumeyer2010). Additionally, lack-of-fit test at the 95% level comparing the nonlinear regression models to ANOVA was conducted to test appropriateness of model fit (Ritz and Streibig Reference Ritz and Streibig2005). Differences among parameters and estimates were compared using standard errors, t-tests, and F-tests at the 5% significance level (Knezevic et al. Reference Knezevic, Streibig and Ritz2007).
Results and Discussion
Interactions between experimental runs and main effects were not detected; therefore, data were pooled across runs. Nonsignificant (P > 0.05) results of lack-of-fit tests indicated appropriateness of model fit for all herbicides and species except for clopyralid, for which data were unable to be fitted to the log-logistic model. Plant response to clopyralid was relatively low for all three species compared to responses to the other herbicides, and not even the highest doses resulted in a 50% reduction in aboveground biomass (Table 1). This may have been due to the doses used in this study; a greater range of responses may have been observed had a different rate of titration been used. However, it is also possible that clopyralid has limited activity on the three study species, as has been reported by previous research on canola and closely related species (Blackshaw Reference Blackshaw1989; Geronimo Reference Geronimo1978). Here, it was found that clopyralid at the highest dose (512 g ae ha−1) reduced canola and okra biomass by only 29%, and squash biomass by 32%, compared to the nontreated controls (Table 1).
Table 1. Effect of clopyralid dose on aboveground biomass of canola, squash, and okra (2- to 3-leaf stage) in greenhouse conditions.a

a Values represent means (± standard error) pooled across experimental runs, expressed as a percent of the nontreated controls.
b Abbreviation: NTC, nontreated controls.
Aminopyralid and triclopyr had the greatest effect on canola biomass, followed by AMCP and picloram (Table 2; Figure 2). AMCP (ED50 = 112.9 g ae ha−1) was roughly twice as active as picloram (ED50 = 227.7 g ae ha−1). However, canola was 1.9-fold and 3.1-fold more sensitive to aminopyralid (ED50 = 60.3 g ae ha−1) and triclopyr (ED50 = 37.3 g ae ha−1) than AMCP, respectively. The slope at the inflection point (b) was higher for triclopyr compared to that for aminopyralid and picloram but not AMCP (Table 2); this suggests that canola was more sensitive to increases in dose of triclopyr than of aminopyralid and picloram near their respective ED50 values (Table 2).
Table 2. Log-logistic regressiona parameter estimates (± standard error) for aboveground biomass of canola, squash, and okra (2- to 3-leaf stage), based on from whole-plant, dose-response experiments comparing herbicides under greenhouse conditions.

a Log-logistic model: Y = d/1 + exp[b(logx – loge)], where Y is the response (aboveground biomass as a percent of the nontreated controls), x is the triclopyr rate, b is the slope of the inflection point, d is the upper limit of the curve, and e is the inflection point of the fitted line. The parameter e is also the effective dose rate (ED50), defined as the herbicide dose (g ae ha−1) required to cause 50% response (i.e., 50% reduction in aboveground biomass).
b ED50 estimates followed by the same letter within each species are not different according to t-tests and F-tests at the 5% significance level.
c Abbreviation: AMCP, aminocyclopyrachlor.

Figure 2. Aboveground biomass of canola 20 d after treatment with one of three pyridine carboxylic acid herbicides or aminocyclopyrachlor. Aminocyclopyrachlor, aminopyralid, and picloram were applied at 0, 0.25, 1, 4, 16, 64, 256, and 512 g ae ha−1. Triclopyr was applied at 0, 1, 4, 16, 64, 300, 650, and 1,300 g ae ha−1. Data were fitted to a three-parameter log-logistic regression model: Y = d/1 + exp[b(logx – loge)], where Y is the response (% aboveground dry biomass reduction), x is the triclopyr rate, b is the slope of the inflection point, d is the upper limit of the curve, and e is the inflection point of the fitted line (equivalent to the herbicide dose [g ae ha−1] required to cause 50% reduction in aboveground biomass [ED50]).
Squash was most sensitive to AMCP and triclopyr, followed by aminopyralid and picloram (Table 2; Figure 3). AMCP and triclopyr had similar activities on squash, with ED50 values of 6.6 and 7.8 g ae ha−1, respectively (Table 2). Picloram (ED50 = 23.3 g ae ha−1) and aminopyralid (ED50 = 21.1 g ae ha−1) also had similar activities on squash, and both were approximately 3.5 times less active than AMCP. However, although squash was less sensitive to aminopyralid than triclopyr across application rates, the ED50 values of those herbicides were not significantly different (Table 2; Figure 3). The inflection point slopes between herbicides did not differ, indicating a similar sensitivity to increases in dose near their respective ED50 values.

Figure 3. Aboveground biomass of squash 20 d after treatment with one of three pyridine carboxylic acid herbicides or aminocyclopyrachlor. Aminocyclopyrachlor, aminopyralid, and picloram were applied at 0, 0.25, 1, 4, 16, 64, 256, and 512 g ae ha−1. Triclopyr was applied at 0, 1, 4, 16, 64, 300, 650, and 1,300 g ae ha−1. Data were fitted to a three-parameter log-logistic regression model: Y = d/1 + exp[b(logx – loge)], where Y is the response (% aboveground dry biomass reduction), x is the triclopyr rate, b is the slope of the inflection point, d is the upper limit of the curve, and e is the inflection point of the fitted line (equivalent to the herbicide dose [g ae ha−1] required to cause 50% reduction in aboveground biomass [ED50]).
Aminopyralid had the greatest activity in okra, followed by AMCP, picloram, and triclopyr (Table 2; Figure 4). Aminopyralid produced the lowest ED50 value in okra (10.3 g ae ha−1) but it was not different from that of AMCP (ED50 = 14.6 g ae ha−1). The ED50 value resulting from picloram (ED50 = 17.3 g ae ha−1) was roughly 1.7-fold greater than that caused by aminopyralid but it was also not different from that of AMCP (Table 2). Triclopyr had the least activity on okra, with an ED50 value that was 8.6-fold greater than aminopyralid and 5.1-fold greater than picloram. The inflection point slopes were greater for AMCP and picloram than for triclopyr, indicating a higher sensitivity to changes in dose near the ED50 value (Table 2).

Figure 4. Aboveground biomass of okra 20 d after treatment with one of three pyridine carboxylic acid herbicides or aminocyclopyrachlor. Aminocyclopyrachlor, aminopyralid, and picloram were applied at 0, 0.25, 1, 4, 16, 64, 256, and 512 g ae ha−1. Triclopyr was applied at 0, 1, 4, 16, 64, 300, 650, and 1,300 g ae ha−1. Data were fitted to a three-parameter log-logistic regression model: Y = d/1 + exp[b(logx – loge)], where Y is the response (% aboveground dry biomass reduction), x is the triclopyr rate, b is the slope of the inflection point, d is the upper limit of the curve, and e is the inflection point of the fitted line (equivalent to the herbicide dose [g ae ha−1] required to cause 50% reduction in aboveground biomass [ED50]).
In this study, canola was more sensitive to aminopyralid than to AMCP and picloram, whereas okra was more sensitive to aminopyralid than picloram and triclopyr. Greater sensitivity to aminopyralid compared to AMCP, picloram, and triclopyr has been observed in other species such as soybean (Solomon and Bradley Reference Solomon and Bradley2014). Interestingly, both canola and okra were more tolerant of picloram than aminopyralid, despite the two molecules differing by only one chlorine group (Figure 1). Russian knapweed [Acroptilon repens (L.) DC.] has also been shown to be more sensitive to aminopyralid than picloram (Enloe et al. Reference Enloe, Kyser, Dewey, Peterson and DiTomaso2008; Enloe and Kniss Reference Enloe and Kniss2009a, Reference Enloe and Kniss2009b). In contrast, picloram and aminopyralid behaved similarly in squash, and are reported to have similar activity in other species such as tropical soda apple and Canada thistle [Cirsium arvense (L.) Scop.; Enloe et al. Reference Enloe, Lym, Wilson, Westra, Nissen, Beck, Moechnig, Peterson, Masters and Halstvedt2007; Ferrell et al. Reference Ferrell, Mullahey, Langeland and Kline2006]. AMCP was highly active in squash and okra, suggesting that it may provide effective control of weeds or increased risk to crops in the Cucurbitaceae and Malvaceae families. Conversely, AMCP had little activity in canola (Table 2). Flessner et al. (Reference Flessner, McElroy, Cardoso and Martins2012) also found differential AMCP activity among plant families, reporting that cotton was more sensitive to AMCP than cantaloupe (Cucumis melo L.) or eggplant (Solanum melongena L.).
Despite multiple cases of differential synthetic auxin herbicide sensitivity among plant species, causal mechanisms are not well understood because evidence in the literature is conflicting. For example, canola’s tolerance to picloram and sensitivity to 2,4-D has been shown to be caused by differential translocation (Hallmen Reference Hallmen1974). Similarly, differential sensitivity to AMCP was reported for members of Asteraceae and was linked to differences in absorption, translocation, and metabolism (Bell et al. Reference Bell, Burke and Prather2011). However, herbicides such as picloram and aminopyralid that have similar structures should exhibit comparable absorption and translocation according to a mathematical model combining the weak acid and intermediate permeability theories (Kleier Reference Kleier1988). For instance, differential activity of canola to picloram and clopyralid was not due to differences in translocation, absorption, or metabolism (Hall and Vanden Born Reference Hall and Vanden Born1988). Similarly, Israel et al. (Reference Israel, Everman and Richardson2015) reported that absorption and translocation of AMCP were similar among species such as water lettuce (Pistia stratiotes L.), alligatorweed [Alternanthera philoxeroides (Mart.) Griseb.], and water hyacinth [Eichhornia crassipes (Mart.) Solms], despite their differential response to this herbicide. Whereas differential metabolism of synthetic-auxin herbicides is a common mechanism of differential activity, target-site sensitivity (transport inhibitor response 1 [TIR1]) is also a possible mechanism that is species-specific (Grossmann Reference Grossmann2010). Despite the possible mechanisms for differential plant response, the current level of understanding of PCA herbicides and AMCP does not allow for confident prediction of an undocumented species’ sensitivity.
Although the responses of plants under greenhouse conditions may not directly correspond to plant growth in the field, greenhouse studies are useful for exploring plant responses to herbicides (Mangla et al. Reference Mangla, Sheley, James and Radosevich2011; Novoplansky and Goldberg Reference Novoplansky and Goldberg2001). These results support the need for additional research among the AMCP and PCA herbicides to understand the potential differences in herbicide selectivity. Clarifying these issues would benefit practitioners in selecting the most appropriate product for weed control operations as many of these herbicides have similar use patterns.
Acknowledgments
We thank Michael Durham for assistance in conducting experiments. This research received no specific grant from any funding agency, commercial or not-for-profit sectors. No conflicts of interest have been declared.








