Introduction
Seed germination is a critical part of the regeneration niche, which affects species assembly in plant communities (Grubb, Reference Grubb1977; Poschlod et al., Reference Poschlod, Abedi, Bartelheimer, Drobnik, Rosbakh, Saatkamp, van der Maarel and Franklin2013). Temperature is the most important environmental factor governing the capacity and speed of the germination process in the presence of water (Probert, Reference Probert and Fenner2000; Bewley et al., Reference Bewley, Bradford, Hilhorst and Nonogaki2013). Therefore, thermal niche breadth of germination seems to be related to species occurrence across different ecosystems (e.g. Ranieri et al., Reference Ranieri, Pezzini, Garcia, Chautems and França2012; Marques et al., Reference Marques, Atman, Silveira and Lemos-Filho2014; Rosbakh and Poschlod, Reference Rosbakh and Poschlod2015; Tudela-Isanta et al., Reference Tudela-Isanta, Ladouceur, Wijayasinghe, Pritchard and Mondoni2018; Picciau et al., Reference Picciau, Pritchard, Mattana and Bacchetta2019). Such ‘temperature window’ describes seed germination of a given species or population (Labouriau, Reference Labouriau1978; Alvarado and Bradford, Reference Alvarado and Bradford2002), thus bounding the limits of the regeneration niche (Thompson et al., Reference Thompson, Gaston and Band1999; Porceddu et al., Reference Porceddu, Mattana, Pritchard and Bacchetta2013).
Temperatures which combine maximal germination capacity (G%) and higher germination rate (GR, reciprocal of germination time) comprise an optimal range, splitting the temperature window into sub- and supra-optimal ranges (Bradford, Reference Bradford2002; Bewley et al., Reference Bewley, Bradford, Hilhorst and Nonogaki2013). Hence, the base temperature (Tb) is the sub-optimal limit under which there is no growth, while the ceiling temperature (Tc) marks the supra-optimal limit in which germination ceases because temperatures are too high (Garcia-Huidobro et al., Reference Garcia-Huidobro, Monteith and Squire1982). Within this context, it is mandatory to better understand the thermal limits of germination, especially as seed and germination traits are still under-studied in most plant communities (Jiménez-Alfaro et al., Reference Jiménez-Alfaro, Silveira, Fidelis, Poschlod and Commander2016). In Brazil, there is a large knowledge gap regarding seed ecology and germination traits, mainly among Amazon species (Ribeiro et al., Reference Ribeiro, Teixido, Barbosa and Silveira2016).
Thermal limits of seed germination seem to vary between 10 and 40°C in tropical forest species throughout the world (Larcher, Reference Larcher2000; Dürr et al., Reference Dürr, Dickie, Yang and Pritchard2015). Nevertheless, few studies have investigated such limits using controlled temperatures with seeds of Amazon species (Ferraz and Varela, Reference Ferraz, Varela, Higuchi, Santos, Sampaio, Marenco, Ferraz, Sales, Saito and Matsumoto2003; Bastos et al., Reference Bastos, Ferraz, Lima Junior and Pritchard2017; Amoêdo and Ferraz, Reference Amoêdo and Ferraz2019), although some have addressed this issue under greenhouse uncontrolled conditions (Silva et al., Reference Silva, Goldman, Magalhães and Moreira1988; Moreira and Moreira, Reference Moreira and Moreira1996). There are many factors limiting seed collection in the region, including the difficult access to native populations and technical restrictions to reach fresh-dispersing propagules in the forest canopy. Moreover, botanical identification can be challenging in such a diverse ecosystem, thus limiting ecophysiological research, including seed germination studies. Despite the generalized difficulties, light requirements have been studied in Amazon seeds (Aud and Ferraz, Reference Aud and Ferraz2012) as well as morphology of seed and seedlings (Camargo et al., Reference Camargo, Ferraz, Mesquita, Santos and Brum2008; Ramos and Ferraz, Reference Ramos and Ferraz2008; Ferraz et al., Reference Ferraz, Albuquerque, Calvi and Farias2012).
Thermal time (θ) requirements for seed germination, which can be defined as the amount of ‘thermal energy’ a seed population requires to reach a certain percentage of germination (usually 50%; see Trudgill et al., Reference Trudgill, Honek, Li and van Straalen2005), has never been investigated in Brazilian Amazon species. Theoretically, the environmental temperature is accumulated over time, reaching temperatures above the base temperature threshold (Tb) which is intrinsic to each species. Hence, θ requirements can be used to establish comparisons among species and/or populations (e.g. Covell et al., Reference Covell, Ellis, Roberts and Summerfield1986; Daibes and Cardoso, Reference Daibes and Cardoso2018), as well as modelling seedling emergence and seed dormancy alleviation (Forcella et al., Reference Forcella, Benech Arnold, Sanchez and Ghersa2000; Bradford, Reference Bradford2005; Orrù et al., Reference Orrù, Mattana, Pritchard and Bacchetta2012).
We aimed to investigate the role of temperature on seed germination of ten tree species occurring in the western Brazilian Amazon. To do so, we carried out germination trials with non-dormant seeds (scarifying them if necessary) under a range of constant temperatures. Specifically, we assessed (1) seed germination (G% and germination rate) under different constant temperatures and (2) parameters of seed germination (cardinal temperatures and thermal time requirement), which allows comparisons among species. We hypothesized that germination would show a high percentage and a rapid rate, favouring seedling recruitment in the moist understory. We also expected that thermal parameters would differ among species, which could further explain their coexistence in the Amazon forest.
Materials and methods
Seed collection
Seeds were collected in non-flooded forest areas in the western Brazilian Amazon, mostly in the Floresta Nacional (Flona) do Jamari, state of Rondônia (Table 1). These forest areas are characterized by a closed and tall canopy rich in woody species. The species used in this study are mostly legume trees with a height of 15 to 40 m. Mature fruits of five to ten individuals per species were collected and taken to the laboratory where seeds were removed from fruits and screened in order to discard malformed and/or predated seeds. Seeds were then stored under laboratory conditions for a few months prior to the germination trials, except for Theobroma cacao, which produces recalcitrant seeds (Venial et al., Reference Venial, Alexandre, Camata, Lopes, Zanotti, Ferreira and Aguilar2017) and thus germination trials were set up immediately after these seeds were harvested.
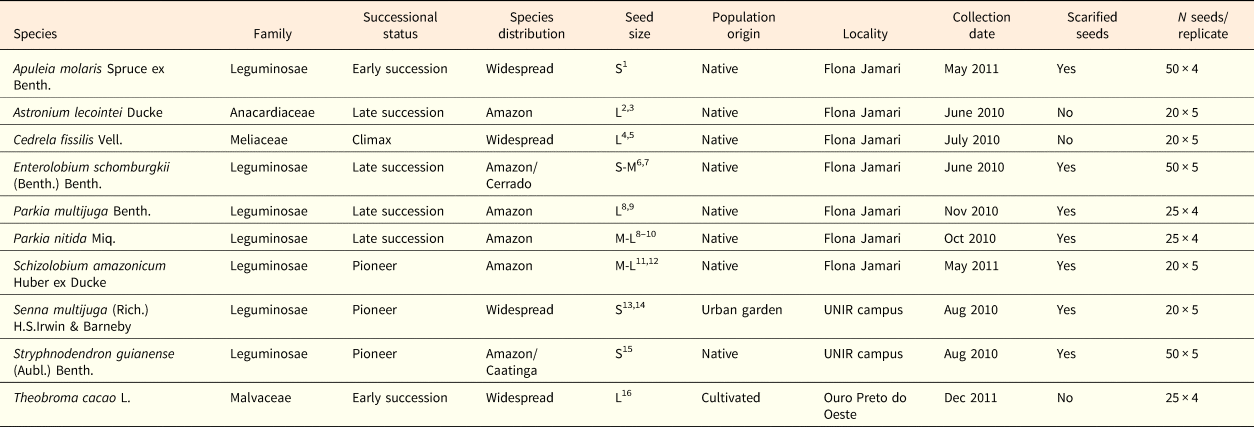
Table 1. Study species, family, distribution, successional status, population origin (native or cultivated) and number of seeds per replicate
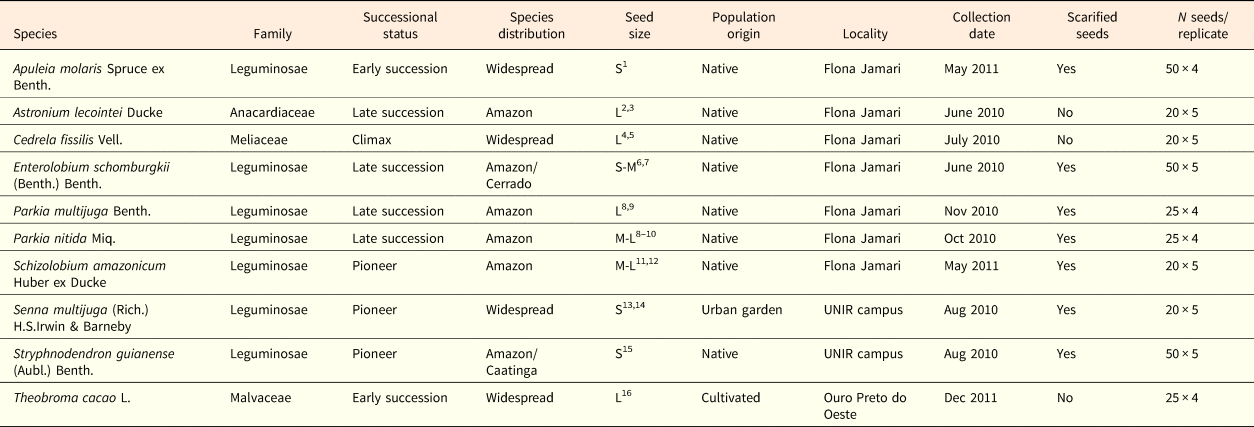
1Reis et al. (Reference Reis, Freitas, Leão and Santos Filho2016); 2Ferraz et al. (Reference Ferraz, Leal Filho, Imakawa, Varela and Piña-Rodrigues2004); 3Cornejo and Janovec (Reference Cornejo and Janovec2010); 4Pereira et al. (Reference Pereira, Navroski, Hoffmann, Grabias, Blum, Nogueira and Rosa2017); 5Angeli et al. (Reference Angeli, Barrichelo and Müller2005); 6Ramos and Ferraz (Reference Ramos and Ferraz2008); 7Bonadeu and Santos (Reference Bonadeu and Santos2013); 8Camargo et al. (Reference Camargo, Ferraz, Mesquita, Santos and Brum2008); 9Carvalho (Reference Carvalho2009); 10Díaz-Bardales (Reference Díaz-Bardales2001); 11Souza et al. (Reference Souza, Rossi, Azevedo and Vieira2003); 12Braga et al. (Reference Braga, Oliveira and Sousa2013); 13Amorim et al. (Reference Amorim, Davide, Ferreira and Chaves2008); 14Carvalho (Reference Carvalho2004); 15Freitas et al. (Reference Freitas, Viegas and Lopes2014); 16Venial et al. (Reference Venial, Alexandre, Camata, Lopes, Zanotti, Ferreira and Aguilar2017). Successional status was classified according to Ferraz et al. (Reference Ferraz, Leal Filho, Imakawa, Varela and Piña-Rodrigues2004) and Amaral et al. (Reference Amaral, Vieira, Almeida, Salomão, Silva and Jardim2009). Species distribution was classified according to the vegetation domain described in Flora do Brasil 2020 (http://floradobrasil.jbrj.gov.br). Seed size was classified from the literature (see below), following the criteria proposed by Cornejo and Janovec (Reference Cornejo and Janovec2010) that is based on seed length: S = small = 0.5–0.99 cm; M = medium = 1–1.99 cm; L = large ≥ 2 cm. Approximate GPS coordinates of sites where seeds were collected: Flona Jamari (9°0´S; 62°44´W); UNIR campus (8°50´S; 63°56´W); Ouro Preto do Oeste (10°44´S; 62°12´W).
All studied species have a broad distribution across the Amazon basin. One species (T. cacao) is cultivated in South and Central America and three species (Apuleia molaris, Cedrela fissilis and Senna multijuga) also occur throughout most of the Brazilian territory. According to the literature, the species used in this study are considered as successional species (Ferraz et al., Reference Ferraz, Leal Filho, Imakawa, Varela and Piña-Rodrigues2004; Amaral et al., Reference Amaral, Vieira, Almeida, Salomão, Silva and Jardim2009). Seed size was classified from information extracted from the literature and based on the criteria established by Cornejo and Janovec (Reference Cornejo and Janovec2010) (Table 1). The forest areas sampled show a tropical rainy climate, similar to most areas across the Amazon basin (see Bastos, Reference Bastos1982), with a mean annual temperature of 25°C, minimum temperature of 18°C, maximum temperature >30°C (Bastos and Diniz, Reference Bastos and Diniz1982), and a mean annual precipitation that can reach over 2000 mm (INMET, 1961–1990). Rainfall is well distributed throughout the year but may be reduced during drier months, from June to August (Marengo et al., Reference Marengo, Liebmann, Kousky, Filizola and Wainer2001).
Germination trials
For all species, germination trials were conducted under 10 constant temperatures (10, 13, 15, 17, 20, 25, 30, 35, 37 and 40°C) in different germination chambers under constant white light, except for Enterolobium schomburgkii, in which experiments at 35 and 37°C were not carried out due to restraints in seed availability. Number of replicates varied between four and five, and number of seeds per replicate varied from 20 to 50 seeds (Table 1). For the germination experiments, seeds were placed in plastic boxes with two layers of filter paper saturated with distilled water. Germination was determined by protrusion of the primary root (Bewley et al., Reference Bewley, Bradford, Hilhorst and Nonogaki2013) and experiments were monitored daily for 30 days. By the end of the trials, non-germinated seeds were visually inspected to assure they were unviable and thus counted as dead.
Some of the study species (legumes, see Table 1) had impermeable seed coats (physical dormancy; see Baskin et al., Reference Baskin, Baskin and Li2000) and physical dormancy was confirmed by preliminary germination tests where seeds showed no signs of imbibition. Thus, seeds of these species were mechanically scarified with sandpaper prior to the germination experiments, except for A. molaris, in which seeds were chemically scarified with sulfuric acid for two minutes.
Germination thermal parameters
Germination capacity (G%) was determined as the proportion of germinated seeds in relation to the total number of seeds sown in the plastic boxes. In addition, we calculated the germination time (t50) and the germination rate (GR, i.e. inverse of germination time) for 50% of germinated seeds at each temperature for each species. Germination time and germination rate were also calculated separately only for the replicates in which G% reached 50%, even though the mean of G% might not reach 50% in some exceptional cases. To calculate germination time, we used a linear interpolation between two points of the cumulative germination curve around the desired percentage of germination (G% = 50) from the y-axis and used a line equation to calculate the estimated time (t50) from the x-axis (Steinmaus et al., Reference Steinmaus, Prather and Holt2000; Soltani et al., Reference Soltani, Ghaderi-Far, Baskin and Baskin2015). We established two optimal temperature ranges: one considering high G% (values over 80%) and one considering the highest GR50 (lowest t50) combined with a high G%, which usually delimits a sub- and a supra-optimal range. Thus, best germination times (t50) were assigned as the lowest numerical value of t50 (days) within the optimal G% range.
We used regression analysis to examine the association between GR50 and temperatures. The base temperature (Tb) was then calculated as the point in which the regression line touches the x-axis (y = 0), thus ceasing the germination process (Steinmaus et al., Reference Steinmaus, Prather and Holt2000). Thermal time (θT) requirement was calculated as the reciprocal of the slope (1/b values) in the regression lines in the sub-optimal range (Garcia-Huidobro et al., Reference Garcia-Huidobro, Monteith and Squire1982; Bradford, Reference Bradford2002). Ceiling temperatures (Tc) could not be precisely obtained mostly due to the small number of points found in the supra-optimal range. Therefore, Tc values were estimated based on which temperature G% was reduced to below 5%.
Data analysis
We used linear models to evaluate how temperatures affected seed germination for each species separately, considering a significance level of α = 0.05. Linear models were carried out with the lme4 package (Bates et al., Reference Bates, Maechler, Bolker and Walker2015) in R software version 3.2.5 (R Core Team, 2016). Post-hoc Tukey's tests were performed to evaluate multiple comparisons among temperatures using the multcomp package (Hothorn et al., Reference Hothorn, Bretz and Westfall2008). Likewise, we used regression analysis and linear models to evaluate how the different temperatures affected GR50 for each species. Tb, θ50 and optimal t50 values were compared between species using linear models after log transformations to fit model assumptions.
Results
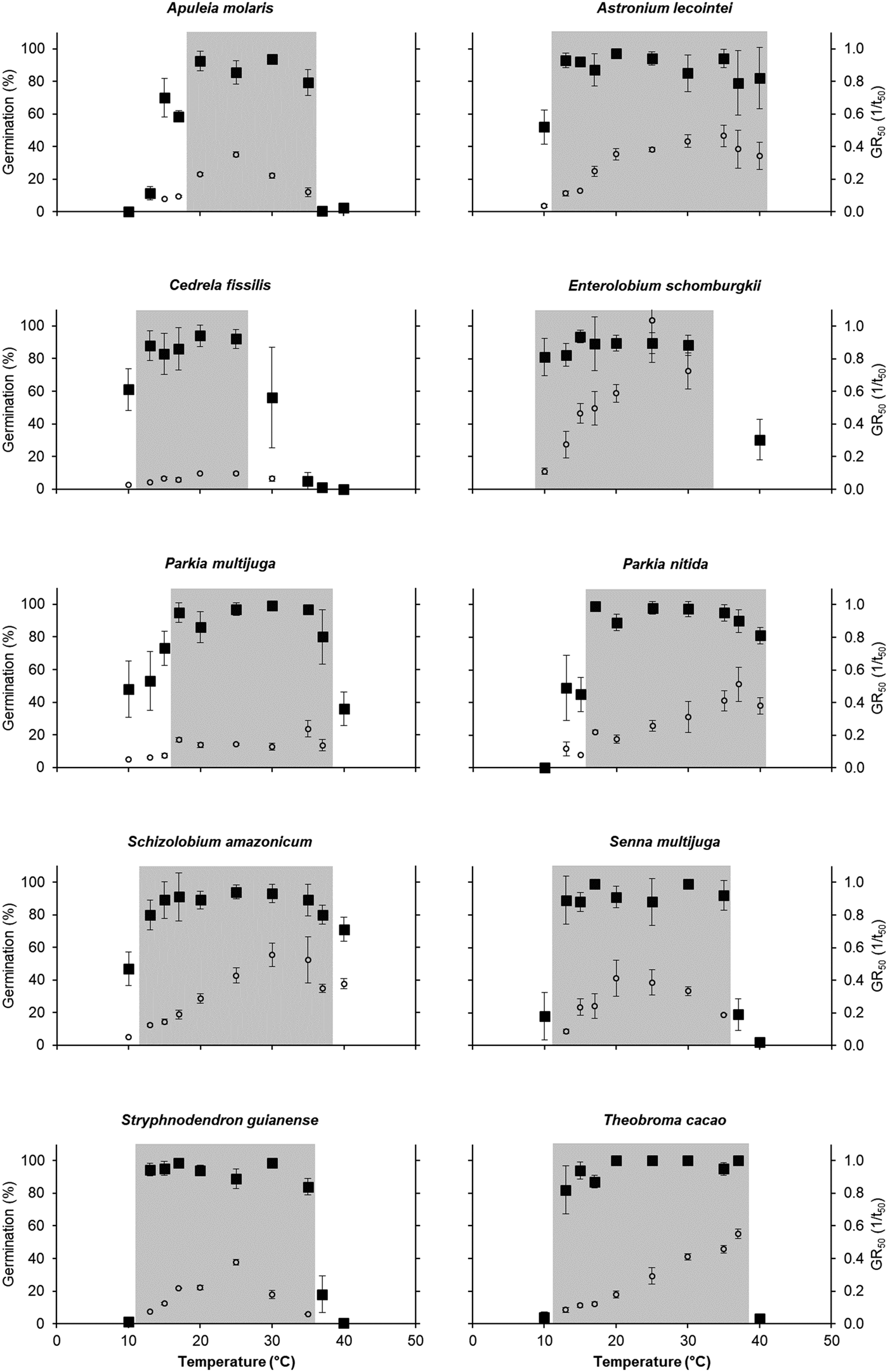
For all species, there was a wide range of temperatures at which germination reached 80–100% (Fig. 1). All species showed germination ≤60% under the lowest temperature tested (10°C), except for E. schomburgkii, which already had germination of over 80% at this treatment. Most species started to show high germination percentages at 13°C; however, Parkia spp. required 17°C and A. molaris required 20°C to reach G% ≥80%. Regarding the hotter temperatures tested, only C. fissilis showed reduced germination (56%) at 30°C, while the other species showed reduced germination only at 37–40°C. Nevertheless, seeds of three species kept high germination at 40°C: Astronium lecointei (82%), Parkia nitida (81%) and Schizolobium amazonicum (71%).

Fig. 1. Germination capacity (G%, black squares) and germination rate (GR50, white circles, 1/t50) under a range of constant temperatures of ten tree species occurring in the western Brazilian Amazon. Optimal ranges for maximal G% are shaded in grey.
Therefore, considering G%, A. lecointei displayed the broader range of optimal temperatures, ranging from 13 to 40°C. The optimal range for S. amazonicum and T. cacao was from 13 to 37°C, while S. guianense and S. multijuga showed higher G% from 13 to 35°C. The species with the shorter optimal range was A. molaris (from 20 to 35°C) and C. fissilis (from 13 to 25°C) (Fig. 1; Table 2). GR values showed a more specific thermal dependence, increasing linearly with temperature up to an optimal point or range (Fig. 1). Astronium lecointei and P. nitida maintained a high G% and relatively high GR50 even under 40°C. For these two species, GR50 increased with increasing temperatures. A similar pattern was found in T. cacao, with increased GR50 up to 37°C, but at 40°C germination abruptly declined to zero (Fig. 1).
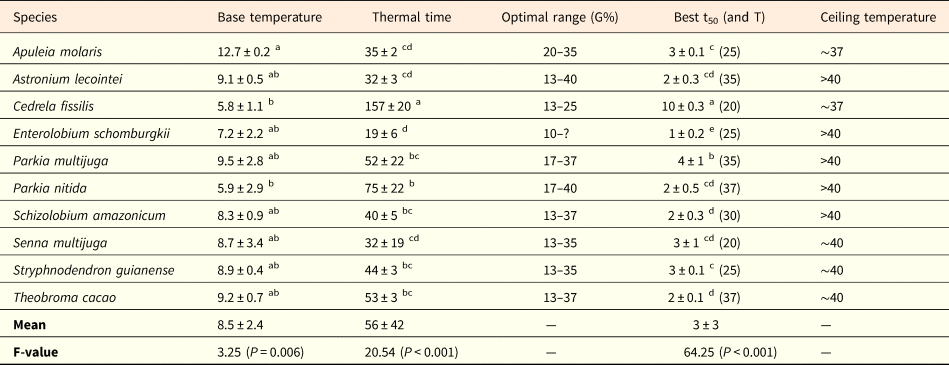
Table 2. Thermal parameters of germination of ten tree species occurring in the western Brazilian Amazon
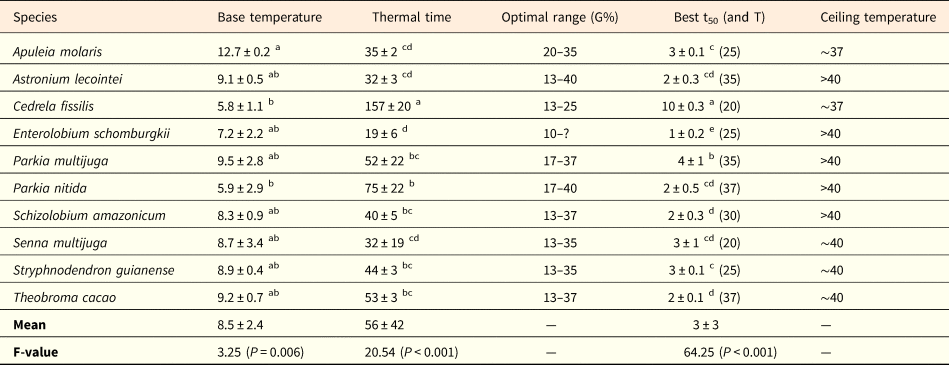
Base temperature (Tb, °C), thermal time requirement (θ50, °C.days), optimal range (temperature intervals, in °C, showing G% ≥80% with no statistical difference among them), best germination time (lowest numerical value of t50, in days; optimal temperature (T) where the best germination time was found is shown in parentheses), and approximate value of ceiling temperature (Tc, °C). All values are means ± standard deviation. Different superscript letters in the columns indicate significant differences among species (P ≤ 0.05). The question mark for E. schomburgkii indicates that optimal range could not be precisely determined for this species, given that temperatures of 35 and 37°C were not tested.
The shortest germination times (lowest numerical value of t50, in days) were found under the temperatures of 20 to 37°C. In the hottest temperatures, P. nitida and T. cacao showed the fastest germination times (t50) under 37°C, while A. lecointei and P. multijuga exhibited fastest t50 under 35°C (Table 2). For most species, t50 varied between 2 and 4 days. Only C. fissilis showed a slower germination time (t50 = 10 days at 20°C), contrary to E. schomburgkii, for which 50% of seeds germinated within 1 day of the experiment (Table 2). Likewise, thermal time requirement (θ50) varied from 32 to 75°C.days in all species, except for C. fissilis, which showed θ50 = 157°C.days, and E. schomburgkii, which showed the lowest θ requirement (θ50 = 19°C.days, see Table 2).
Most values of base temperature (Tb) varied from 7.2 to 9.5°C (Table 2), not differing among each other. The only difference was found between A. molaris, which had the highest Tb value (12.7°C), in relation to C. fissilis and P. nitida (both with Tb ~6°C, see Table 2). Ceiling temperatures (Tc) could not be precisely determined due to small number of temperatures falling in the supra-optimal range. Nevertheless, most species were tolerant to the hotter temperatures tested and would show Tc values >40°C, given that they still germinated more than 30% under the hottest treatment (Fig. 1; Table 2). Senna multijuga, S. guianense and T. cacao showed Tc limits around 40°C, temperature at which G% was nearly zero. For A. molaris and C. fissilis, germination was close to zero at 37°C.
Discussion
In the forest understory, temperatures may vary from 20 to 30°C (Benítez-Malvido and Martínez-Ramos, Reference Benítez-Malvido and Martínez-Ramos2003). However, forest gap openings alter the microclimate, enhancing sunlight availability and temperatures (Denslow, Reference Denslow1980; Chazdon and Pearcy, Reference Chazdon and Pearcy1991; Pearson et al., Reference Pearson, Burslem, Mullins and Dalling2002). Our data show that seeds maintain high G% and low germination times under a wide range of temperatures, with most optimal temperatures varying from 20 to 40°C (see also Ferraz and Varela, Reference Ferraz, Varela, Higuchi, Santos, Sampaio, Marenco, Ferraz, Sales, Saito and Matsumoto2003). Although temperature is a fundamental aspect of niche breadth (Thompson et al., Reference Thompson, Gaston and Band1999), our studied species do not seem to be particularly demanding with regard to their thermal requirements. Therefore, temperatures found in the forest would not be a limiting factor for seed recruitment. Cooler temperatures might cause a decrease in germination time, but G% is maintained >80% for most species, even under 13°C. Even though Amazon seeds rarely face temperatures below 20°C, their base temperature is around 9°C. However, such values are higher than Tb found in temperate species, which face severe winters and tolerate temperatures close to zero (Dürr et al., Reference Dürr, Dickie, Yang and Pritchard2015). Under hotter conditions, Tc values were commonly higher than 40°C, indicating their capacity to tolerate high temperatures.
Even though rainforest species often have non-dormant and large seeds (Vázquez-Yanes and Orozco-Segovia, Reference Vázquez-Yanes, Orozco-Segovia, Medina, Mooney and Vázquez-Yanes1984; Baskin and Baskin, Reference Baskin and Baskin2014; Rubio de Casas et al., Reference Rubio de Casas, Willis, Pearse, Baskin, Baskin and Cavender-Bares2017), seven of our species (all legumes) required scarification as they had water-impermeable seed coats (physical dormancy). The occurrence of this type of dormancy is related to seed size (Rubio de Casas et al., Reference Rubio de Casas, Willis, Pearse, Baskin, Baskin and Cavender-Bares2017), given it offers physical protection to the embryo (Rolston, Reference Rolston1978; Tweddle et al., Reference Tweddle, Dickie, Baskin and Baskin2003; Dalling et al., Reference Dalling, Davis, Schutte and Arnold2011), and is strongly clustered within tropical legumes (Dayrell et al., Reference Dayrell, Garcia, Negreiros, Baskin, Baskin and Silveira2017). Under natural conditions, physical dormancy is usually alleviated as a result of the higher temperatures found in gaps (Moreno-Casasola et al., Reference Moreno-Casasola, Grime and Martínez1994; Geisler et al., Reference Geisler, Pinto, Santos and Paulilo2017), but might also be related to their storage time in soil seed banks (Rodrigues-Junior et al., Reference Rodrigues-Junior, Baskin, Baskin and Garcia2018). Once scarified, seeds behave as non-dormant species, allowing germination parameters based on thermal time models to be calculated (Daibes and Cardoso, Reference Daibes and Cardoso2018).
Species that have a wide distribution range normally have a wide range of optimal temperatures for germination (see Marques et al., Reference Marques, Atman, Silveira and Lemos-Filho2014). However, among the studied species, such a pattern remained unclear: S. multijuga was the only widespread species showing a broad plateau of optimal temperatures, while A. lecointei, a species restricted to the Amazon basin, showed a wide range of optimal temperatures. Apuleia molaris, considered to have a wide distribution range, showed the narrowest range of optimal G% (20–35°C), which suggests that germination niche breadth is not always linked to a broader distributional range (Thompson and Ceriani, Reference Thompson and Ceriani2003). Despite the narrow optimal range, the temperatures at which A. molaris germinates are still within the range of temperatures usually found in the understory. Nevertheless, in forest gaps where temperatures could reach over 40°C (Pearson et al., Reference Pearson, Burslem, Mullins and Dalling2002; Geisler et al., Reference Geisler, Pinto, Santos and Paulilo2017), seed germination would be hampered as G% of A. molaris falls after 35°C.
The most sensitive species to the hotter temperatures was the climax species C. fissilis, for which germination was reduced at 35°C and reached nearly zero at 37°C. Because C. fissilis showed a relatively high germination time (t50 = 10 days), it also had the highest thermal time requirement, irrespective of its widespread distribution. Highest thermal requirement could be related to larger seed size of climax species, which tend to show slow-growing (and shade-tolerant) seedlings (Foster, Reference Foster1986; Swaine and Whitmore, Reference Swaine and Whitmore1988; Souza and Válio, Reference Souza and Válio2001, Reference Souza and Válio2003). In contrast, the small-seeded E. schomburgkii (a late-successional species) had the quickest germination time and lowest thermal time requirement. Other late-successional species, including the large-seeded P. multijuga, germinated most of their seeds within a few days after experiments began and showed similar θ values in comparison with the other species. The inclusion of more climax and late-successional species would be desired to unfold a clearer pattern regarding thermal requirements, successional status, and seed size in tropical forests.
Independent of the successional status, some species (see A. lecointei and P. nitida) showed an enhanced GR50 at the hottest temperatures, maintaining a germination of ≥ 80% even at 40°C. Such unique patterns differ from most theoretical thermal-dependence patterns that have been described, including those for tropical legumes (Daibes and Cardoso, Reference Daibes and Cardoso2018). Therefore, we argue that increasing kinetics of seed germination under hotter temperatures might be an ecological strategy to face forest gaps, thus favouring the rapid recruitment of understory species (Chazdon and Pearcy, Reference Chazdon and Pearcy1991). However, GR-temperature dependence should increase linearly with temperature, reaching a point where it decreases from optimal to supra-optimal ranges until the ceiling temperature (Alvarado and Bradford, Reference Alvarado and Bradford2002; Bewley et al., Reference Bewley, Bradford, Hilhorst and Nonogaki2013). In T. cacao, GR increased up to 37°C and then germination dropped abruptly from 100% to zero at 40°C (Fig. 1). Hence, instead of slowly decreasing GR in the supra-optimal range, there was a short but decisive interval of 3°C that drove seed germination from its maximum capacity to death. Other species (see Table 2) showed Tc values greater than the hottest temperature tested, thus treatments testing temperatures over 40°C are necessary to stipulate their supra-optimal range limits.
Even though most species are tolerant to high temperatures, small differences in their germination requirements, especially of their supra-optimal range, could act as potential drivers of seed recruitment in tropical rainforests (see Daws et al., Reference Daws, Burslem, Crabtree, Kirkman, Mullins and Dalling2002; Pearson et al., Reference Pearson, Burslem, Mullins and Dalling2002), especially in more disturbed sites where temperatures are usually higher. However, temperatures are normally lower than their supra-optimal range in forest understories and would not play such an important role in seed recruitment. At these sites, non-dormant seeds can be quickly recruited, forming seedling banks that compete for light in the understory. Therefore, seed and seedling traits might show complementary trade-offs contemplating all aspects of the regeneration niche in tropical forests (Osunkoya et al., Reference Osunkoya, Ash, Hopkins and Graham1994; Daws et al., Reference Daws, Ballard, Mullins, Garwood, Murray, Pearson and Burslem2007). Thus, intermediate-successional species would be able to germinate in the understory but would also be able to take advantage from forest gaps in disturbed sites and grow into the canopy (Denslow, Reference Denslow1980).
In conclusion, germination temperature limits may be similar among Amazon species but patterns in their thermal requirements seem to be different. Efficient thermal energy use throughout the germination processes leads to increasing germination with higher temperature, thus enhancing the chance of a successful establishment. On the other hand, in the supra-optimal range, delicate limits can be found as temperatures increase, with germination dropping to zero within a short interval of temperatures. This might be fundamental to understand germination responses in the context of habitat loss and fragmentation, given climate and microclimate conditions are altered (Laurence and Williamson, Reference Laurence and Williamson2001). Climate change consequences could also affect the dynamics of forest understories as the Intergovernmental Panel on Climate Change (IPCC) predict, for the Amazon region, an increase in temperature of 3–5°C in the less pessimistic scenario and an increase of up to 8°C in the more pessimistic scenario (Ambrizzi et al., Reference Ambrizzi, Rocha, Marengo, Pisnitchenko, Alves and Fernandez2007; Malhi et al., Reference Malhi, Roberts, Betts, Killeen, Li and Nobre2008). Moreover, other aspects of seed germination should be examined, such as tolerance to moisture, as it may negatively affect germination, especially in large-seeded species (Daws et al., Reference Daws, Crabtree, Dalling, Mullins and Burslem2008). Cardinal temperatures may also change according to the life cycle stage, differing between seed germination and seedling growth (Bastos et al., Reference Bastos, Ferraz, Lima Junior and Pritchard2017). Thus, it remains unclear how recruitment of Amazon species will respond to global change. Additional research is needed to understand whether seed size and successional status explain thermal parameters of seed germination, as well as how the thermal requirements change during seedling establishment. This study helps to start elucidating important questions regarding seed regeneration in the Amazon rainforest.
Author ORCIDs
L. Felipe Daibes, 0000-0001-8065-6736
Acknowledgements
We thank the Programa Petrobras Ambiental for funding this study and the team of the Centro de Estudos Rioterra for the collaboration, especially A. Bastos who coordinated the Project Semeando Sustentabilidade. We thank Dejesus Aparecido (Cidinho) for technical support and seed collection in the field. We also would like to thank the staff of the Floresta Nacional do Jamari and Caio Márcio V.C. de Almeida (CEPLAC-RO) for the collection of cocoa seeds (Theobroma cacao). Undergraduate students received grants from PIBIC/UNIR/CNPq at the Universidade Federal de Rondônia. We thank T.M. Zupo for reviewing the text.