Introduction
Soils of agricultural and early successional environments contain reservoirs of viable seeds (hereafter referred to as ‘seed banks’) that provide opportunities for population growth during years with low reproductive success (Fenner and Thompson, Reference Fenner and Thompson2005; Alexander et al., Reference Alexander, Pilson, Moody-Weis and Slade2009) and that conserve genetic information potentially beneficial for population persistence in variable environments (England et al., Reference England, Whelan and Ayre2003). Seed-bank persistence is influenced by traits received from parents by genetic transmission, and therefore, seed populations maturing on plants with different evolutionary histories can vary in capacities for seed-bank persistence (reviewed by Baskin and Baskin, Reference Baskin and Baskin1998).
Venable and Brown (Reference Venable and Brown1988) proposed that seed-bank persistence evolves interdependently with seed size and capacity for long-distance dispersal, such that strong selection for one trait coincides with weak selection for the other two. This hypothesis builds on evidence that seed size, seed-bank persistence and seed dispersal each represent strategies to improve seedling recruitment by increasing the proportion of seeds completing germination in environments conducive to seedling emergence (Cohen, Reference Cohen1966; Comins et al., Reference Comins, Hamilton and May1980; Gross, Reference Gross1984). Although previous studies identified negative relationships between seed size and seed-bank persistence among co-occurring species of certain floras (Thompson et al., Reference Thompson, Band and Hodgson1993, Reference Thompson, Jalili, Hodgson, Hamzeh'ee, Asri, Shaw, Shirvany, Yazdani, Khoshnevis, Zarrinkamar, Ghahramani and Safavi2001; Rees, Reference Rees1996; Funes et al., Reference Funes, Basconcelo, Diaz and Cabido1999; Cerabolini et al., Reference Cerabolini, Ceriani, Caccianiga, Andreis and Raimondi2003; Wang et al., Reference Wang, Jiao, Jia and Wang2011; Zhao et al., Reference Zhao, Wu and Cheng2011), additional studies failed to find correlations between seed size and seed-bank persistence among plant species of specific environments (Leishman and Westoby, Reference Leishman and Westoby1998; Moles et al., Reference Moles, Hodson and Webb2000; Yu et al., Reference Yu, Sternberg, Kutiel and Chen2007). Inverse relationships between seed size and seed-bank persistence have been reported among closely related species within genera (de Jong et al., Reference de Jong, Isanta and Hesse2013) and among individuals within populations (Schutte et al., Reference Schutte, Regnier and Harrison2008b); however, intra-specific and intra-genera studies on relationships between seed size and seed-bank persistence are rare. Thus, studies using closely related taxonomic units do not provide conclusive evidence for interdependent evolution among seed traits as proposed by Venable and Brown (Reference Venable and Brown1988).
Longevity in soil results from a suite of characteristics that collectively inhibit germination while preventing mortality due to physiological ageing, pathogen attack and predation (Fenner and Thompson, Reference Fenner and Thompson2005). Seed deterrents to predators and pathogens include chemicals with antiherbivory, antifungal and bactericidal properties (Banko et al., Reference Banko, Cipollini, Breton, Paulk, Wink and Izhaki2002; Xu et al., Reference Xu, Zhang, Wang and Lu2010); physically restrictive embryo-covering structures (Paulsen et al., Reference Paulsen, Colville, Kranner, Daws, Hogstedt, Vandvik and Thompson2013); and symbiotic relationships with beneficial microorganisms (Kremer, Reference Kremer1986). Protection from physiological ageing is afforded by antioxidant defence mechanisms that remove potentially damaging molecular species (Corbineau, Reference Corbineau2012) and embryo-covering structures that prevent imbibition injury (McDonald, Reference McDonald1999). Dalling et al. (Reference Dalling, Davis, Schutte and Arnold2011) proposed that seed defences to mortality evolve concurrently with germination constraints. Empirical support for interdependent evolution of defence and germination constraints was provided by Goggin et al. (Reference Goggin, Powles and Steadman2011) who determined that high-dormancy and low-dormancy seeds of Lolium rigidum Gaudin differed with respect to constitutive physiological responses to abiotic stress, such that high-dormancy seeds were better equipped to survive environmental stresses after imbibition as compared to low-dormancy seeds. Khan et al. (Reference Khan, Cavers, Kane and Thompson1997) determined that differences in seed dormancy among biotypes of Panicum miliaceum L. corresponded with differences in protection from imbibition injury derived from embryo-covering structures. These examples of concurrent variation in seed defence and seed dormancy suggest that measurements of seed defence can clarify alleged associations between seed-bank persistence and seed size.
Abutilon theophrasti Medic. is a problematic annual weed in crop production systems across temperate regions of North America, Europe and Asia (Warwick and Black, Reference Warwick and Black1988). The success of A. theophrasti in agricultural fields has been attributed to the longevity of seeds in the soil (Cardina and Norquay, Reference Cardina and Norquay1997), which is promoted by hard seed coats that prevent imbibition of water necessary for germination (LaCroix and Staniforth, Reference LaCroix and Staniforth1964), a condition referred to as ‘physical seed dormancy’ (Baskin and Baskin, Reference Baskin and Baskin1998). The pervasiveness and perniciousness of A. theophrasti in agricultural systems overshadows this plant species' history as a crop grown for fibre, food and medicine (Spencer, Reference Spencer1984; Medovic and Horvath, Reference Medovic and Horvath2012). Crop, weedy and crop–weedy mixed hybrids (hereafter referred to as ‘mixed’) accessions of A. theophrasti from across a broad geographic region have been maintained (Kurokawa et al., Reference Kurokawa, Shimizu, Uozumi and Yoshimura2003; Sosnoskie, Reference Sosnoskie2005). Many of the preserved accessions differ in plant growth and architecture, as well as seed morphology and seed dormancy (Kurokawa et al., Reference Kurokawa, Shimizu, Uozumi and Yoshimura2003; Sosnoskie, Reference Sosnoskie2005).
The primary defence structure against mortality in A. theophrasti seeds is the seed coat (Davis et al., Reference Davis, Schutte, Iannuzzi and Renner2008), which is the principal protectant in seeds of many species (Mohamedyasseen et al., Reference Mohamedyasseen, Barringer, Splittstoesser and Costanza1994). Coats protect seeds by physically blocking predators and pathogens from nutritional reserves (Kremer et al., Reference Kremer, Hughes and Aldrich1984), by limiting expulsion of chemical cues used by adversarial organisms (Van der Wall, Reference Van der Wall1998) and by storing chemical defences (Banko et al., Reference Banko, Cipollini, Breton, Paulk, Wink and Izhaki2002; Xu et al., Reference Xu, Zhang, Wang and Lu2010). Protective properties of seed coats can be measured through a variety of ways (e.g. mechanical strength, chemical composition). However, seed-coat thickness provides a straightforward assessment of the cumulative defence effects of seed coats, as was shown by Davis et al. (Reference Davis, Schutte, Iannuzzi and Renner2008) who determined that differences among species in seed-coat thickness were positively associated with seed-bank persistence, and by Gardarin et al. (Reference Gardarin, Durr, Mannino, Busset and Colbach2010) who determined that mortality in seed banks decreased with increasing seed-coat thickness.
The hypothesis regarding interdependent evolution of germination constraints and seed defences proposed by Dalling et al. (Reference Dalling, Davis, Schutte and Arnold2011) has yet to be integrated with the hypothesis on concurrent evolution of seed-bank persistence and seed size proposed by Venable and Brown (Reference Venable and Brown1988). Separation between these hypotheses limits understanding of the traits associated with seed survival in the soil. The objective of this study was to determine if seed characteristics of A. theophrasti accessions vary in manners consistent with the predictions of both Venable and Brown (Reference Venable and Brown1988) and Dalling et al. (Reference Dalling, Davis, Schutte and Arnold2011). With respect to this objective, we hypothesized that: (1) variation in seed morphology among A. theophrasti accessions corresponds with differences in seed-coat allocation, such that seed coats are thicker for smaller-seeded accessions compared to larger-seeded accessions; (2) A. theophrasti accessions characterized by smaller seeds and thicker seed coats are more dormant and more persistent than accessions with larger seeds and thinner seed coats; and (3) statistical models with both seed-coat thickness and seed-size data better explain variability in seed-bank persistence and seed dormancy than statistical models with only seed-size data.
Materials and methods
Plant material
Sixteen A. theophrasti accessions were selected from a collection maintained at The Ohio State University (OSU collection; Table 1). The OSU collection is described by Sosnoskie (Reference Sosnoskie2005) and includes accessions originally collected by Dr R.N. Andersen and by the N.I. Vavilov Institute of Plant Industry. With accession labels corresponding to those in Sosnoskie (Reference Sosnoskie2005), A. theophrasti accessions used in this study were: Andersen (A)-6, A-15, A-23, A-25, A-39, and Vavilov (V)-499213, V-499215, V-499218, V-499222, V-499224, V-499231, V-499232, V-499234, and V-499244, V-499247 and V-499252. This set of accessions comprised ‘weedy’, ‘crop’ and ‘mixed’ biotypes. In addition, weedy accessions were collected from agricultural fields at the University of Illinois, Crop Sciences Research and Education Centre (CSREC; latitude = 40.049, longitude = − 88.233).
Table 1 Abutilon theophrasti accessions that served as maternal sources of seeds used in this study. Information from Sosnoskie (Reference Sosnoskie2005)
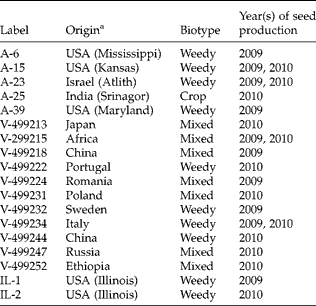
a State/provenance provided in parentheses. State/provenance information is not available for Vavilov (V) accessions.
To obtain sufficient quantities of seeds for the experiments described below, all accessions were grown for one generation in an isolated field (10 m by 45 m) at CSREC. Beginning 17 June 2009 and 20 June 2010, three plants per accession were grown in rows (5 m length) that were spaced 2 m apart. On 18 September 2009 and 2 September 2010, mature fruits (dehisced capsules) were collected from all plants, dried at 25°C and manually crushed to expel seeds. Seeds were separated from chaff with combinations of sieving and forced-air separation. Cleaned seeds were then stored in moisture-proof containers at 3–5°C until use. In this study, a ‘seed lot’ was defined as a population of seeds with a common maternal accession and a shared maturation year. Thus, ten seed lots were produced in 2009 and 12 seed lots were produced in 2010 (Table 1).
Genetic analysis
A. theophrasti flowers can be self- or cross-pollinating (Andersen, Reference Andersen1988). To account for the potential of depleted genetic diversity caused by a dominant pollen source (Sork and Smouse, Reference Sork and Smouse2006), genetic variation among seed lots produced during a specific year was determined using the intersimple sequence repeat (ISSR) technique (Godwin et al., Reference Godwin, Aitken and Smith1997), which was previously used to detect genetic polymorphisms among A. theophrasti accessions (Kurokawa et al., Reference Kurokawa, Shimizu, Uozumi and Yoshimura2003). Plants for ISSR were produced by germinating seeds of each seed lot in Petri dishes that were lined with moistened filter papers and that were placed in a growth chamber set to 25/15°C day/night temperatures, 12 h photoperiods. Germinated seeds with 1-cm radicles were then transferred to 10-cm-diameter pots containing growing media (Metro-Mix 360; Sun Gro Horticulture, Agawam, Massachusetts, USA). Transplanted seedlings were placed into a growth chamber set to 25/15°C day/night temperatures, 16 h photoperiods, with photosynthetic photon flux density within the chamber approximately 200 μmol m− 2s− 1. Plants were allowed to grow for 28 d. Leaf tissues were then collected and stored at − 20°C until DNA isolation.
DNA was isolated using a commercial DNA extraction kit (DNAeasy® Plant Mini Kit, Qiagen, Venlo, Netherlands). Following extraction, total DNA was quantified using a spectrophotometer (Nanodrop ND-1000, Thermo Scientific, Wilmington, Delaware, USA). Based on spectrophotometer results, DNA samples were diluted to 25 ng μl− 1 for further analyses. Polymerase chain reaction (PCR) amplification was carried out using an initial denaturation of 7 min at 94°C, followed by 45 cycles at 45°C for 30 s, 94°C for 30 s, 52°C for 45 s, 72°C for 2 min, and a final extension for 7 min at 72°C in a Techne TC-412 (Barloworld Scientific US Ltd, Burlington, New Jersey, USA). PCR product was diluted accordingly and run on a 6% acrylamide gel for 12 h. Gels were stained for 30 min using SYBR® Green Nucleic Acid Gel Stain and visualized. Ten primers from Kurokawa et al. (Reference Kurokawa, Shimizu, Uozumi and Yoshimura2003) were used for the initial ISSR analysis, which included two randomly selected individuals from each of two seed lots. Five primers that produced a clear polymorphic banding pattern in the initial analyses were selected for the analysis of the entire sample set of seed lots. For the analysis of all seed lots, DNA from five individuals within each accession was pooled. The primers used in the final ISSR analysis included (AG)8T, (GA)8TG, (AC)8TG, (GA)8T, (AG)8G and were purchased from Integrated DNA Technologies (IDT, Coralville, Iowa, USA).
Electrophoretic data were scored for the presence (1) or absence (0) of each PCR fragment (compared to all populations). A binary matrix of 1 and 0 was constructed for each population sample. Data matrices were used to construct similarity matrices, using simple matching coefficients. Genetic similarity dendrograms were constructed by further analysis of similarity data, using the UPGMA (unweighted pair group method for arithmetic averages) cluster analysis in the NTSYS-PC 2.02 computer program (Rohlf, Reference Rohlf1997).
Seed dormancy
Within 1 month of harvest, seed lots were subjected to germination assays conducted in a growth chamber at 25/15°C day/night temperatures, 12 h photoperiods, with photosynthetic photon flux density approximately 45 μmol m− 2s− 1 within the chamber. Experimental units for germination assays were Petri dishes (10 cm diameter, 1.5 cm height) containing a single layer of moistened filter papers upon which 20 seeds were placed. The experimental design was a randomized complete block design with eight replications. Germination was monitored daily for 14 d, and germinated seeds were removed immediately from dishes. Seeds that failed to germinate by the end of the 14-d period were tested for viability with tetrazolium (TZ) staining assays, using a 1.0% aqueous solution of 2,3,5-triphenyl-tetrazolium chloride (Peters, Reference Peters2000). Dormancy was expressed as the percentage of total viable seeds that failed to germinate, with the total number of viable units being equal to the sum of the germinated seeds and viable, non-germinated seeds.
Seed fates after burial
Seed fates, including seedling emergence, seed-bank persistence and seed mortality, were determined with field burial trials that were conducted at CSREC. Field burial trials ran for approximately 1 year (17 November 2009 to 26 October 2010; 10 November 2010 to 4 October 2011). Immediately before burial, percentages of viable seeds were determined for samples from each seed lot (60 seeds lot− 1) using TZ staining assays described above. Experimental units for field burial trials were trays (5-cm width, 5-cm length, 5-cm depth; 100 seeds tray− 1) fabricated from aluminium mesh (1.5 mm square opening) and filled with soil [Flanagan silt loam (Fine, smectitic, mesic Aquic Argiudolls)] that was previously sieved through a 2 mm screen to remove A. theophrasti seed contaminates. A. theophrasti seeds were buried to a depth of 3 cm by: (1) depositing a layer of soil (1 cm thickness) across the bottoms of trays, (2) placing seeds on top of the deposited soil layer, and (3) filling trays with soil so that tray tops formed a lip 1 cm above the soil surface.
Experimental units were arranged in randomized complete blocks with four replicates. The study area was protected from surface-foraging predators by a mesh fence (6.4 mm square openings) and was kept free of unwanted vegetation with combinations of hand weeding and herbicide applications (glyphosate at 0.94 kg ai ha− 1). At weekly intervals beginning 1 March, emerged seedlings were counted and removed without soil disturbance by uprooting entire seedlings with forceps. Approximately 1 year after burial, mesh trays were excavated and ungerminated seeds were recovered with a mechanical elutriation procedure described by Wiles et al. (Reference Wiles, Barlin, Schweizer, Duke and Whitt1996). Recovered seeds were tested for viability with TZ staining assays, and resulting data were used to calculate the following fates: emergence (proportion of viable seeds added at the start of the experiment that produced a seedling), persistence (proportion of viable seeds added at the start of the experiment that were recovered after 1 year) and mortality (proportion of viable seeds added at the start of the experiment that did not emerge or persist).
Seed form and seed-coat thickness
For each seed lot, seed mass was determined for 40 individual seeds using a high-precision balance that enabled measurements to the nearest 0.1 mg. Seed morphological characteristics were quantified using digital images of magnified seeds (10 × magnification; 12 seeds lot− 1). Magnified seeds were positioned twice to show the flattened, broad face and the hilar face. Image analysis software (Rasband, Reference Rasband2007) was used to determine seed length (length of transect running through the long axis of the broad face), seed width (length of transect running through the short axis of the broad face), seed depth (length of transect running through the short axis of the hilar face) to the nearest 0.1 mm.
Seed-coat thickness and additional seed width data were obtained for 12 individual seeds lot− 1 using images obtained with a field-emission environmental scanning electron microscope (EDAX XL 30 ESEM-FG, FEI Company, Hillsboro, Oregon, USA) maintained by the Imaging Technology Group of the University of Illinois Beckman Institute. To prepare seeds for imaging, seeds were embedded in paraffin, sectioned along longitudinal axes with a scalpel, and sputter-coated with a nanolayer of gold/palladium (Delton Desk II TSC, Denton Vacuum Co., Moorestown, New Jersey, USA). For each seed, image analysis software was used to measure both seed width and the thickness of the seed coat macrosclerid layer in an area opposite the hilum to the nearest 0.1 μm. Seed-coat thickness was then divided by seed width to provide measurements of seed defence as proportions of overall seed allocation for individual seeds. Additional data on seed defence allocation was obtained by dividing seed-coat thickness by seed mass, using seed lot means. Hereafter, coat proportions based on seed width are referred to as ‘coat:width ratios’, and coat proportions based on seed mass are referred to as ‘coat:mass ratios’.
Statistical analyses
Hypothesis 1: Variation in seed morphology among A. theophrasti accessions corresponds with differences in seed-coat allocation such that seed coats are thicker for smaller-seeded accessions compared to larger-seeded accessions
All statistical analyses were performed using the open source statistical software program R (v.2.12.1, The R Foundation for Statistical Computing, http://www.r-project.org). Seed-lot effects on morphological traits, including seed length, width, mass and coat thickness, were determined with analyses of variance (ANOVA). Visual inspections of residuals plotted against fitted values indicated absences of relationships between variances and predicted values, and thus, data fulfilled ANOVA assumptions of homoscedasticity. Using seed-lot means, linear associations between morphological traits were assessed using Pearson correlation coefficients with Bonferroni-corrected P values. Data for each year were analysed separately, thereby allowing for an assessment of repeatability of alleged relationships between seed traits.
Hypothesis 2: A. theophrasti accessions characterized by smaller seeds and thicker seed coats are more dormant and more persistent than accessions with larger seeds and thinner seed coats
Dimensionality of morphology, size and coat thickness data was reduced using principal component analysis (Johnson, Reference Johnson1998) implemented with the prcomp function in R. Resulting principal component scores for the first rotation were utilized as independent variables in generalized linear mixed models for the binary response variables ‘persistence’ and ‘dormancy’, which were coded as persistent = 1, non-persistent = 0; and dormant = 1, germinable = 0. These models were developed with the R package lme4 using binomial distributions and logit link functions. Principal component scores were fixed effects, and random effects were experimental replicates.
Hypothesis 3: Statistical models with both seed-coat thickness and seed-size data better explain variability in seed-bank persistence and seed dormancy than statistical models with only seed-size data
Logistic regression models with single predictor variables (seed mass, seed length, seed-coat thickness) and with two predictor variables (seed length and seed-coat thickness, seed mass and seed-coat thickness) were compared using information-theoretic model selection (Burnham and Anderson, Reference Burnham and Anderson2002). Models were fit with the R function glm using binomial distributions and logit link functions. The R function glm was selected, in part, because it allows for determination of variance inflation factors using the R package car. Information on variance inflation was necessary because of possible collinearity (Zuur et al., Reference Zuur, Ieno and Elphick2010) between the predictor variables seed length and seed-coat thickness, as well as seed mass and seed-coat thickness. Preliminary analyses indicated that the relationships between seed traits, seed-bank persistence and seed dormancy were similar between years. Accordingly, logistic regression models used data combined across years and, in addition to seed morphology variables, included terms for year and replicate within year. The relative empirical support for candidate models was determined from ranks of Akaike's Information Criterion values corrected for small sample sizes (AICc). AICc includes a penalty term that increases as more model parameters are added (Burnham and Anderson, Reference Burnham and Anderson2002). Therefore, AICc allows for relative assessments of candidate models according to the balance between enhanced explanatory power that results from the addition of model parameters, and the risk of poor predictive performance that arises from too many model parameters relative to the number of observations. In this study, AICc values were used to assess model relative likelihoods with Akaike weights (w i ) in relation to the model that minimized AICc (Burnham and Anderson, Reference Burnham and Anderson2002).
Results
Genetic analysis
The number of bands produced by each primer set ranged from 6 to 28. A total of 63 bands were scored and 85.7% of the bands were polymorphic among accessions. In 2009, the genetic similarity of the seed lots ranged from 0.69 to 0.84 and averaged 0.78. In 2010, genetic similarity of the seed lots ranged from 0.71 to 0.93 and averaged 0.84. Genetic similarity dendrograms indicated that seed lots did not separate according to Andersen (A) and Vavilov (V) accessional origins (Fig. 1) and that seed lots did not separate according to the seed traits measured in this study (data not shown). These results suggest that the number of primers used in the ISSR analyses was insufficient to detect genetic polymorphisms in seed traits potentially controlled by a limited number of genes. Nonetheless, ISSR analyses indicated that A. theophrasti seed lots of a particular year represented genetically distinct seed populations produced under common conditions.
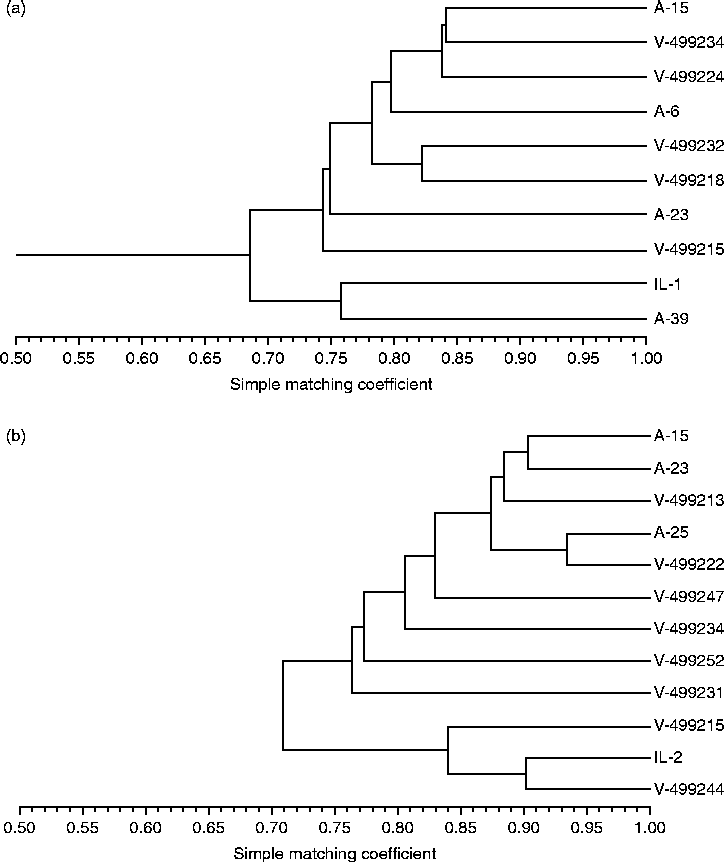
Figure 1 Dendrogram of ten Abutilon theophrasti seed lots in 2009 (a) and 12 A. theophrasti seed lots in 2010 (b). Dendrograms were developed with the UPGMA cluster analysis in the NTSYS-PC 2.02 computer program using intersimple sequence repeat (ISSR) markers obtained with five primers.
Seed morphology and seed defence
Seed lot influenced morphological traits of individual seeds, including seed length, seed width, seed mass and seed-coat thickness (Table 2). Seed length, width and mass were not correlated with direct measurements of seed-coat thickness (Table 3). However, measurements of seed-coat thickness expressed as a ratio of seed width (coat:width ratio) and as a ratio of seed mass (coat:mass ratio) were negatively correlated with morphological variables including seed width and seed mass, which indicated that coats constituted greater fractions of the whole as seed size decreased. Negative associations between seed size and coat:width ratio, as well as seed size and coat:mass ratio were observed in both 2009 and 2010.
Table 2 Ranges of seed-lot means and seed-lot effects on morphological traits, seed-bank fates and dormancy rates for the Abutilon theophrasti seed lots used in the study
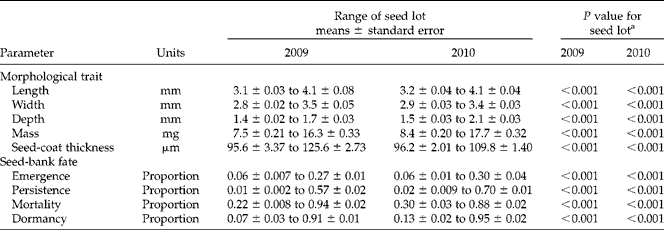
a For morphological traits, P values are for F-tests for significance in variation among seed lots in the specified parameter. For seed-bank fates and dormancy, P values are for likelihood ratio tests for seed-lot effects on the specified parameter.
Table 3 Pearson correlation coefficients for linear relationships between morphological trait means for Abutilon theophrasti seed lots produced in 2009 (above diagonal) and 2010 (below diagonal). Significance levels indicated as: *P < 0.05, **P < 0.01, ***P < 0.001
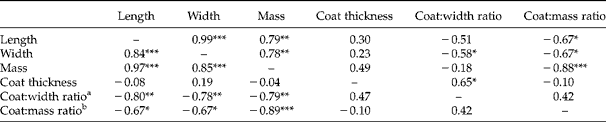
a Coat:width ratio is seed-coat thickness in proportion to seed width.
b Coat:mass ratio is seed-coat thickness in proportion to individual seed mass.
Principal component analyses reduced data for seed length, seed width, seed mass, coat:width ratio and coat:mass ratio to a single factor that accounted for 73% of the variation in seed traits among seed lots in 2009 and 91% of variation in seed traits among seed lots in 2010. Relationships between first rotation principal component scores and seed traits indicated that higher-factor scores described seed lots characterized by larger, heavier seeds with proportionately thinner seed coats (Fig. 2). Lower-factor scores described seed lots characterized by smaller, lighter seeds with proportionately thicker seed coats.
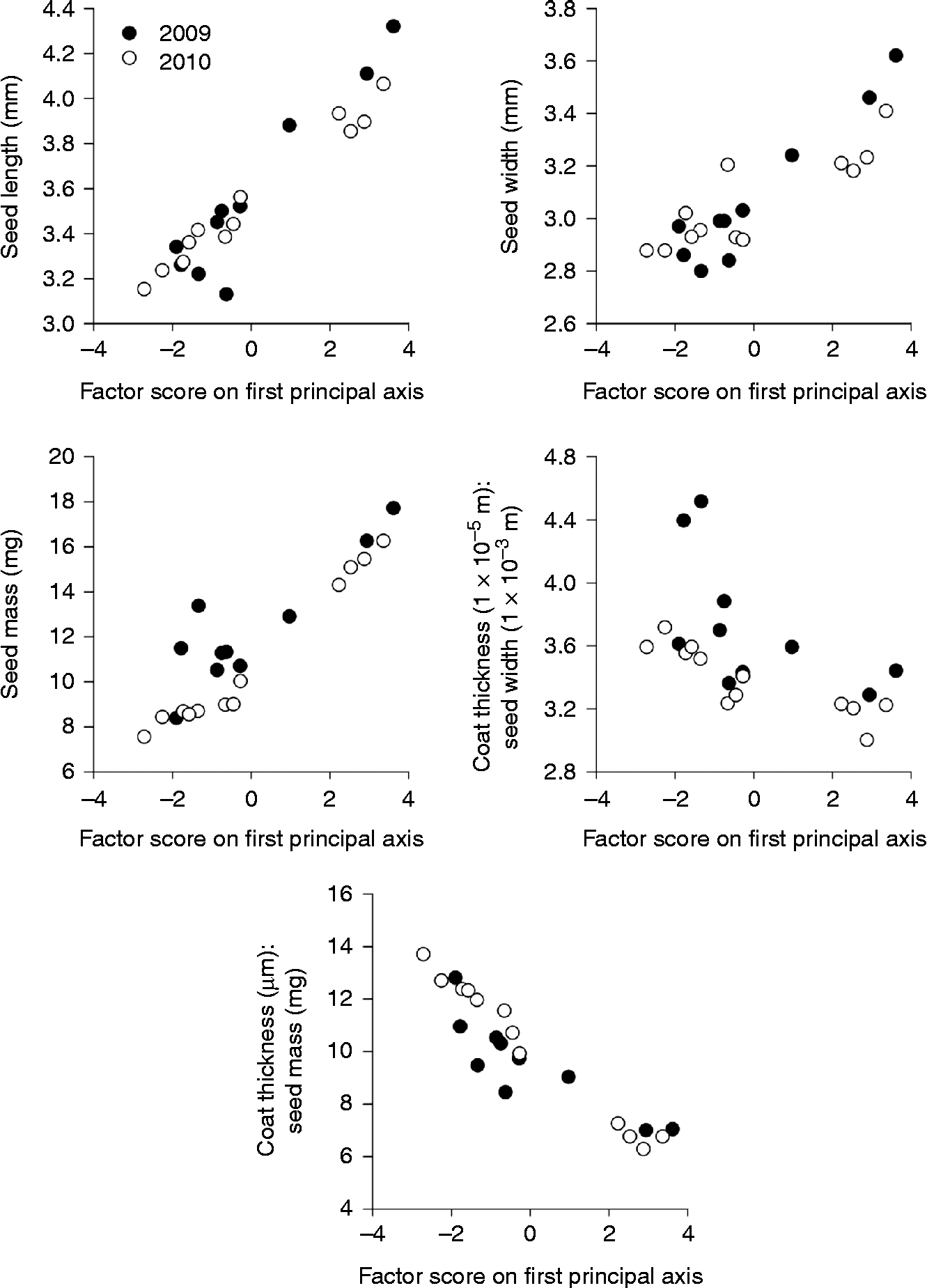
Figure 2 Relationship between first rotation factor scores of principal component analyses and seed trait means for Abutilon theophrasti seed lots produced in 2009 (filled circles, ●) and 2010 (open circles, ○). The ratio ‘coat thickness:seed width’ is seed-coat thickness in proportion to seed width. The ratio ‘coat thickness:seed mass’ is seed-coat thickness in proportion to individual seed mass.
Seed-bank fate and seed dormancy
Seed lot influenced the proportion of buried seeds that persisted, the proportion of buried seeds that emerged and the proportion of buried seeds that perished (Table 2). Pearson correlation coefficients indicated that variation in persistence followed inverse associations with mortality (2009, r= − 0.90, df = 8, P< 0.001; 2010, r= − 0.93, df = 10, P< 0.001); however, correlations were not significant between emergence and mortality (2009, r= − 0.12, df = 8, P= 0.73; 2010, r= − 0.38, df = 10, P= 0.21) and between emergence and persistence (2009, r= − 0.31, df = 8, P= 0.38; 2010, r= 0.04, df = 10, P= 0.91). Persistence in soil seed-banks was positively correlated with dormancy in controlled environments in 2009 (r= 0.64, df = 8, P= 0.04) and in 2010 (r= 0.85, df = 10, P < 0.001). Dormancy was influenced by seed lot in both 2009 and 2010 (Table 2).
Relationships between seed morphology and seed performance
Logistic regression models for the binary response variable ‘persistence’ indicated that differences in seed-bank persistence among seed lots coincided with the concurrent variation in seed length, width, mass, coat:width ratio and coat:mass ratio that was summarized by factor scores on first principal axes (Table 4). One-unit increases in first rotation factor scores decreased the odds of persistence by a factor of 0.63 in 2009 and 0.69 in 2010. Logistic regression models for the binary response variable ‘dormancy’ revealed that differences in dormancy among seed lots also coincided with the concurrent variation in seed length, width, mass, coat:width ratio that was summarized by factor scores on first principal axes (Table 4). One-unit increases in first rotation factor scores decreased the odds of dormancy by a factor of 0.78 in 2009 and 0.61 in 2010. Because one-unit increases in first rotation factor scores corresponded with increasing seed size and decreasing allocation to seed coats (Fig. 2), these logistic regression models indicate that larger seeds with proportionately thinner coats were less persistent and less dormant than smaller seeds with proportionately thicker seed coats.
Table 4 Summary of logistic regression models for the responses of 1-year seed-bank persistence and seed dormancy of Abutilon theophrasti to increasing first rotation factor scores of principal component analysis. Increasing PCA axis scores corresponded with increasing seed size and decreasing allocation to seed coats (Fig. 2). Binary dependent variables were coded as ‘persistent’ or ‘dormant’=1, and ‘non-persistent’ and ‘germinable’=0
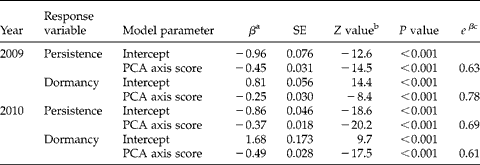
a Parameter estimate with standard error (SE).
b Z-statistic for maximum likelihood parameter estimate, which is equal to [β/SEβ)], where β is the parameter estimate.
c Exponentiated value of the parameter estimate for PCA axis score, which describes changes in probability in persistence or dormancy when PCA axis score increases by one unit.
Addition of seed-coat thickness data to statistical models for seed-size effects on persistence
Parameters of logistic regression models with multiple predictor variables for seed trait effects on seed-bank persistence were without bias caused by collinearity, as indicated by variance inflation factors (VIF) that were lower than the recommended cut-off value of 3 (Table 5) (Zuur et al., Reference Zuur, Ieno and Elphick2010). Variance inflation factors were also less than 3 for predictor variables in multiple logistic regression models for seed-trait effects on seed dormancy. Logistic regression models that included seed-coat thickness data with seed-mass data featured reduced AICc compared to logistic regression models with single predictor variables of seed mass, seed-coat thickness or seed length, and compared to models with seed-coat thickness and seed length as predictor variables (Table 5). Thus, given the set of models evaluated, a logistic regression model with predictor variables comprising seed-coat thickness and seed mass best explained variability in seed-bank persistence and seed dormancy among seed lots.
Table 5 Comparison of logistic regression models for seed trait effects on seedbank persistence and seed dormancy of Abutilon theophrasti
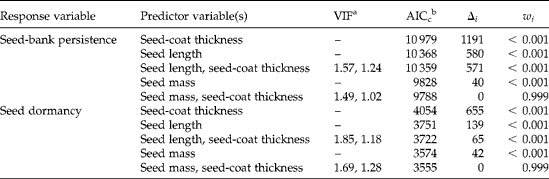
a Variance inflation factors presented in order corresponding with the left-to-right listing of predictor variables. VIF values less than 3 indicate that logistic regression model parameters were not influenced by bias caused by collinearity among covariates (Zuur et al., Reference Zuur, Ieno and Elphick2010). Collinearity assumptions do not pertain to logistic regression models with single predictor variables.
b Explanation of model selection criteria abbreviations: AICc, Akaike information criterion, adjusted for small sample size; Δi, Akaike differences, the difference between a given model and the model that minimized AIC; w i, Akaike weights, the relative likelihood of a model, given the set of candidate models.
Discussion
We predicted that variation in seed size among A. theophrasti seed lots coincides with differences in seed-coat thickness, seed dormancy and seed-bank persistence, such that A. theophrasti seed lots characterized by smaller seeds and thicker seed coats exhibit greater levels of seed-bank persistence and seed dormancy compared to A. theophrasti seed lots distinguished by larger seeds and thinner seed coats. With seed-coat thickness expressed as a proportion of overall seed allocation, results of this study supported our overall hypothesis. Therefore, we propose that seed traits in A. theophrasti accessions vary in manners consistent with predictions for both interdependent evolution of germination constraints and seed defences (Dalling et al., Reference Dalling, Davis, Schutte and Arnold2011) and concurrent evolution of seed-bank persistence and seed size (Venable and Brown, Reference Venable and Brown1988). We acknowledge that focus on one species precludes broad generalizations on associations among seed size, seed-bank persistence and seed defence, but we believe that this study presents evidence supporting a framework that can be tested on additional species, including species with physiological seed dormancy mechanisms. Covariance among seed traits justifies subsequent studies that can further clarify interdependent evolution of seed morphology, seed defence and seed-bank persistence at the level of the gene. Such studies are important because neither this, nor previous studies on associations between seed size and seed persistence eliminated possibilities for pleiotropy (Thompson et al., Reference Thompson, Band and Hodgson1993, Reference Thompson, Jalili, Hodgson, Hamzeh'ee, Asri, Shaw, Shirvany, Yazdani, Khoshnevis, Zarrinkamar, Ghahramani and Safavi2001; Rees, Reference Rees1996; Funes et al., Reference Funes, Basconcelo, Diaz and Cabido1999; Cerabolini et al., Reference Cerabolini, Ceriani, Caccianiga, Andreis and Raimondi2003; Schutte et al., Reference Schutte, Regnier and Harrison2008b; Wang et al., Reference Wang, Jiao, Jia and Wang2011; Zhao et al., Reference Zhao, Wu and Cheng2011; de Jong et al., Reference de Jong, Isanta and Hesse2013).
Expectations for inverse associations between seed size and seed-bank persistence arise from assumptions of trade-offs between traits with fitness consequences. Such assumptions are commonplace in evolutionary ecology and have become critical for understanding plant regeneration strategies. Trade-offs between seed size and seed-bank persistence have yet to be widely accepted because previous studies presented data that supported each of the following conclusions: seed size and seed-bank persistence are negatively correlated (Thompson et al., Reference Thompson, Band and Hodgson1993, Reference Thompson, Jalili, Hodgson, Hamzeh'ee, Asri, Shaw, Shirvany, Yazdani, Khoshnevis, Zarrinkamar, Ghahramani and Safavi2001; Funes et al., Reference Funes, Basconcelo, Diaz and Cabido1999; Cerabolini et al., Reference Cerabolini, Ceriani, Caccianiga, Andreis and Raimondi2003; Peco et al., Reference Peco, Traba, Levassor, Sanchez and Azcarate2003; Schutte et al., Reference Schutte, Regnier and Harrison2008b; Wang et al., Reference Wang, Jiao, Jia and Wang2011; Zhao et al., Reference Zhao, Wu and Cheng2011); seed size and seed-bank persistence are negatively correlated only if seeds feature specialized structures for dispersal (Rees, Reference Rees1996); negative associations between seed size and seed-bank persistence are conditioned by habitat (Thompson et al., Reference Thompson, Bakker, Bekker and Hodgson1998); seed size and seed-bank persistence are not associated (Leishman and Westoby, Reference Leishman and Westoby1998; Moles et al., Reference Moles, Hodson and Webb2000; Yu et al., Reference Yu, Sternberg, Kutiel and Chen2007). Many of these previous studies quantified seed-bank persistence by utilizing germination indices based entirely, or partly, on seedling emergence data. Such data do not account for species-specific environmental controls on germination and emergence (Saatkamp et al., Reference Saatkamp, Affre, Dutoit and Poschlod2009). Although replacing emergence-based persistence data with direct measurements of persistence is the ideal strategy for determining alleged trade-offs between seed size and seed-bank persistence, the continued use of emergence-based persistence data can be improved with seed-coat thickness data, which are measurements of seed defence allocation (Davis et al., Reference Davis, Schutte, Iannuzzi and Renner2008; Gardarin et al., Reference Gardarin, Durr, Mannino, Busset and Colbach2010). Seemingly inconsistent seed-bank persistence and seed defence data (i.e. short-lived seeds with thick seed coats) would suggest an experimental bias and would call for a re-evaluation of experimental procedures. Also, because the inclusion of seed-coat thickness data improves the performance of statistical models for seed-bank persistence responses to increasing seed size, supplementing seed-size data with coat-thickness data may clarify the reported inconsistencies regarding associations between seed size and seed-bank persistence.
Trade-offs between seed size and seed-bank persistence were previously studied in the context of evolutionary changes above the level of the species (Thompson et al., Reference Thompson, Band and Hodgson1993, Reference Thompson, Jalili, Hodgson, Hamzeh'ee, Asri, Shaw, Shirvany, Yazdani, Khoshnevis, Zarrinkamar, Ghahramani and Safavi2001; Rees, Reference Rees1996; Funes et al., Reference Funes, Basconcelo, Diaz and Cabido1999; Cerabolini et al., Reference Cerabolini, Ceriani, Caccianiga, Andreis and Raimondi2003; Wang et al., Reference Wang, Jiao, Jia and Wang2011; Zhao et al., Reference Zhao, Wu and Cheng2011). Results of our study suggest that species-level comparisons can provide empirical evidence of trade-offs in seed traits, which is consistent with the intra-specific focus of some of the studies that underlie the hypothesis for interdependent evolution of seed size, seed-bank persistence and seed dispersal (Venable and Brown, Reference Venable and Brown1988). Such studies include Venable and Lawlor (Reference Venable and Lawlor1980), Stanton (Reference Stanton1985) and Winn (Reference Winn1985). Intra-specific analyses may be preferred to inter-specific analyses because some traits regulating persistence are phylogenetically conserved (Baskin and Baskin, Reference Baskin and Baskin1998), which can confound evolutionary insights from studies that investigate seed traits using a broad range of species. Population-level variation in seed-bank persistence has been reported for arable weeds with annual life histories (Brainard et al., Reference Brainard, DiTommaso and Mohler2007), reflecting strong selection for diversified emergence timing in frequently disturbed environments (Childs et al., Reference Childs, Metcalf and Rees2010). Population-level variation has also been reported for seed size (Michaels et al., Reference Michaels, Benner, Hartgerink, Lee, Rice, Willson and Bertin1988; Vaughton and Ramsey, Reference Vaughton and Ramsey1998), potentially reflecting selection pressures caused by environmental conditions during seedling establishment (Moles and Leishman, Reference Moles, Leishman, Leck, Parker and Simpson2008), but this may also represent selection caused by seed predation (Moegenburg, Reference Moegenburg1996). Natural occurrences of population-level variation for both seed size and seed-bank persistence are opportunities for testing the hypothesis of Venable and Brown (Reference Venable and Brown1988) using intra-specific comparisons that are independent of the influences of artificial selection that is associated with cultivated varieties.
Compared to previous studies on A. theophrasti seed-bank dynamics (Davis et al., Reference Davis, Cardina, Forcella, Johnson, Kegode, Lindquist, Lusche, Renner, Sprague and Williams2005; Schutte et al., Reference Schutte, Davis, Renner and Cardina2008a), our results suggest that some A. theophrasti seed lots used in this study were poorly suited for survival in the conditions of the field trial. Specifically, seed-bank mortality rates in this study were as great as 0.94, which was nearly double previously reported values for 1-year mortality in A. theophrasti seed banks (Schutte et al., Reference Schutte, Davis, Renner and Cardina2008a). Also, the maximum rate of persistence in this study (0.58) was equal to a previously reported mean for A. theophrasti seed banks across the US Midwest (Davis et al., Reference Davis, Cardina, Forcella, Johnson, Kegode, Lindquist, Lusche, Renner, Sprague and Williams2005). Relatively low levels of persistence and high levels of mortality in this study may have been caused by inadequate regulation of germination, as suggested by the positive correlation between persistence and dormancy. Interestingly, previous studies determined that A. theophrasti plants inhabiting agricultural fields can produce seed lots with dormancy rates of 0.10 to 0.19 (Baloch et al., Reference Baloch, DiTommaso and Watson2001; Nurse and DiTommaso, Reference Nurse and DiTommaso2005). Such low levels of dormancy, combined with evidence for a positive association between dormancy and persistence, suggest that A. theophrasti plants in agricultural fields can produce seed lots with low probabilities for survival in the local soil environments.
Overall mean seed-coat thickness in our study (109.0 μm) was comparable to previously reported seed-coat thickness for A. theophrasti (112.0 μm; Davis et al., Reference Davis, Schutte, Iannuzzi and Renner2008). Similarly, previous reports of individual seed mass for A. theophrasti ranged from 4.4 to 17.0 mg (Warwick and Black, Reference Warwick and Black1986; Zhang and Hamill, Reference Zhang and Hamill1997; Baloch et al., Reference Baloch, DiTommaso and Watson2001; Nurse and DiTommaso, Reference Nurse and DiTommaso2005), which was comparable to the range of seed mass observed in this study. However, unlike our results, Baloch et al. (Reference Baloch, DiTommaso and Watson2001) observed a positive association between seed mass and seed dormancy for seeds collected from ten A. theophrasti individuals inhabiting a single agricultural field. Nurse and DiTommaso (Reference Nurse and DiTommaso2005) also observed a positive association between individual seed mass and seed dormancy among seeds collected from a single A. theophrasti population. Inconsistent correlations between seed size and seed dormancy reported for A. theophrasti may reflect differences in the underlying causes of seed-size variability between this and previous studies. Specifically, the processes driving variation in seed size among individuals within populations are likely different than those driving variation among populations adapted to different locations. For example, differences in resource availability and resource acquisition can correspond with variability in seed allocation (Winn, Reference Winn1991; Zas et al., Reference Zas, Cendan and Sampedro2013) and, thus, differences in seed size among individuals within a population may be non-adaptive and reflect limits on maternal control on seed size. Non-adaptive variation in seed size induced by the maternal environment is likely to occur with A. theophrasti populations. This was indicated by Nurse and DiTommaso (Reference Nurse and DiTommaso2005) who determined that A. theophrasti individuals grown in resource-rich environments produce heavier seeds compared with those grown in resource-limited environments.
A. theophrasti accessions with different ancestries provided us an opportunity to study relationships between two theoretical models on the evolution of traits promoting seed-bank persistence. Specifically, we linked a previous model predicting interdependent evolution of seed size and seed-bank persistence (Venable and Brown, Reference Venable and Brown1988) with more recent evidence indicating concurrent variation in seed dormancy and seed defence (Dalling et al., Reference Dalling, Davis, Schutte and Arnold2011). In so doing, we showed that: (1) data on seed-coat thickness, a seed defence trait, can improve the explanatory power of statistical models for seed-size effects on persistence and physical seed dormancy; and (2) intra-specific comparisons can be useful for providing evidence of alleged seed-trait trade-offs. Improved knowledge of the evolutionary trade-offs between the traits regulating seed survival will assist in the development of a comprehensive framework for understanding intra- and inter-specific diversity observed in seed traits. Furthermore, knowledge of associations between seed traits may contribute to improved mechanistic knowledge of seed survival in the soil. Comprehensive evaluations of seed defences, coupled with measurements of seed morphology and seed fate in a phylogenetic context will likely lead to a better understanding of the endogenous factors regulating seed-bank dynamics, which are central to population dynamics of annual plant species, including many arable weeds.
Acknowledgements
We thank J. Cardina of the Department of Horticulture and Crop Science at The Ohio State University for kindly providing seeds, S. Robinson of the Imaging Technology Group of the University of Illinois Beckman Institute for his expert guidance in environmental scanning electron microscopy, and R. Creamer of the Department of Entomology, Plant Pathology and Weed Science at New Mexico State University for assistance with ISSR analyses.
Financial support
This work was supported by the USDA Agricultural Research Service. Mention of trade names or commercial products in this article is solely for the purpose of providing scientific information and does not imply recommendation or endorsement by the US Department of Agriculture.
Conflicts of interest
None.









