Introduction
Production of seeds with variable germination behaviour is a mechanism present in many plant species to cope with changing environmental conditions, and it is a widespread strategy in Mediterranean wild species (Pérez-García, Reference Pérez-García1993, Reference Pérez-García2009). Phenotypic variation of a trait can be the result of genetic and/or environmental influences. Individuals from the same population may show differences in seed morphology or germination, which can be the result of micro-environmental factors during seed maturation and maternal genotype (Pérez-García, Reference Pérez-García1993; Bewley and Black, Reference Bewley and Black1995). Therefore, seed germination is subject to strong selection pressure and, consequently, is likely to be highly sensitive to climatic changes (Walck et al., Reference Walck, Hidayati, Dixon, Thompson and Poschlod2011). A better understanding of variation of seed germination and viability within a population is important, as phenotypic plasticity might provide a buffer against climate change (Fernández-Pascual and Jiménez-Alfaro, Reference Fernández-Pascual and Jiménez-Alfaro2014; Hudson et al., Reference Hudson, Ayre and Ooi2015).
Seed heterogeneity may be associated with ecological strategies that have evolutionary significance. Morphological heterogeneity within a population may occur in seed size, shape or colour (Baskin and Baskin, Reference Baskin and Baskin1998; Imbert, Reference Imbert2002; Matilla et al., Reference Matilla, Gallardo and Puga-Hermida2005; Zaidi et al., Reference Zaidi, González-Benito and Pérez-García2010), and has been related to physiological properties, including dormancy (Duran and Retamal, Reference Duran and Retamal1989; Rodríguez et al., Reference Rodríguez, Barrero, Corbineau, Gubler and Benech-Arnold2015), germination (Puga-Hermida et al., Reference Puga-Hermida, Gallardo, Rodriguez-Gacio and Matilla2003; Mira et al., Reference Mira, González-Benito, Ibars and Estrelles2011b; Reference Mira, Arnal and Perez-Garcia2017), and longevity (Kochanek et al., Reference Kochanek, Steadman, Probert and Adkins2009; Niedzielski et al., Reference Niedzielski, Walters, Luczak, Hill, Wheeler and Puchalski2009; Nagel and Borner, Reference Nagel and Borner2010). Seed characteristics may also vary within plant and even fruit (Venable, Reference Venable1985; Guzzon et al., Reference Guzzon, Orsenigo, Gianella, Müller, Vagge, Rossi and Mondoni2018).
Regarding seed germination, there is a narrow correlation between seed mass and germination characteristics in some species (Milberg et al., Reference Milberg, Andersson, Elfverson and Regner1996; Baloch et al., Reference Baloch, DiTomaso and Watson2001; Matilla et al., Reference Matilla, Gallardo and Puga-Hermida2005). Most studies have reported greater viability and vigour for heavier seeds compared with lighter seeds of the same species (Khan, Reference Khan2004; Lopes Souza and Fagundes, Reference Lopes Souza and Fagundes2014). However, some authors have informed that higher germination could not be clearly linked to heavier seeds (Pérez-García et al., Reference Pérez-García, Iriondo and Martínez-Laborde1995; Delgado et al., Reference Delgado, Serrano, López and Acosta2008; Genna and Pérez, Reference Genna and Perez2016), and even in some cases, the lightest seeds reached the highest germination percentages (Pérez-García, Reference Pérez-García2009; Zaidi et al., Reference Zaidi, González-Benito and Pérez-García2010).
Intra-specific variation in seed longevity is an important functional trait that has been scarcely studied. There are some reports of highly variable longevity among seed lots of the same cultivar (Niedzielski et al., Reference Niedzielski, Walters, Luczak, Hill, Wheeler and Puchalski2009; Nagel and Borner, Reference Nagel and Borner2010) or wild seed lots (Kochanek et al., Reference Kochanek, Steadman, Probert and Adkins2009). Also, seed characteristics such as size and weight have been related to longevity variation among species, and Venable and Brown (Reference Venable and Brown1988) proposed that strong selection for seed longevity in the soil coincides with weaker selection for seed size, as adaptations to heterogeneity in the environment. However, the variation of seed longevity within a species and its relationship with seed size has been rarely studied.
Hirschfeldia incana (L.) Lagr.-Foss., Fl. Tarn (Brassicaceae) is an annual herbaceous plant widely distributed throughout the Mediterranean and Irano-Turanian regions. It is also a frequent weed in a large number of crops and invasive species in diverse localities of the world (Gómez-Campo, Reference Gómez-Campo and Castroviejo1993; USDA, 2017). Its short life cycle, its ability to prosper in highly disturbed environments, and the high number of seeds produced per plant showing differences in seed mass provide a unique model to study the mass-dependent response of germination.
We hypothesize that there is a relationship between seed size, germination and viability loss during storage of H. incana, being likely that seeds with deep dormancy would show a higher longevity. The objectives of the study were: (1) to identify primary dormancy differences among seed lots related to seed size; (2) to determine whether seed longevity varied among seed size and storage conditions; and (3) if seed viability loss was related to electrolyte leakage, measured using the conductivity test.
Materials and methods
Seed collection
Experiments were performed on seeds of H. incana collected from the same wild population in Soto del Real (Madrid, Spain), in July 2014. The sample was taken randomly across the extent of the population, collecting 5–10 fruits (siliqua) from 40–50 individuals that were kept mixed. Size of the population was about 200 individuals. Seeds were all collected at full maturation, when fruits were about to open. Fruits were dried under laboratory conditions and seeds collected.
Seeds were separated according to their size in order to study its effect on seed germination and longevity. Visibly deficient seeds were excluded. Three categories of seed size were established using sieves: small seeds (diameter <500 μm); intermediate seeds (diameter = 500–630 μm); and large seeds (diameter >630 μm). Seeds of the three categories were weighted in 12 replicates of 110 seeds. Seeds were stored under laboratory conditions [at approximately 23°C, in darkness, at 20% relative humidity (RH)] until their use, in October 2014.
Seed storage experiments
Seeds were equilibrated within an air-tight plastic box with a saturated solution of NaCl (75% RH, ‘humid conditions’); or a box with a saturated solution of MgCl2 (33% RH, ‘dry conditions’) at 25°C (Vertucci and Roos, Reference Vertucci and Roos1993) for 3 days. When seeds were equilibrated, the two boxes were stored at 35°C for 1 month (humid storage) or 3 years (dry storage). Storage conditions were chosen to study the effect of high temperature and humid or dry environments on seed viability loss, and resemble environmental conditions of the natural population during summer, time of seed dispersal.
Within each plastic box, seeds of the three categories (small, intermediate and large) were stored in subsamples of 110 seeds. Subsamples were used for determination of seed germination, dormancy level, viability, water content and electrolyte leakage at an interval of 2 to 7 days (humid storage) or an interval of 84 to 365 days (dry storage). Seed water content (WC) was evaluated twice during the storage period. WC was calculated by the low constant temperature oven method (ISTA, 2017) on three replicates and expressed as percentage of g H2O g–1 dry weight (DW).
Seed germination
Seed germination was evaluated with four replicates of 25 seeds incubated in glass Petri dishes (9 cm diameter) on top of two sheets of filter paper previously moistened with 4 ml of distilled water before and during storage experiments. Filter papers were re-wetted regularly with distilled water as required. Incubation conditions were 25°C with a 16-hour photoperiod provided by cool white fluorescent tubes with an irradiance of 35 μmol m2 s–1. Samples were checked every 1–5 days and germinated seeds were counted and removed. Emergence of the radicle was the criterion for germination.
Dormancy was evaluated by applying gibberellic acid (1000 mg l–1) for 24 h to non-germinated seeds after 21 days of incubation in germination chambers. Dormancy level was calculated as the percentage of viable but non-germinated seeds previously to the application of gibberellic acid. Viability after different times of storage was defined as final percentage of germinated seeds, after gibberellic acid was added.
Electrolyte leakage
Electrolyte leakage was determined by placing three replicates of 10 mg of seeds into 10 ml deionized water at 20°C and measuring the conductivity of the medium with a conductivity meter (EC-Meter GLP 31) after 16 h. Results are expressed as mS g–1 DW and represent the mean of three measurements ± standard error (SE).
Data analysis
Seed viability loss, the response to storage time in terms of percentage germination, was modelled using the glm function with a binomial distribution available in the statistical package R (R Core Team, 2015). Time for germination percentage to decrease to 50% of maximum germination (i.e. longevity, P50) was calculated from the modelled curves for each treatment using the dose.p function available in R (R Core Team, 2015). ANOVA was used to compare viability loss curves with the test statistic for F-tests.
One-way factorial ANOVA was used to test differences among seeds lots on weight, water content, initial parameters of germination and P50 (Table 1). Square roots of germination values were arcsine-transformed prior to statistical analysis (untransformed data appear in Table 1). Where ANOVA indicated a significant effect (P < 0.01), a multiple comparison test was carried out with Tukey's test at P < 0.05.
Table 1. Seed diameter and weight, initial seed germination (germ), initial dormancy level, initial seed viability, seed water content (WC) and seed longevity (P50) of three Hirschfeldia incana seed categories: small seeds, intermediate seeds (Int), and large seeds; stored at 35°C and two humidity conditions: 75% RH (humid) or 33% RH (dry)

Data are expressed as mean values ± standard error. In each column, mean values followed by the same letters are not significantly different by pair-wise comparison (P < 0.05).
Results
Differences in H. incana seed size were related to differences in seed weight and WC during storage (Table 1). Small seeds had a higher WC than large seeds when stored at the same conditions of relative humidity (12.8 and 10.7%, respectively, at 75% RH, P < 0.05).
Seed lots showed differences in the initial dormancy level, but initial viability (final germination after dormancy break) was similar, 77% on average (Table 1). Viability loss during storage at 35°C and humid or dry conditions is shown in Fig. 1. Significant differences were found when comparing viability curves of seed with different size, both at humid (P < 0.05) and dry storage (P < 0.01). P50 was lower for small seeds than intermediate or large seeds, both at humid and dry storage (Table 1).

Fig. 1. Seed viability loss during storage at 35°C and 75% RH (A, Humid) or 33% RH (B, Dry) of three Hirschfeldia incana seed accessions: small (long dashed line,●); intermediate (dotted curve,○); and large seeds (continuous curve, ▼). Each data point represents the percentage of final germination, after gibberellic acid was added, for a particular treatment, storage time and replicate.
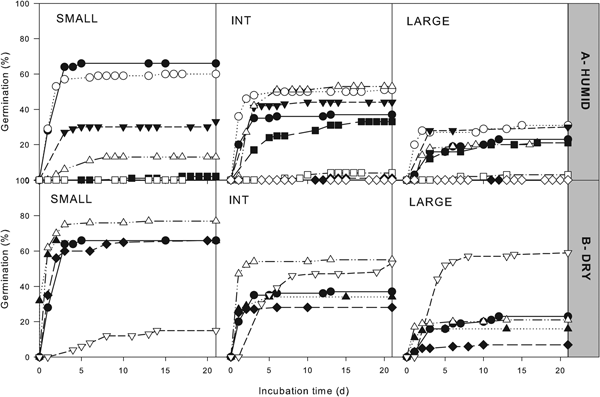
The cumulative germination curves for each seed lot are shown in Fig. 2. Curves up to 21 days of incubation, which represent germination of non-dormant seeds, were used to observe germination speed and estimate graphically the number of days required to reach 50% of germination (T 50). Initial T 50 for non-aged seeds was: 1.1 days for small seeds, 0.8 days for intermediate seeds, and 2.2 days for large seeds. Short storage in humid or dry storage slightly increased germination speed, but as storage continued germination slowed down (T 50 increased), and differences could be detected depending on seed size. Small seeds showed the greatest increase in T 50 with ageing, being, for example, 5 days after 706 days of storage at 33% RH while it was 3 days for both intermediate and large seeds (Fig. 2B).

Fig. 2. Germination time courses for non-dormant seeds (without treatment with gibberellic acid) stored at 35°C and humid (A) or dry (B) conditions. Three Hirschfeldia incana seed accessions were studied: small, intermediate and large seeds. Values represent the average of four replicates after different storage times representative of the experiment. Humid conditions: 0 (●), 4 (○), 7 (▼), 11 (△), 14 (■), 18 (□), 20 (◆) and 27 (◇) days of storage. Dry conditions: 0 (●), 88 (△), 172 (◆), 341 (▲) and 706 (▽) days of storage.
The relationship between viability and conductivity is shown in Fig. 3. There was a negative linear relationship between electrolyte leakage and seed germination for both humid storage (R 2 = 0.60) (Fig. 3A) and dry storage (R 2 = 0.62) (Fig. 3B). When taking all seeds lots into account, the slope of the relationship between conductivity and germination was –47% g mS–1 in humid and –27% g mS–1 in dry storage. At humid storage, the correlation between electrolyte leakage and seed germination was higher when studying each individual seed lot (R 2 = 0.95–0.98). At the same germination percentage, conductivity decreased with seed size. For example, when seed germination during storage decreased to 80% of the initial value, the conductivity was 0.7 mS g–1 DW for small seeds, 0.4 mS g–1 DW for intermediate seeds and 0.3 mS g–1 DW for large seeds.

Fig. 3. Relationships between electrolyte leakage (mS g–1 DW) and viability (final germination, %) of seeds stored at 35°C and humid (A, Humid) or dry conditions (B, Dry) of three Hirschfeldia incana seed accessions: small (long dashed line,●); intermediate (dotted line,○); and large seeds (short dashed line,▼). Values are the average of three replicates ± standard error. At humid storage (A), linear regression was fitted for each seed lot (R 2 = 0.95–0.98) and the complete dataset (R 2 = 0.60). At dry storage (B), linear regression was fitted for the complete dataset (R 2 = 0.62).
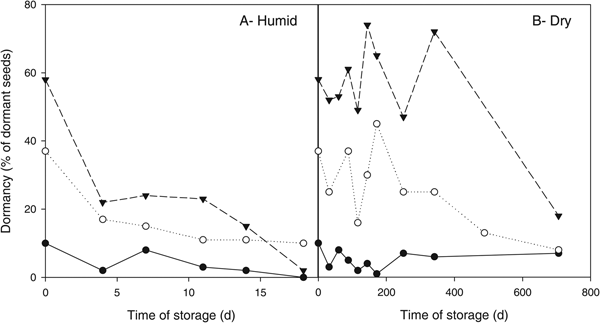
Changes in dormancy during storage are shown in Fig. 4, up to the storage time when seed lots maintained at least 40% viability. Small seeds showed low dormancy (13% initial value, Table 1) and no remarkable changes during storage (Fig. 4). Intermediate and large seeds showed a high initial dormancy level (50 and 72%, respectively) which was released in humid conditions in a short period of time. For example, after 4 days dormancy of intermediate seeds was down to 25% (Fig. 4A). Under dry conditions, dormancy level was highly irregular up to around 350 days, ranging from 60 to 90% for large seeds and from 9 to 61% for intermediate seeds. Moreover, during a long time of storage under dry conditions, large and intermediate seeds lose their dormancy.

Fig. 4. Dormancy level of seeds during storage at 35°C and humid (A, Humid) or dry conditions (B, Dry) of three Hirschfeldia incana seed accessions: small (●), intermediate (○) and large seeds (▼). Each data point represents the percentage of viable but non-germinated seeds after storage, previously to the application of gibberellic acid. Data points presented are those for storage times when seeds maintained at least 40% viability.
Discussion
It is known that seed viability is influenced by genotype, environment during seed development and seed storage conditions (Clerkx et al., Reference Clerkx, Blankestijn-De Vries, Ruys, Groot and Koornneef2004; Fessel et al., Reference Fessel, Vieira, da Cruz, de Paula and Panobianco2006; Acikgoz et al., Reference Acikgoz, Sincik, Wietgrefe, Surmen, Cecen, Yavuz, Erdurmus and Goksoy2013; Hampton et al., Reference Hampton, Boelt, Rolston and Chastain2013). Our results confirm that humidity during storage is an important parameter affecting seed viability, and that the higher the seed water content the faster ageing occurs at a given temperature (Mira et al., Reference Mira, Estrelles and Gonzalez-Benito2015; Reference Mira, Hill, Gonzalez-Benito, Ibanez and Walters2016). Moreover, membrane permeability was related to loss of seed viability in H. incana, as has been previously reported for some cultivated Brassica species (Mirdad et al., Reference Mirdad, Powell and Matthews2006; Demir et al., Reference Demir, Mavi, Kenanoglu and Matthews2008; Matthews et al., Reference Matthews, Demir, Celikkol, Kenanoglu and Mavi2009; Lazar et al., Reference Lazar, Mira, Pamfil and Martinez-Laborde2014) and wild species of Brassicaceae (Mira et al., Reference Mira, Estrelles, Gonzalez-Benito and Corbineau2011a). Our data indicate that electrolyte leakage increased linearly with ageing at both humid and dry conditions, but that the slope of the relationship between conductivity and germination is steeper when seeds were stored in humid rather than in dry conditions.
Intra-specific variability of seed longevity has been little studied, and our results indicate that even within the same population, viability loss of H. incana varied among seeds lots classified by size. Small seeds were shorter-lived than larger seeds under both humid and dry storage, indicating that storage behaviour in humid conditions was predictive of the relative longevity in dry conditions similar to those used for long-term conservation (Hay and Whitehouse Reference Hay and Whitehouse2017). Differences on viability loss curves were greater in dry than in humid storage. Longevity studies using a high humidity storage produce an accelerated loss of germination that might mask differences among seed lots. When longevity was evaluated as membrane permeability by the conductivity test, however, differences among seeds of different size were detected only under humid storage. Previous reports identify differences in conductivity among cultivars of Brassica oleracea and Pisum sativum that related to seed heterogeneity in colour (Atak et al., Reference Atak, Kaya, Kaya, Kaya and Khawar2008; Demir et al., Reference Demir, Mavi, Kenanoglu and Matthews2008). If mechanisms of ageing are different under different storage conditions (Mira et al. Reference Mira, González-Benito, Hill and Walters2010, Reference Mira, Hill, Gonzalez-Benito, Ibanez and Walters2016), correlating seed characteristics with longevity will depend on both the viability loss parameters analysed and the storage conditions at which longevity was evaluated.
Seed characteristics such as size and weight were hypothesized to be related to the variation in longevity among species, with larger seeds having shorter longevity (Venable, Reference Venable1985). Previous reports have indicated that species producing smaller seeds tend to persist for longer in the natural environment (Thompson et al., Reference Thompson, Band and Hodgson1993; Funes et al., Reference Funes, Basconcelo, Diaz and Cabido1999), although this might not reflect their longevity but only their particular germination requirements (Probert et al., Reference Probert, Daws and Hay2009). In the Brassicaceae family there was no relationship between seed weight and longevity when comparing among species (Mira et al., Reference Mira, Estrelles and Gonzalez-Benito2015), and these findings were consistent with several previous reports using a wide variety and number of species (Priestley, Reference Priestley1986; Medeiros et al., Reference Medeiros, Probert, Sader and Smith1998; Walters et al., Reference Walters, Wheeler and Grotenhuis2005; Probert et al., Reference Probert, Daws and Hay2009; Schwienbacher et al., Reference Schwienbacher, Marcante and Erschbamer2010). However, studies on longevity variability within a species are rare. Smaller seeds (size and weight) of H. incana had lower longevity than larger ones. When equilibrated at the same relative humidity, small seeds acquired higher water content, which contributes to faster ageing. As seed lipid content greatly influences water content, we hypothesize that small seeds might have a lower lipid content than larger seeds. Moreover, we know that pre-zygotic environment greatly influences seed development, seed constituents (Lee et al., Reference Lee, Kwak, Yoon and Hay2017), and also seed longevity (Kochanek et al., Reference Kochanek, Steadman, Probert and Adkins2011; Mondoni et al., Reference Mondoni, Orsenigo, Dona, Balestrazzi, Probert, Hay, Petraglia and Abeli2014). Previous reports on species from the Asteraceae family found an inverse relationship between seed size and longevity among individuals within a population (Schutte et al., Reference Schutte, Regnier and Harrison2008; Genna and Pérez, Reference Genna and Perez2016), which is contrary to our findings. However, it was suggested that this relationship was affected by the environment during seed development and seed burial in the field (Schutte et al., Reference Schutte, Regnier and Harrison2008). So, we hypothesize that differences in the environment during seed development might produce variations in seed size and lipid composition, which might ultimately affect seed water content and, therefore, longevity (Schutte et al., Reference Schutte, Regnier and Harrison2008; Kochanek et al., Reference Kochanek, Steadman, Probert and Adkins2011; Lee et al., Reference Lee, Kwak, Yoon and Hay2017).
The relationship between longevity and dormancy in seeds has not been studied in detail. Deeper dormancy has been correlated with low longevity in Arabidopsis seed populations (Nguyen et al., Reference Nguyen, Keizer, van Eeuwijk, Smeekens and Bentsink2012). Our results on a single population contradict this previous statement, as seeds with the lowest longevity showed a low level of dormancy (small seeds). However, longevity of H. incana was interlinked with seed size, water content and, probably, seed composition. It is likely that deeply dormant seeds have to stay alive for longer than non-dormant seeds, hence they may show higher resistance to ageing. Consistently, studies in Aegilops neglecta showed that dormant seeds were longer lived than non-dormant seeds (Guzzon et al., Reference Guzzon, Orsenigo, Gianella, Müller, Vagge, Rossi and Mondoni2018).
Our study marks the importance of careful viability loss evaluation during storage, so that low germination is not misunderstood with dormancy and variability within a species and population is taken into account. Several authors have identified that differences in dormancy across seed lots may sometimes be mistaken as differences in viability (Pérez-García et al., Reference Pérez-García, González-Benito and Gómez-Campo2007; Mira et al., Reference Mira, Estrelles, Gonzalez-Benito and Corbineau2011a; van Hintum and van Treuren, Reference van Hintum and van Treuren2012; van Treuren et al., Reference van Treuren, de Groot and van Hintum2013). Therefore, assessing seed viability by laboratory germination tests, as established by International Standards (ISTA, 2017), require to be accompanied by parallel studies on intra-specific variability, especially in wild species.
The conductivity test may be used to evaluate the quality of H. incana seeds and allows the identification of a dormant (non-germinating) seed lot as viable.
Intermediate and large seeds of H. incana showed a deeper dormancy than small seeds, which was partially released after a short storage at 35°C and humid conditions. Under dry storage conditions, irregular changes in the dormancy level were observed for over a year, and longer times of storage had a dormancy-breaking effect through dry after-ripening. It has been reported before that some species show a highly irregular germination during storage at controlled conditions, due to endogenous dormancy cycles (Froud-Williams et al., Reference Froud-Williams, Hilton and Dixon1986; Rawat and Thapliyal, Reference Rawat and Thapliyal2003; Gutterman and Gendler, Reference Gutterman and Gendler2005). Production of heterogeneous seeds with different germination behaviours is a widespread strategy to cope with changing environmental conditions in Mediterranean wild species (Pérez-García, Reference Pérez-García1993, Reference Pérez-García2009). However, the capacity to overcome environmental barriers also increases a species’ colonization potential and persistence (Gioria et al., Reference Gioria, Pysek and Moravcova2012). Hirschfeldia incana is a Mediterranean wild species, found all over the world as a weed of agricultural systems or invasive in urban areas (Lee et al., Reference Lee, Patel, Conlan, Wainwright and Hipkin2004; DiTomaso and Healy, Reference DiTomaso and Healy2007; USDA, 2017). A successful strategy for H. incana resilience is, on the one hand, to have seeds that germinate immediately after seed dispersal, small seeds with low dormancy and short longevity. Simultaneously, larger seeds with greater dormancy and longevity will form part of the soil seed bank. Storage conditions studied here will resemble environmental conditions of the wild population during summer, time of seed dispersal. So, a summer rain will release some dormancy, allowing for germination. Dormant seeds in the soil bank might have endogenous dormancy cycles and, with time and after-ripening, increase sensitivity to environmental conditions promoting germination and decrease perception of conditions repressing germination (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006). This dual germination strategy has been identified in H. incana seeds of different harvest years by Castro et al. (Reference Castro, Figueroa and Escobedo2016), and could explain the species’ high persistence and spread in the ecosystem.
Author ORCIDs
Sara Mira, 0000-0002-0164-5156
Acknowledgements
The authors would like to thank Dr Miguel Ibáñez, Statistics Department, UPM, for advice on the statistical analysis. The authors are very grateful to the anonymous reviewers for their careful reading and their extremely useful comments on an earlier version of the manuscript.
Financial support
This work was supported by a grant from the Spanish Ministerio de Educacion y Ciencia (CGL2006-10536).