Introduction
Artemisia adamsii is a perennial forb or subshrub belonging to the Asteraceae and is distributed across the eastern Eurasian steppe including Mongolia. It colonizes lands degraded by inappropriate utilization, such as overgrazing or abandonment of cultivation, and is thus recognized as an indicator of rangeland degradation (Fernández-Giménez, Reference Fernández-Giménez2000; Tuvshintogtokh and Ariungerel, Reference Tuvshintogtokh, Ariungerel, Yamamura, Fujita and Maekawa2013). As A. adamsii has low palatability to most livestock, its predominance in overgrazed grasslands is a serious concern for rangeland management in the Mongolian steppe (Okayasu et al., Reference Okayasu, Okuro, Jamsran and Takeuchi2012; Bruegger et al., Reference Bruegger, Jigjsuren and Fernández-Giménez2014). In addition, pollen from Artemisia species is known as a cause of pollinosis in many parts of the world (D'Amato et al., Reference D'Amato, Cecchi, Bonini, Nunes, Annesi-Maesano, Behrendt, Liccardi, Popov and Van Cauwenberge2007; Tang et al., Reference Tang, Sun, Yin and Li2015), and A. adamsii is also recognized as an allergen among Mongolians (Konagaya, Reference Konagaya, Yamamura, Fujita and Maekawa2013). Despite its harmfulness to livestock farming and human health, to date little effort has been made to control this species (but see Yoshihara et al., Reference Yoshihara, Koyama, Undarmaa and Okuro2015; Koyama et al., Reference Koyama, Kubo, Yoshihara, Jamsran and Okuro2016).
Seed germination is a critical stage for plant establishment, and therefore comprehension of the germination characteristics of weeds can facilitate the development of effective weed management (Kurstjens, Reference Kurstjens2007; Chauhan and Johnson, Reference Chauhan and Johnson2010). For example, in a no-till cropping system, mature cover crop residue applied on the soil surface as a mulch was reported to reduce light quantity and soil temperature, and thereby suppressed or delayed weed germination (Teasdale and Mohler, Reference Teasdale and Mohler1993; Mirsky et al., Reference Mirsky, Ryan, Curran, Teasdale, Maul, Spargo, Moyer, Grantham, Weber and Way2012). In addition, living cover crops are known to have a greater suppressive effect on weed germination than cover crop residue because living parts absorb red light and reduce the ratio of red to far-red light sufficiently to inhibit phytochrome-mediated seed germination (Teasdale and Daughtry, Reference Teasdale and Daughtry1993; Teasdale et al., Reference Teasdale, Brandsaeter, Calegari, Neto, Upadhyaya, Blackshaw, Upadhyaya and Blackshaw2007). The germination characteristics of A. adamsii, however, have not been investigated, and the environmental factors inducing or suppressing its germination are still unclear.
Phytotoxic inhibition of germination can be utilized for weed control. Many plants produce secondary metabolites (allelochemicals) and release them into the environment through leachates, litter decomposition, root exudates and volatilization; in this way, they can inhibit seed germination and the growth of other plants (Rice, Reference Rice1984). Autotoxicity, the inhibition of germination and growth due to phytotoxic compounds contained in the body of the plant itself, has been observed in many crop and wild species (Chou, Reference Chou1999; Singh et al., Reference Singh, Batish and Kohli1999). Autotoxicity in weed species has the potential to be utilized as a natural bioherbicide (Singh et al., Reference Singh, Batish and Kohli1999; Qasem and Foy, Reference Qasem and Foy2001; Hegab et al., Reference Hegab, Khodary, Hammouda and Ghareib2008). It has been shown that many Artemisia species contain bioactive compounds possessing anti-fungal, anti-bacterial and phytotoxic functions (Tan et al., Reference Tan, Zheng and Tang1998; Chon and Nelson, Reference Chon and Nelson2010), some of which have autotoxic activity. For example, the germination of Artemisia tridentata and Artemisia cana was inhibited by aqueous extracts and volatile compounds of their own leaves (Hoffman and Hazlett, Reference Hoffman and Hazlett1977), germination of Artemisia vulgaris was inhibited by aqueous extracts of its own plant body (Onen, Reference Onen2007) and, in Artemisia annua, germination was inhibited by artemisinin, a chemical compound contained in its own plant body that has anti-fungal, anti-bacterial and phytotoxic activities (Jessing et al., Reference Jessing, Duke and Cedergreeen2014). More recently, Arroyo et al. (Reference Arroyo, Pueyo, Pellissier, Ramos, Espinosa-Ruiz, Millery and Alados2018) reported that the germination of Artemisia herba-alba was inhibited by both volatile and water-soluble chemicals produced by this plant itself. As for A. adamsii, it is likely to contain bioactive compounds because this species has been used as a folk medicine and its essential oil has anti-bacterial activities (Horváth et al., Reference Horváth, Acs and Kocsis2013; Randalova et al., Reference Randalova, Sambuunyam, Zhigzhitzhapova and Radnaeva2017); moreover, its volatile chemicals are known to affect the growth of other species (Tsubo et al., Reference Tsubo, Nishihara, Nakamatsu, Cheng and Shinoda2012). The aforementioned studies strongly suggest that A. adamsii exhibits the autotoxic inhibition of germination, but no studies have investigated this to date.
In this study, we thus investigated the germination characteristics and autotoxic inhibition of germination of A. adamsii, a weed colonizing degraded lands in the Mongolian steppe that is harmful to livestock farming and human health. We also discussed the applicability of these characteristics to the control of this species. In addition to its practical value, autotoxicity has been suggested to have some ecological consequences; some pioneer invaders in disturbed lands have autotoxic activity on germination, which restricts self-regeneration and can mediate the secondary succession (Jackson and Willemsen, Reference Jackson and Willemsen1976; Kobayashi et al., Reference Kobayashi, Morimoto, Shibata, Yamashita and Numata1980; Alias et al., Reference Alias, Sosa, Escudero and Chaves2006; Li et al., Reference Li, Fan, Yin and Wei2014). To discuss the contribution of autotoxicity in A. adamsii to the secondary succession in the Mongolian steppe, we also investigated the allelopathic effect of A. adamsii on the germination of some native grassland species.
Materials and methods
We performed four germination experiments to test the germination characteristics and autotoxic inhibition of germination in A. adamsii (Experiments 1–3), and to evaluate the phytotoxicity of A. adamsii residue to the germination of species native to the Mongolian steppe (Experiment 4). Seeds of A. adamsii and of four species native to the Mongolian steppe, Artemisia frigida, Agropyron cristatum, Salsola collina and Stipa krylovii, were collected in late September to early October in 2011, 2014 and 2015 in a semi-arid grassland near Bayan-Unjuul (47°2.3′ N, 105°57.3′ E; elevation, 1215 m), which is approximately 130 km southwest of Ulaanbaatar, the capital of Mongolia. In this region, vegetative growth usually begins in May and ends in September, during which time approximately 85% of the annual precipitation falls (Fig. S1). Seeds of each species were randomly collected from several populations, and collected seeds were air-dried and stored in paper bags at 5°C in a refrigerator until the experiments started.
Experiment 1: Germination characteristics of A. adamsii
Germination characteristics of A. adamsii were tested in the period from April to August in 2012 for seeds collected in 2011. Germination tests were conducted in a temperature gradient incubator (TG-300CCFL-3LE; EYELA, Tokyo, Japan) placed in a dark room using seven temperature treatments (5, 10, 15, 20, 25, 30 and 35°C) under two continuous light conditions, red and far-red light, and in the dark condition which was created by shading a container put into the incubator. For red and far-red light treatments, LED lamps were used (EP30-A0E0-00, 660 nm, and EP30-A0F0-00, 740 nm; EDISON, Taipei, Taiwan).
Before the germination tests, seeds were sterilized by immersion in 80% ethanol for 1 min and rigorously rinsed using distilled water. Two 5-cm Petri dishes filled with 0.75% (w/v) agar (gelling temperature, 30–31°C; Nacalai Tesque, Kyoto, Japan) were prepared for each treatment and 50 seeds were sown in each dish. The numbers of germinated seeds were counted every day in the dark room using a green safe light so as to avoid the induction of germination. Germination was defined as the time when the radicles first appeared, and germinated seeds were discarded after counting. Germination tests were ceased when new germination was not observed for five consecutive days. After the germination tests, the viability of ungerminated seeds was tested by the triphenyltetrazolium chloride (TTC) staining method. Seeds were cut to expose the embryo and then immersed in 0.1% (w/v) TTC solution (2,3,5-triphenyltetrazolium chloride; Wako, Japan) in the dark at 30°C for 24 h. Embryos that turned red were classified as viable.
Experiment 2: Autotoxic inhibition of germination in A. adamsii
Autotoxic activity of the residue of A. adamsii on germination was tested in 2017. To confirm the phytotoxicity of the residue, effects on the germination of lettuce (Lactuca sativa cv. Melbourne MT) and radish (Raphanus sativa cv. Comet), which are highly sensitive to allelochemicals and used as standard indicator species (Wu et al., Reference Wu, Pratley, Lemerle, Haig and An2001), were also tested. The seeds of A. adamsii used for the test were collected in 2015, and the seeds of lettuce and radish were obtained from a seed dealer. Germination was tested in a growth chamber (LH-60FL12-DT; Nippon Medical & Chemical Instruments, Osaka, Japan) under 20°C with continuous light treatment by a fluorescent lamp, as the germination of A. adamsii required light and peaked at about 20°C in Experiment 1. As the germination of radish is inhibited by light, its germination was tested in a shading container put into the growth chamber.
Germination was tested on agar medium filled in 5-cm Petri dishes with two different treatments: agar medium containing and not containing the residue of A. adamsii (Fig. S2A). Agar medium not containing residue was prepared in the same manner as described in Experiment 1. As for agar medium containing residue, dried residue of A. adamsii was embedded in the agar medium. The residue of A. adamsii that was collected in grassland near Bayan-Unjuul in early May 2016 just before the plant growing season was gently oven-dried at 35°C for more than 5 days and stored in a desiccator at room temperature until the experiment started. The residue was then cut into 1- to 3-cm pieces, and 100 mg of this cut residue was put into each Petri dish. This amount of residue is equivalent to 51 g m−2 when shown as a ground area basis, which is comparable to the amount of A. adamsii residue in grasslands dominated by A. adamsii. The A. adamsii community with an above-ground biomass of 51 g m−2 has vegetation cover of about 60% (Fig. S3). Autoclaved agar was kept at 40–45°C in a water bath, and 5 ml of agar was added to each Petri dish to embed the residue. After gelatinizing, the same procedure but with the addition of 4 ml of agar was repeated twice, and prepared Petri dishes were stored in a refrigerator at 5°C overnight until the germination test.
Before the germination test, seeds of A. adamsii, lettuce and radish were sterilized by immersion in 80% ethanol for 1 min and rigorously rinsed using distilled water. Five 5-cm Petri dishes were prepared for each treatment in each tested species, and 20 seeds were sown in each Petri dish. The numbers of germinated seeds were counted every day, until new germination was not observed for three consecutive days. Germination of radish was checked in a dark room using a green safe light so as to avoid the photoinhibition of germination. The procedure for checking germination followed those in Experiment 1. After finishing the germination test of A. adamsii in agar medium containing residue, ungerminated seeds were transplanted to new agar medium not containing residue and continued incubation in the growth chamber of the same environment as germination test were performed to confirm seed viability.
Experiment 3: Contribution of volatile compounds to the autotoxicity of A. adamsii
Germination of A. adamsii was tested in the presence or absence of volatile compounds from A. adamsii residue in 2017. Seeds and residue of A. adamsii employed for the test were from similar sources to those used in Experiment 2. Five 5-cm Petri dishes filled with agar medium were prepared for each of the two treatments: treatment with and without volatile compounds of the residue. After sterilizing using 80% ethanol and rinsing with distilled water, 20 seeds were sown in each Petri dish. After seeding, each Petri dish was put in an 8.5-cm Petri dish and had a lid put on it (Fig. S2B), followed by placement in a growth chamber at 20°C with continuous light treatment by a fluorescent lamp. In the treatment with volatile compounds of the residue, 289 mg of A. adamsii residue was put into the 8.5-cm Petri dish together with the seeded Petri dish. This amount of residue is equivalent to that in Experiment 2 when considering the ground area basis. The numbers of germinated seeds were counted every day, until new germination was not observed for three consecutive days. The definition of and procedure for checking germination followed those in Experiment 1.
After the germination test, samples in Petri dishes for the treatment with volatile compounds of the residue were moved into new 8.5-cm Petri dishes without residue and continued to undergo testing of germination to confirm seed viability (Fig. S2B). However, as new germination was not observed even 3 days after moving, ungerminated seeds were transplanted to 5-cm Petri dishes filled with new agar medium and continued to undergo testing of germination until new germination was not observed for three consecutive days.
Experiment 4: Phytotoxicity of A. adamsii residue on germination of grassland species
The effect of A. adamsii residue on the germination of five grassland species native to the Mongolian steppe, namely, A. adamsii, A. frigida, A. cristatum, S. collina and S. krylovii, was tested in 2017. Artemisia frigida is a perennial forb or subshrub and is both palatable and nutritious to livestock. This species is found in grasslands with moderate to heavy grazing intensity (Gao et al., Reference Gao, Wang, Han, Patton and Nyren2005), and emerges during the mid-vegetation recovery after land disturbance (Li et al., Reference Li, Tsujimura, Sugimoto, Davaa and Sugita2006). Two grass species (Poaceae), A. cristatum and S. krylovii, are both highly palatable to livestock, and they are sensitive to grazing (Bat-Oyun et al., Reference Bat-Oyun, Shinoda, Cheng and Purevdorj2016) and to land degradation, especially in the case of S. krylovii (Li et al., Reference Li, Tsujimura, Sugimoto, Davaa and Sugita2006). Salsola collina, an annual forb belonging to Amaranthaceae, is moderately palatable to livestock and is found in early vegetation recovery after land degradation (Li et al., Reference Li, Tsujimura, Sugimoto, Davaa and Sugita2006). Seeds were collected in 2015, except for in the case of A. cristatum, seeds of which were collected in 2014. All seeds were stored in a refrigerator at 5°C until the experiment.
Five 5-cm Petri dishes were prepared for each species for each of the two treatments: agar medium containing and not containing A. adamsii residue. Each Petri dish was seeded with 20 seeds and placed in a growth chamber at 20°C with continuous light. The rest of the procedure in the germination test followed that in Experiment 2.
Data analysis and statistical tests
Germination percentage was calculated as the number of germinated seeds divided by that of germinable seeds (the sum of germinated seeds and ungerminated viable seeds) in Experiment 1. In Experiments 2–4, the germination percentage was calculated by dividing the number of germinated seeds by that of seeds tested, with hypothesizing that most of tested seeds were viable. Actually, viability of tested seeds of A. adamsii exceeded 95% in Experiment 1, and the number of germinated seeds exceeded 80% of tested seeds in most of the tested species and treatments in Experiments 2–4. In addition, the relative difference in germination percentage was more important than the absolute germination percentage, except in Experiment 1, and the relative difference in germination percentage can be judged without testing the viability of ungerminated seeds when hypothesizing that the seed viability was consistent among treatments.
The optimum temperature for the germination of A. adamsii under red light was estimated by applying a quadratic function to the relationship between temperature and germination percentage (Experiment 1). Germination time defined as the days until the accumulated number of germinated seeds reached 50% of the final germination number was estimated by applying the logistic function shown below to the time course of accumulated number of seeds germinated (Experiment 4):
where t, N and M represent days after seeding, accumulated number of seeds germinated until t and final germination number, respectively, and a and b are constant values. All fittings of quadratic and logistic functions were performed using the Kaleida Graph 4.0 software (Synergy Software, Reading, PA, USA).
A generalized linear model (GLM) followed by likelihood-ratio test were used to test the differences in germination percentage and in germination date among treatments. GLM with a binomial distribution and the logistic link function was applied for analysing germination percentage, and GLM with a gaussian distribution and the identity link function was for germination date. GLM and likelihood-ratio test were performed by JMP 13 software (SAS Institute, Cary, NC, USA). When multiple comparison was needed, we adjusted P-values using sequential Bonferroni method.
Results and Discussion
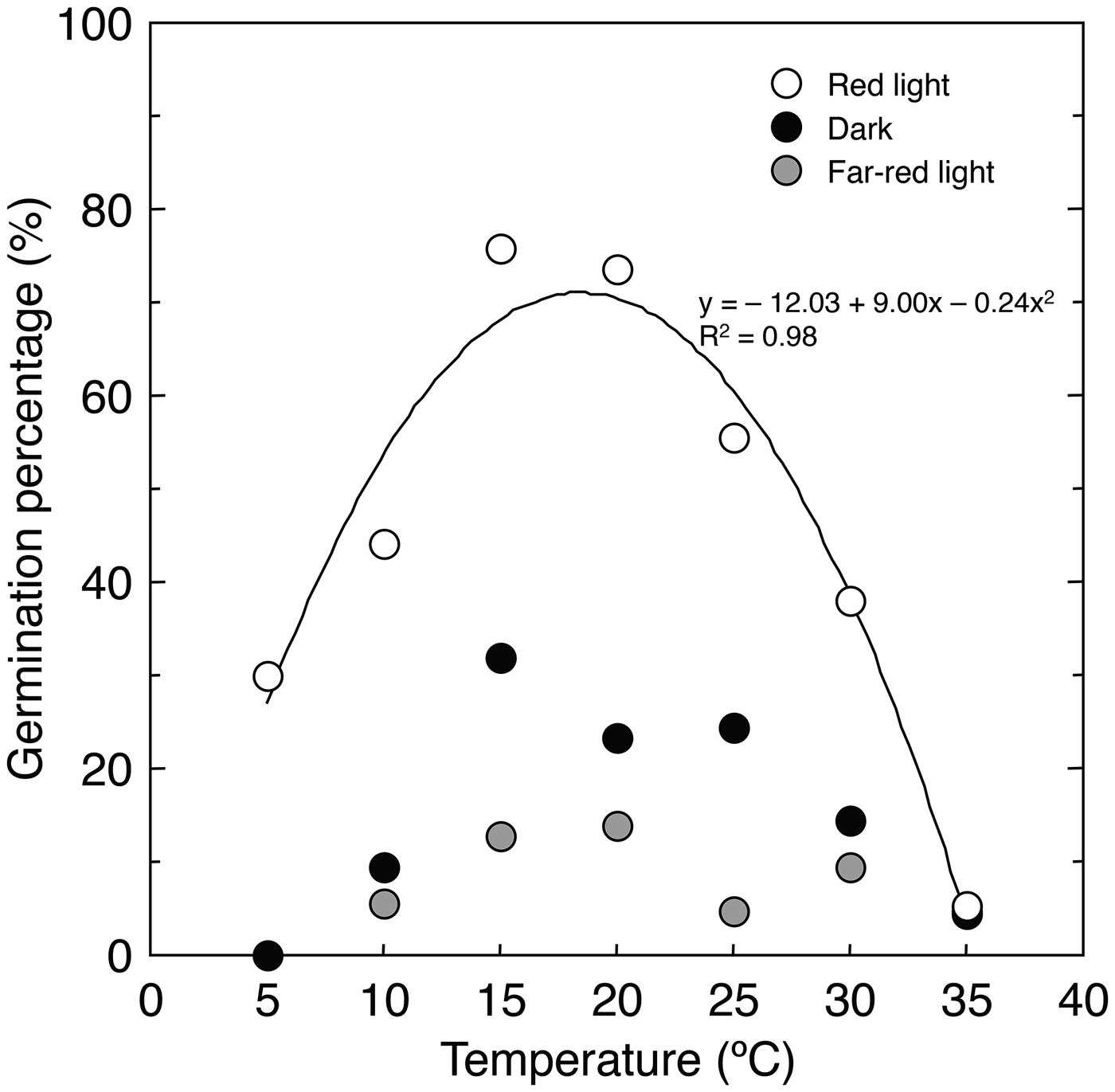
The germination percentage of A. adamsii was significantly higher under red light than in the dark and under far-red light at all temperatures except for 35°C, at which little germination was observed under all light conditions (P < 0.05, Pearson's χ2 test followed by sequential Bonferroni adjustment) (Fig. 1). This means that the germination of this species can be limited when seeds are buried in the soil or covered with vegetation because sunlight penetrates only 4–10 mm of soil and green leaves absorb red light at a much higher proportion than far-red light (Baskin and Baskin Reference Baskin and Baskin2014). Limited germination of A. adamsii under far-red light may reflect the survivorship strategy of this species that colonizes degraded lands: for example, investigating the germination of a Mediterranean grassland species, Dobarro et al. (Reference Dobarro, Valladares and Peco2010) found that increasers, the species becoming abundant in the presence of grazing, had lower germination percentages at low red:far-red ratios, and pointed out that this potentially reflects the ability of those species to avoid germination under highly competitive conditions. It is likely that A. adamsii also uses light quality as a key indicator to germinate in suitable sites.
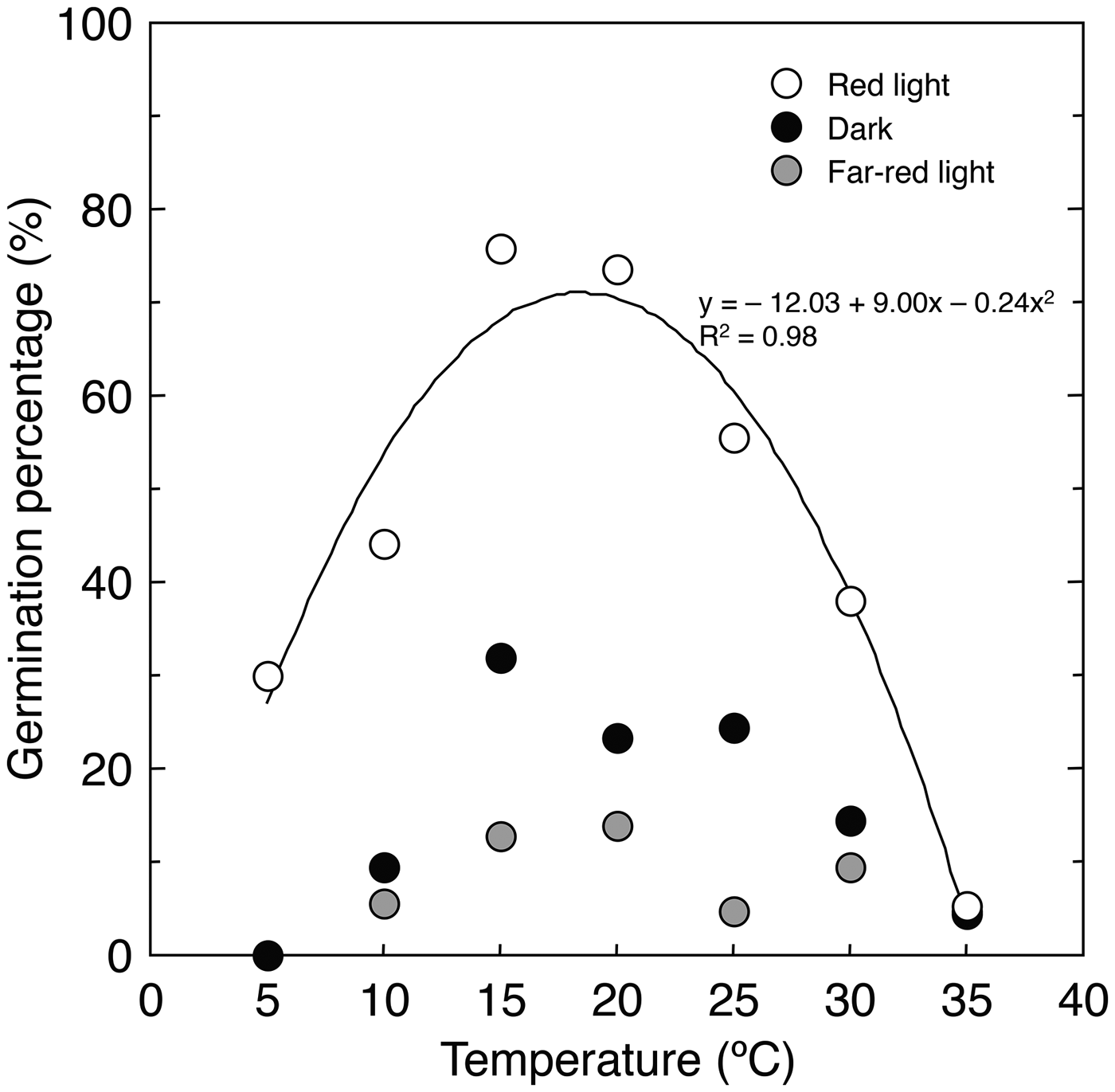
Fig. 1. Germination percentage of A. adamsii at different temperatures and light conditions.
Germination under red light was clearly temperature dependent, with an optimal temperature of about 19°C (Fig. 1). This means that the germination of A. adamsii is limited by temperature when the light conditions are appropriate. The optimal temperature was near the monthly mean temperature of the growing area in the period from June to August, during which monthly precipitation is much higher than that in other periods (Fig. S1). This can allow this species to germinate particularly in this period. As A. adamsii requires light and an appropriate temperature for germination, many of seeds that have been dispersed would be ungerminated. Ungerminated seeds sometimes form soil seed banks (Chambers and MacMahon, Reference Chambers and MacMahon1994), but nearly no seeds were found for A. adamsii in the soil near the area where seeds were collected (Kinugasa and Oda, Reference Kinugasa and Oda2014). This may be because seeds of A. adamsii lose viability easily by ageing, predation or infection by pathogens. In a related species, Artemisia genipi, the germination percentage rapidly dropped from 99% to 2% upon seed burial for one winter in the field (Schwienbacher and Erschbamer, Reference Schwienbacher and Erschbamer2002). Similarly, in A. tridentata, germinable seeds in the soil decreased and disappeared in 6 months after seed dispersal (Young and Evans, Reference Young and Evans1989), although the reduction in seed viability was found to differ among subspecies, burial depths and regions (Wijayratne and Pyke, Reference Wijayratne and Pyke2012). As A. adamsii is a perennial plant, it might rely less on buried seeds at maintaining its population. The regeneration of A. adamsii through seed germination might be controlled effectively by suppressing the production and germination of seeds in one or several seasons.
The germination of A. adamsii was greatly suppressed when tested on agar medium containing its own residue, although the germination of allelochemical-sensitive species, lettuce and radish, was little reduced (Fig. 2). This implies that the phytotoxicity of A. adamsii residue is highly species specific. Ungerminated seeds of A. adamsii in the presence of its own residue started to germinate rapidly after being transplanted to the agar medium not containing residue (Fig. S4) and the final germination percentage reached a high enough level not to differ significantly from that of the control (Fig. 2). This indicates that the autotoxic inhibition of germination of this species is sub-fatal. Allelopathic inhibition of germination has been widely observed, but its physiological mechanism is not well understood. One suggested mechanism is the suppression of respiratory ATP production, which is caused by the inhibition of enzyme activities involved in glycolysis and the oxidative pentose phosphate pathway, by the induction of mitochondrial uncoupling, or by the inhibition of reserve mobilization (Weir et al., Reference Weir, Park and Vivanco2004; Kupidłowska et al., Reference Kupidłowska, Gniazdowska, Stępień, Corbineau, Vinel, Skoczowski, Janeczko and Bogatek2006; Cheng and Cheng Reference Cheng and Cheng2015). In addition, allelochemicals are suggested to induce the accumulation of reactive oxygen species (ROS), which causes lipid peroxidation (Oracz et al., Reference Oracz, Bailly, Gniazdowska, Côme, Corbineau and Bogatek2007; Ishii-Iwamoto et al., Reference Ishii-Iwamoto, Pergo Coelho, Reis, Moscheta and Bonato2012; Radhakrishnan et al., Reference Radhakrishnan, Alqarawi and Abd Allah2018). ROS may further cause an imbalance in hormones relating to germination and dormancy; the synthesis of ethylene that induces germination decreases, while the concentration of abscisic acid, which induces seed dormancy, increases, forcing seeds to become artificially dormant (Bogatek and Gniazdowska, Reference Bogatek and Gniazdowska2007).
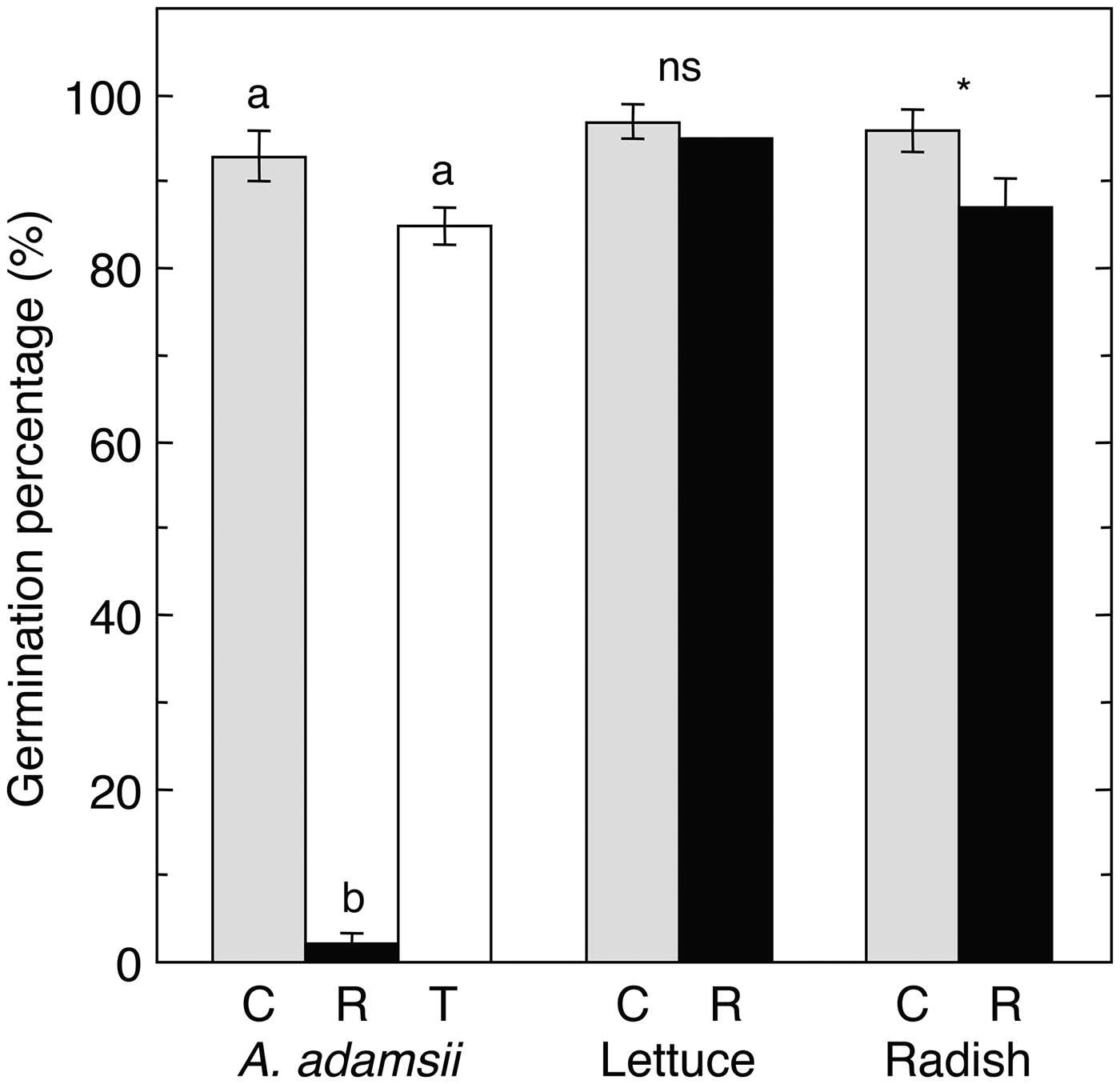
Fig. 2. Allelopathic inhibition of germination by A. adamsii residue in A. adamsii (autotoxicity), lettuce and radish. C and R represent germination tests in agar medium not containing residue and containing residue, respectively. In A. adamsii, viability of ungerminated seeds in R was tested by transplanting them into new agar medium not containing residue, and the overall germination percentage including germination in R was calculated (T). Different lower case letters above columns indicate statistically significant differences in A. adamsii (likelihood ratio test on GLM followed by sequential Bonferroni adjustment, P = 0.05). *P < 0.05; ns, no statistically significant difference (likelihood ratio test on GLM, P = 0.05). Error bars represent ± SE (n = 5).
The germination of A. adamsii was significantly suppressed when it was tested on agar medium in a closed environment containing A. adamsii residue (P < 0.05, likelihood ratio test on GLM) (Fig. 3); the suppression continued even after the seeds were moved into closed environment not containing residue (Fig. S2B). These suppressive effects are in line with the process by which volatile allelochemicals reach plants as proposed in earlier studies. That is, volatiles can reach plants directly and/or indirectly by fixing in the soil by dew or rain (Reigosa et al., Reference Reigosa, Sanchez-Moreiras and Gonzalez1999; Arimura et al., Reference Arimura, Shiojiri and Karban2010). It has been shown that volatile allelochemicals such as terpenoids are frequently present in plants in arid regions, while water-soluble allelochemicals such as phenolics are common in plants in cool temperate regions (Chou, Reference Chou1999; Reigosa et al., Reference Reigosa, Sanchez-Moreiras and Gonzalez1999), although the ecological significance of these geographical differences is still unclear. Seeds of A. adamsii that were ungerminated even after moving into the closed environment not containing residue started to germinate when they were transplanted on agar medium not containing residue, and the germination percentage finally reached more than 80% of that of seeds in the control (Fig. 3). This indicated that the autotoxic inhibition of germination by volatile compounds in this species is sub-fatal.

Fig. 3. Autotoxic inhibition of germination by volatile compounds of A. adamsii. After testing germination in the presence of volatile compounds, seed viability was tested by moving samples in Petri dishes to conditions without residue (Fig. S2B). Ungerminated seeds were further tested for viability by transplanting into new agar medium. Error bars represent ± SE (n = 5).
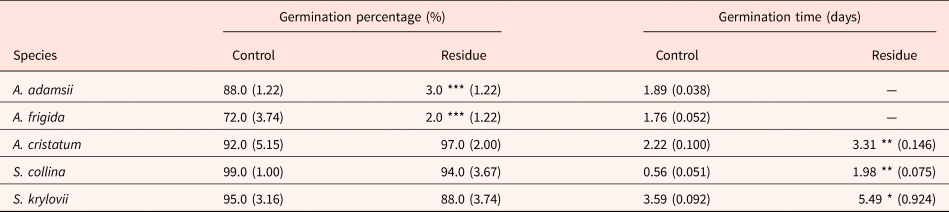
The germination percentage of plant species native to the Mongolian steppe was not significantly inhibited by A. adamsii residue, except for in A. frigida and A. adamsii itself (Table 1, Fig. S5). The germination time of those uninhibited species was delayed by A. adamsii residue, but the delay was at most 2 days. The greater autotoxicity in A. adamsii than in other grassland species might have some adaptive meaning to A. adamsii itself. Recently, Renne et al. (Reference Renne, Sinn, Shook, Sedlacko, Dull, Villarreal and Hierro2014) demonstrated the adaptive significance of autotoxicity by testing the hypothesis that the intraspecific inhibition of germination by phytochemicals will be greater than the interspecific inhibition if autotoxicity has adaptive meaning. They tested 12 wild species and found that the inhibition of germination was greater within species than among species, and thus concluded that the autotoxicity is an ‘eavesdrop-and-wait’ competition-avoidance strategy performed by chemically recognizing potential competitors. In our study, moreover, the germination inhibition in A. adamsii was sub-fatal, which may support the conclusion that the autotoxicity in this species is ecologically adaptive. Several advantages other than the avoidance of intraspecific competition have been proposed for autotoxicity; for example, plants that have autotoxicity can disperse seeds over longer distances by inhibiting germination near the parent plants or can avoid inbreeding (Reigosa et al., Reference Reigosa, Sanchez-Moreiras and Gonzalez1999; Singh et al., Reference Singh, Batish and Kohli1999).
Table 1. Germination percentage and germination time of species native to the Mongolian steppe tested in agar medium not containing A. adamsii residue (Control) and that containing residue (Residue)
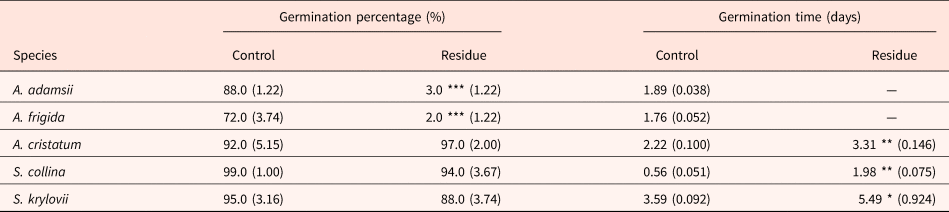
Values in parentheses represent SE (n = 5). As little germination was observed in A. adamsii and A. frigida in the agar medium containing residue, germination time was not calculated. *P < 0.05, **P < 0.01 (likelihood ratio test on GLM).
The results of the present study may demonstrate the ecological consequence of A. adamsii as a factor promoting plant succession after disturbance in the Mongolian steppe. The light requirement and autotoxic inhibition of germination in A. adamsii (Figs 1 and 2) imply that the self-regeneration of this species within its own community through seeds is strongly limited. On the other hand, in tested grassland species other than A. adamsii, the light requirement for germination and phytotoxic inhibition of germination by A. adamsii residue were low, except in A. frigida (Table 1, Fig. S6; Bai et al., Reference Bai, Romo and Young1995; Ronnenberg et al., Reference Ronnenberg, Wesche, Pietsch and Hensen2007; Kinugasa et al., Reference Kinugasa, Hozumi, Nishizima, Ishitobi and Miyawaki2016), indicating that those species can establish themselves within A. adamsii communities. As A. adamsii has low palatability to most livestock in Mongolia, plants growing within its community can be protected from herbivory and their survival would be enhanced, especially for highly palatable grass species like S. krylovii and A. cristatum. Such facilitative (or nursing) effect by unpalatable species against palatable species under grazing conditions has been reported repeatedly (McNaughton Reference Mcnaughton1978; Callaway, Reference Callaway1992; Smit et al., Reference Smit, Den Ouden and Müller-Schärer2006, Reference Smit, Vandenberghe, Den Ouden and Müller-Schärer2007; Graff et al., Reference Graff, Aguiar and Chaneton2007), and it has been pointed out that it contributes to the structure, diversity and dynamics of plant communities in grazed lands (Olff et al., Reference Olff, Vera, Bokdam, Bakker, Gleichman, De Maeyer and Smit1999; Callaway et al., Reference Callaway, Kikvidze and Kikodze2000, Reference Callaway, Kikodze, Chiboshvili and Khetsuriani2005; Danet et al., Reference Danet, Kefi, Meneses and Anthelme2017). The nursing effect of A. adamsii in combination with its limited self-regeneration might facilitate species transition in disturbed lands occupied by this species.
The germination characteristics of A. adamsii shown in this study may contribute to the improvement of the attempt to control A. adamsii by prescribed burning (Yoshihara et al., Reference Yoshihara, Koyama, Undarmaa and Okuro2015; Koyama et al., Reference Koyama, Kubo, Yoshihara, Jamsran and Okuro2016). One of the concerns in prescribed burning is the increasing germination of some species by improving the light environment and breaking dormancy (Hatcher and Melander, Reference Hatcher and Melander2003), implying that the germination of light-requiring seeds of A. adamsii would be induced by burning. To diminish the recruitment of A. adamsii after prescribed burning, it might be preferable to perform prescribed burning in early spring before the soil temperature reaches the optimal for its germination (about 19°C) and/or in late summer when germination has been completed. Although the residue of A. adamsii selectively suppressed the germination of this species itself and did not suppress the germination of two palatable grass species, its applicability as a bioherbicide is likely to be low because the autotoxic suppression of germination was sub-fatal (Figs 2 and 3). As the longevity of A. adamsii seeds is expected to be short as already discussed in the present study, applying extracts from residue or residue itself for several years might deplete the viability of A. adamsii seeds that have been dispersed.
In summary, the germination of A. adamsii was shown to be light-demanding and temperature-dependent. The residue of A. adamsii showed autotoxic but sub-fatal suppression of germination, and the chemicals contributing to that suppression were shown to be aqueous and volatile. Phytotoxicity of A. adamsii residue on the germination of grassland species was low, except for in A. frigida, a related species. The applicability of the observed sub-fatal autotoxicity to the control of this species is likely to be low, but the germination characteristics elucidated in this study should contribute to the development of a strategy for controlling this species. The autotoxicity in A. adamsii germination was suggested to have ecological consequence to facilitate species transition from A. adamsii to other species in degraded lands occupied by A. adamsii. As low-palatability A. adamsii can act as a nurse plant of palatable species under grazing conditions, invasion of A. adamsii into disturbed grasslands may accelerate the recovery of such grasslands in terms of improving pasture quality.
Supplementary Material
To view Supplementary Material for this article, please visit: https://doi.org/10.1017/S0960258519000163
Acknowledgements
We thank Dr E. Nishihara, Tottori University, Japan, for providing useful advice on the bioassay for allelopathy. We thank Enago (www.enago.jp) for the English language review.
Financial support
This work was supported in part by the project ‘Assessment and control of dust emission in degraded drylands of East Asia’ funded by the Japan Ministry of Education, Culture, Sports, Science and Technology and by the Joint Research Program of Arid Land Research Center, Tottori University.