Introduction
Survival and persistence of plant populations strongly depend on reproduction, thus seed germination and seedling establishment are the most critical stages in the life of plant individuals (Grubb, Reference Grubb1977; Kitajima and Fenner, Reference Kitajima, Fenner and Fenner2000). Without successful germination and establishment, populations are threatened by extinction. Germination processes are affected by the biotic environment, e.g. by competition from the established vegetation (Bakker and de Vries, Reference Bakker and de Vries1992; Kitajima and Fenner, Reference Kitajima, Fenner and Fenner2000) or by a litter layer (e.g. Jensen and Gutekunst, Reference Jensen and Gutekunst2003; Loydi et al., Reference Loydi, Eckstein, Otte and Donath2013), and by abiotic environmental conditions – the main abiotic drivers of germination processes being temperature and water availability (Baskin and Baskin, Reference Baskin and Baskin2001; Fenner and Thompson, Reference Fenner and Thompson2005). Most species require specific environmental conditions for the germination process and these factors are decisive for subsequent seedling establishment (Baskin and Baskin, Reference Baskin and Baskin2001).
Floodplain grasslands are hydrologically highly dynamic ecosystems, characterized by the interplay of wet conditions during regularly or irregularly occurring floods and dry conditions over the summer (Hölzel and Otte, Reference Hölzel and Otte2001). In directly inundated (functional) floodplains this gives rise to highly variable soil water potentials in time (i.e. within years) and space (i.e. along flooding gradients), resulting in a distinct zonation of plant communities (Leyer, Reference Leyer2005) which is also determined by seed and germination traits (Leyer and Pross, Reference Leyer and Pross2009). Due to the tightly intermingled vegetation zones, species density is high and floodplains harbour many rare and endangered species (Donath et al., Reference Donath, Hölzel and Otte2003; Toogood et al., Reference Toogood, Joyce and Waite2008). However, species-rich floodplain meadows have declined strongly over the past centuries due to habitat losses, mainly caused by land-use changes (e.g. Wesche et al., Reference Wesche, Krause, Culmsee and Leuschner2012), river regulations (e.g. Tockner and Stanford, Reference Tockner and Stanford2002) and river training (Brunotte et al., Reference Brunotte, Dister, Günther-Diringer, Koenzen and Mehl2009). Consequently, these meadows are of high conservation value and protected by the Habitats Directive (92/43/EEC, habitat type 6440: Alluvial meadows of river valleys of the Cnidion dubii) and subject to various restoration measures (Donath et al., Reference Donath, Bissels, Hölzel and Otte2007; Schmiede et al., Reference Schmiede, Otte and Donath2012).
Climate change might pose an additional threat to the persistence of species-rich floodplain meadows. Regional climate change projections indicate higher temperatures and an increasing risk for summer droughts for the late 21st century due to less summer precipitation in western and northern Germany in relation to the reference period 1961–1990 (Jacob et al., Reference Jacob, Göttel, Kotlarski, Lorenz and Sieck2008; Görgen et al., Reference Görgen, Beersma, Brahmer, Buiteveld, Carambia, de Keizer, Krahe, Nilson, Lammersen, Perrin and Volken2010). Accordingly, reduced river discharges during summers are projected for the large rivers Rhine (Görgen et al., Reference Görgen, Beersma, Brahmer, Buiteveld, Carambia, de Keizer, Krahe, Nilson, Lammersen, Perrin and Volken2010) and Elbe (Conradt et al., Reference Conradt, Koch, Hattermann and Wechsung2012). This, in turn, would lower the water table in the adjacent floodplain with negative effects on the soil water potential. In combination with increased transpiration at higher temperatures, these changes could induce severe drought-stress in plants of floodplain meadows (Jensen et al., Reference Jensen, Reisdorff, Pfeiffer, v. Oheimb, Schmidt, Schmidt, Schrautzer, Meyer-Grünefeldt, Härdtle, Storch and Claussen2011). Additionally, increased temperatures could affect seed longevity, a prelude for building up viable seed banks (Ooi, Reference Ooi2012). As temperature and water availability are important drivers for the germination process, their changes will very likely affect this early stage in plant life (Walck et al., Reference Walck, Hidayati, Dixon, Thompson and Poschlod2011). Consequently, species abundance and population dynamics might be altered, leading to distribution shifts or extinctions (Thuiller et al., Reference Thuiller, Lavorel, Araújo, Sykes and Prentice2005). To estimate future distribution and abundance of plant species, it is essential to gain knowledge on their specific requirements for seedling recruitment (e.g. Adler and HilleRisLambers, Reference Adler and HilleRisLambers2008; Walck et al., Reference Walck, Hidayati, Dixon, Thompson and Poschlod2011).
Recently, the impact of climate change on vegetation has received increasing attention. Different experimental approaches have already been conducted, focusing on CO2 (e.g. Edwards et al., Reference Edwards, Clark and Newton2001; Rasse et al., Reference Rasse, Peresta and Drake2005), temperature (e.g. Klanderud and Totland, Reference Klanderud and Totland2005; Hudson et al., Reference Hudson, Henry and Cornwell2011) or precipitation (e.g. Yahdjian and Sala, Reference Yahdjian and Sala2002; Beier et al., Reference Beier, Beierkuhnlein, Wohlgemuth, Penuelas, Emmett, Korner, de Boeck, Christensen, Leuzinger, Janssens and Hansen2012) and their effects on single species or plant communities. Some greenhouse and common garden experiments simulated drought (e.g. Jentsch et al., Reference Jentsch, Kreyling, Elmer, Gellesch, Glaser, Grant, Hein, Lara, Mirzae, Nadler, Nagy, Otieno, Pritsch, Rascher, Schadler, Schloter, Singh, Stadler, Walter, Wellstein, Wollecke and Beierkuhnlein2011; Weisshuhn et al., Reference Weisshuhn, Auge and Prati2011) or flooding scenarios (e.g. van Eck et al., Reference van Eck, Lenssen, van de Steeg, Blom and de Kroon2006). While all of these approaches focused on the mature plant, little work has been done considering the early life stages. Here, one possible approach is to investigate germination at different water availabilities with seeds exposed to different water potentials (e.g. Fyfield and Gregory, Reference Fyfield and Gregory1989; Swagel et al., Reference Swagel, Bernhard and Ellmore1997; Akhalkatsi and Lösch, Reference Akhalkatsi and Lösch2001). To date, most studies in which water potentials were manipulated focused on germination traits of single plant species, whereas comparative studies on a larger number of species are scarce (but see Evans and Etherington, Reference Evans and Etherington1990). Moreover, rare plant species and the influence of hydrological factors on their germination have only rarely been investigated (but see Geissler and Gzik, Reference Geissler and Gzik2008) and, to our knowledge, only one study investigated whether germination differs between species indicative of different habitats (Evans and Etherington, Reference Evans and Etherington1990). However, analysing the responses of plant functional groups based on key life-history traits to climatic changes appears to be a promising approach (Ooi, Reference Ooi2012).
Therefore, the objective of our study was to test how floodplain meadow species, preferring contrasting habitats with respect to soil moisture, respond (in terms of germination) to different water potentials. We exposed seeds of 20 floodplain meadow species to a water potential gradient ranging from no water limitation to the permanent wilting point. The overall aim was to understand possible impacts of climate change on the early life stage of floodplain meadow plants. We also included seeds of five of these species from populations at the River Elbe to test the effect of seed origin on germination characteristics. To include different aspects of the germination of species, we analysed: (1) germination percentage; (2) mean germination time; and (3) synchrony of germination. Germination percentage simply measures the recruitment success of a batch of seeds, and population fitness increases with increasing germination. In contrast, the success of recruitment does not necessarily monotonically increase with mean germination time or synchrony. Rather, the benefit of early or late germination (i.e. low or high germination time) and germination in one batch or scattered germination over time (i.e. high and low synchrony) may vary with environmental context.
Our research questions and hypotheses were: (1) Do reduced water potentials affect the germination patterns of all plant species? We hypothesize that all study species show reduced germination percentages at reduced water potentials. Further, we expect that germination will take longer and will be less synchronous at reduced water potentials. (2) Does the temperature regime affect germination? We expect germination time to decrease with increasing temperature. (3) Do seeds originating from populations along the Elbe differ in their response to reduced water potentials compared to those from populations along the Rhine? We expect germination of seeds from the River Elbe, where annual precipitation is lower, to be more successful at reduced water potentials than germination of seeds from the Rhine. (4) Does seed germination of floodplain plant species indicative of wet habitats differ to those floodplain plant species indicative of dry habitats? We hypothesize that seed germination of plants indicative of wet habitats will decrease more strongly, to be slower and less synchronous at reduced water potentials than that of plants indicative of dry habitats.
Methods
Study species
We selected 20 species (four from each of five plant families) occurring in floodplain grasslands along the River Rhine (see Table 1). These species consist of typical and rare floodplain meadow plants, such as Galium boreale, Pseudolysimachion longifolium or Peucedanum officinale (according to Burkart, Reference Burkart2001) and more common and widespread grassland species, such as Plantago media, Linaria vulgaris or Galium verum. Moisture requirements of the study species were classified according to the Ellenberg indicator value for moisture (Ellenberg et al., Reference Ellenberg, Weber, Düll, Wirth, Werner and Paulißen1992). Moisture indicator values of the selected species varied from 3 (indicative of dry habitats) to 9 (indicative of wet habitats). We sought to include pairs of genetically related species with different preferences for soil moisture to attain a phylogenetically balanced design. Plant nomenclature follows Wisskirchen and Haeupler (Reference Wisskirchen and Haeupler1998).
Table 1 Study species with information on plant family, Ellenberg indicator value for moisture (EIV moist, ind=species with ‘indifferent behaviour’), indicated habitat, seed viability (%) and germination capacity under outdoor conditions (%)

# According to Burkart (Reference Burkart2001) typical flood meadow species (river corridor plants).
a Tetrazolium chloride test with 25 seeds per replicate (n= 2).
b Tested in common garden, 50 seeds per replicate (n= 3).
Seed collection
Seeds from 13 of the 20 species were hand-collected from autochthonous populations in floodplain meadows along the northern Upper Rhine, Germany (49°50′N 8°25′E–49°51′N 8°23′E). The seeds of six species (Filipendula ulmaria, F. vulgaris, Linaria vulgaris, Plantago media, Sanguisorba minor, Veronica teucrium) were obtained from a commercial supplier (Rieger & Hoffmann GmbH, Blaufelden-Raboldshausen, Germany) due to insufficient amounts of seeds from natural populations along the River Rhine. Seeds of Galium palustre originated from floodplain meadows at the Middle Elbe, Germany, (52°32′N 11°59′E–52°49′N 12°03′E) as seeds of this species could not be found along the River Rhine nor ordered from a commercial supplier. Hand-collected seeds were sampled between August and October 2010 depending on species-specific seed maturation. Freshly matured seeds were collected from at least two populations of a minimum of 20 individuals. For comparing the germination characteristics of seeds from the Rhine with seeds from the Elbe, seeds of five species (Centaurea jacea, Galium verum, Inula britannica, Pseudolysimachion longifolium, Silaum silaus) were additionally collected in autumn 2010 at the Middle Elbe (52°32′N 11°59′E–52°49′N 12°03′E). These seeds were also collected from at least two populations of at least 20 individuals (with the exception of S. silaus from which only one population was available).
The area along the northern Upper Rhine where the seeds were sampled has a mean annual precipitation of 643 mm and a mean annual temperature of 10.6°C (1981–2010 Gernsheim, DWD). The area at the middle Elbe has a lower mean annual precipitation of 555 mm and a mean annual temperature of 9.0°C (1981–2010 Boizenburg, DWD).
Seed handling and germination tests
After collection, seeds were manually cleaned, air-dried and stored in darkness at room temperature (approximately 20°C) until the start of the experiment in December 2010. Viability of seeds was tested for each population (25 per replicate, n= 2) with 1% tetrazolium chloride solution.
To test the germination capacity under outdoor conditions, seeds were sown into trays (50 per replicate, n= 3) with sterile standard garden soil and placed in a common garden (50°32′12″N 8°41′35″E, 172 m above sea level) at the same time as the climate chamber experiment started. The sowing date in January 2011 ensured cold wet stratification. Seeds were watered regularly and seedlings were counted and removed at least every other week for 2 years.
Experimental design
We used a factorial experimental design to study the effects of species (20 species), water potential (0, − 0.25, − 0.5, − 1.0, − 1.5 MPa) and temperature (day/night: 15/5°C and 20/10°C) on seedling emergence. Each treatment combination (species × temperature × water potentials) was replicated five times, resulting in 1000 experimental units. Additionally, for the comparison of the germination patterns of seeds from along the River Rhine with those of seeds from along the River Elbe, the seeds of the above-mentioned five species from the Elbe were also treated in five replicates with the five water potentials (0, − 0.25, − 0.5, − 1.0, − 1.5 MPa) at both temperatures (15/5°C and 20/10°C), resulting in another 250 Petri dishes.
We used the osmotic agent mannitol (Euro OTC Pharma GmbH, Bönen, Germany) to establish defined water potentials. Mannitol concentrations of 0.1, 0.2, 0.4 and 0.6 mol l− 1 were prepared to generate water potentials of approximately − 0.25, − 0.5, − 1.0 and − 1.5 MPa, respectively (according to Swagel et al., Reference Swagel, Bernhard and Ellmore1997). As a control (full water availability = water potential of 0 MPa) we used distilled water.
Fifty seeds of each species (25 seeds of Peucedanum officinale, due to its large seed size) were placed in sterile Petri dishes with one piece of filter paper moistened with 3 ml of d-mannitol solution or distilled water. In order to reduce evaporation, five Petri dishes were sealed together in a plastic bag.
As a stratification measure, the seeds were exposed to cold wet conditions for 5 weeks in climate chambers (Rumed type 3401, Rubarth Apparate GmbH, Laatzen, Germany) at 5°C to ensure breaking of potential seed dormancy. For incubation, we exposed the seeds to 12 h light and 12 h darkness and two diurnally fluctuating temperatures (15/5°C and 20/10°C) to simulate different spring temperature conditions.
Germination was defined as protrusion of the radicle. Germination was checked twice a week and seedlings were removed. After 4 weeks of incubation, germination decreased and Petri dishes were checked only once a week. While checking the Petri dishes for seedlings, the amount of mannitol solution in the Petri dishes was controlled. In order to keep osmotic potentials constant during the experiment, Petri dishes were carefully washed with 7.5 ml of mannitol solution or distilled water (control), emptied and re-filled with 3 ml of new mannitol solution or distilled water after 4 weeks of incubation. After 8 weeks of incubation the experiment ended, since almost no further germination was observed.
Germination parameters
As response variables, we calculated the germination percentage (%), mean germination time (days) and synchrony of germination (an index ranging from 0–1, without units) per replicate (according to Ranal and Santana, Reference Ranal and de Santana2006; Ranal et al., Reference Ranal, de Santana, Ferreira and Mendes-Rodrigues2009). The germination percentage is the percentage of all germinated seeds from the initial number of seeds. Mean germination time and synchrony of germination were calculated based on seedling counts over time (Ranal et al., Reference Ranal, de Santana, Ferreira and Mendes-Rodrigues2009). Mean germination time is a measurement of the weighted average length of time required for germination (Ranal and Santana, Reference Ranal and de Santana2006). The unit depends on the counting frequency and is days (d) in this study. The mean germination time
![]() $$\bar {>t} $$
is:
$$\bar {>t} $$
is:
where t i is the time from the start of the experiment to the ith observation day and n i is number of seeds germinated in the ith time, and k is the last time of germination (for details, see Ranal et al., Reference Ranal, de Santana, Ferreira and Mendes-Rodrigues2009).
The synchrony of germination indicates the germination variability over time, ranging from 0 to 1 (high values indicate highly synchronous germination). The synchrony of germination, Z, is:
 $$\begin{eqnarray} \end{eqnarray}$$
$$\begin{eqnarray} \end{eqnarray}$$
where n i is number of seeds germinated in the ith time.
Data handling and statistical analyses
For data analysis, plants with an Ellenberg moisture value of 3 or 4 were classified as indicators of dry habitats, and species with a moisture value of 7, 8 or 9 as indicators of wet habitats. Species with an intermediate Ellenberg moisture value of 5 or 6, or a so-called indifferent behaviour towards moisture (see Ellenberg et al., Reference Ellenberg, Weber, Düll, Wirth, Werner and Paulißen1992), were not included in the comparison between these two groups of species in our study.
For most species the osmotic threshold for germination was − 1.0 or at least − 1.5 MPa (see Fig. 1). To avoid zero inflation, the osmotic potentials of − 1.0 and − 1.5 MPa had to be omitted from the analyses. Moreover, we had to exclude the results of Galium palustre, due to the extremely low germination percentage of the seeds of this species in the climate chamber experiment (1.0 ± 0.5%).
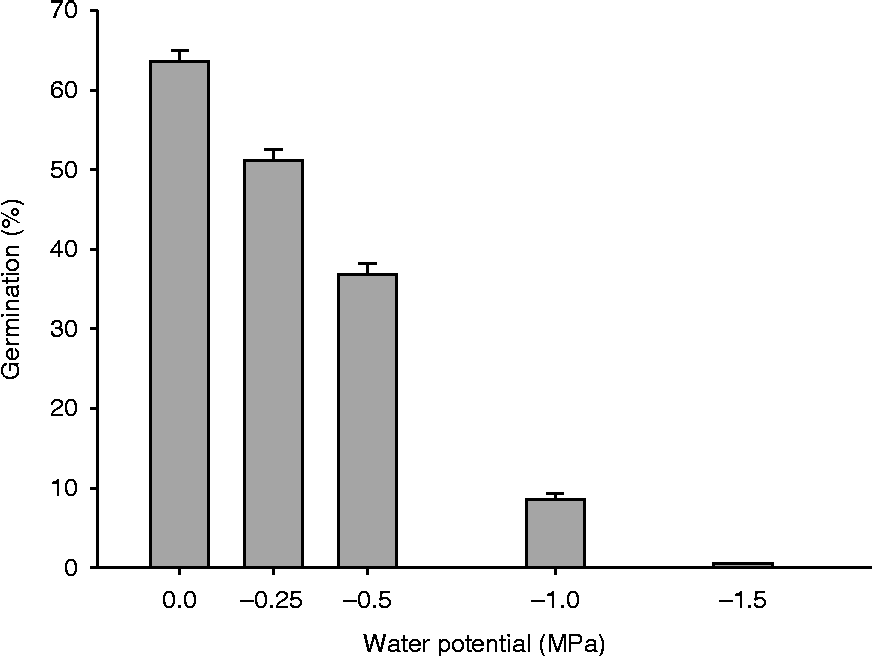
Figure 1 Germination percentage at the tested water potentials (mean+SE); including all treatments and species.
The effects of the experimental predictor variables species, water potential and temperature on germination percentage, germination time and synchrony of germination were analysed by three-way analyses of variance (ANOVAs). To account for inherent effects of family identity, we first calculated one-way ANOVAs with the factor plant family for every transformed (see below) response variable [germination percentage (F 4,565= 39.6, P< 0.001), germination time (F 4,565= 80.3, P< 0.001) and synchrony of germination (F 4,565= 51.4, P< 0.001)] and used the residuals for the three-way ANOVAs. As a measure of the relative contribution of each factor and interaction to the total variability in germination percentage, germination time and synchrony of germination, we used the ratio of the sum of squares of the factor or interaction of interest to the total sum of squares [i.e. variance contribution (vc) for all factors, their interactions and the error]. Following the three-way ANOVA, we conducted: (1) Tukey HSD-tests for the interaction of species× water potential, to analyse whether reducing the water potential to − 0.25 and − 0.5 MPa affected the seed germination percentages on the species level; and (2) contrast analyses to analyse if the species indicative of wet and dry habitats differ in their response to reduced water potential conditions. For the contrast analyses, the germination responses (germination percentage, germination time and synchrony of germination) of the seeds from species indicative of wet habitats were tested against the germination responses of the seeds from species indicative of dry habitats separately for the water potentials − 0.25 and − 0.5 MPa (but not for the non-stress control conditions).
For the analysis of the effects of seed origin (Rhine versus Elbe) on the germination parameters, we conducted ANOVAs with the factors species, water potential, temperature and origin. Data were transformed to approximate normal distribution and variance homogeneity [germination percentage: arcsin (square root/100); mean germination time and synchrony: log+1]. All statistical tests were conducted using STATISTICA 10 (StatSoft Inc., Tulsa, Oklahoma, USA).
Results
General characteristics of seed material
Viability (%) of the seeds was generally high (Table 1). Most species had>85% viable seeds, exceptions were Filipendula ulmaria (50 ± 2%), Galium palustre (56 ± 3%), Linaria vulgaris (60 ± 12%) and Sanguisorba minor (70 ± 2%). Under outdoor conditions 13 species had germination percentages of>70%, whereas low germination percentages were exhibited by Galium boreale (24 ± 9%), G. palustre (19 ± 1%) and Peucedanum officinale (20 ± 5%) (Table 1).
Germination in response to water potential, temperature and origin
Generally, germination percentage was significantly reduced at lower water potentials and totally ceased at a water potential of − 1.5 MPa (Fig. 1). The three-way ANOVA indicated that germination percentage varied significantly among species, this factor explained 41% of the total variation (vc; Table 2). Furthermore, germination percentage was significantly influenced by the water potential (vc = 30%; Table 2). At a water potential of − 0.5 MPa, the germination of most study species was significantly reduced. Only the germination of four species was not susceptible to a water potential of − 0.5 MPa: Centaurea jacea, Inula britannica, Sanguisorba minor and S. officinalis (P values>0.05, Fig. 2). Mean germination time was likewise mostly influenced by the factors species (vc = 43%) and water potential (vc = 27%; Table 2). Additionally, germination time was significantly affected by the temperature regime at which the seeds germinated (vc = 12%; Table 2). On average, seeds needed 13 d for germination at the temperature regime 15/5°C and 17 d at the higher temperature regime 20/10°C (including all species except Galium palustre). Synchrony of germination mainly depended on the factor species (vc = 35%) whereas water potential explained only 11% of the total variation (Table 2).
Table 2 Results of the ANOVA for the climate chamber experiment. Effects of species, water potential and temperature on germination (%), mean germination time (d) and synchrony of germination; including the water potentials 0, −0.25 and −0.5 MPa; df=degrees of freedom, F= variance ratio, P= error probability, vc (%) relative contribution of individual factors and their interactions to total variance


Figure 2 Cumulative germination percentage over time of the study species (except Galium palustre), averaging the data of the two temperature regimes (mean ± SE). Species order as in Table 1. Different letters indicate differences in the final germination percentage between water potential treatments (0, − 0.25 and − 0.5 MPa).
The comparison of seed germination of five species originating from the Rhine and the Elbe indicated species-specific responses. Species identity explained the largest part of the total variation in germination percentages (F 4,240= 299.8, P< 0.001, vc = 48%). The main factor of interest, i.e. origin, had no effect on the germination percentages (F 1,240= 0.04, P= 0.85). Further, the response to the reduced water potential did not differ between origins as no interaction between origin and water potential was detected (F 2,240= 0.71, P= 0.49). Species also explained most of the variation in mean germination time (F 4,239= 742.1, P< 0.001, vc = 60%). Although origin had a significant effect on germination time, it only explained a small part of the variation (F 1,239= 145.8, P< 0.001, vc = 3%). No origin× water potential interaction was found for germination time (F 2,239= 1.1, P= 0.35). Again, in the analysis of synchrony species explained most of the variation (F 4,239= 198.4, P< 0.001, vc = 51%), while origin had a significant, but small impact (F 1,239= 31.9, P< 0.001, vc = 2%) and no origin× water potential interaction was found (F 2,239= 1.3, P= 0.27).
Wet versus dry habitat species
Contrast analyses revealed that the seeds of species indicative of wet habitats had significantly higher germination percentages than species indicative of dry habitats at reduced water potentials (see Table 2 and Fig. 3a). On average, 77 ± 3% (mean ± SE, N= 50) of the seeds from species of wet habitats germinated at a water potential of − 0.25 MPa versus only 62 ± 3% (N= 80) of the seeds from species of dry habitats. At a water potential of − 0.5 MPa, 61 ± 4% (N= 50) of the seeds from species of wet habitats germinated versus only 44 ± 3% (N= 80) of the seeds from species of dry habitats. Besides, the seeds of the species from dry habitats needed approximately 2 d longer for germination at a water potential of − 0.5 MPa (see Table 2 and Fig. 3b) and germinated slightly less synchronously at a water potential of − 0.25 MPa (see Table 2 and Fig. 3c) than the seeds of the species from wet habitats.

Figure 3 Germination percentage, germination time and synchrony, averaging the data of the two temperature regimes (mean+SE) of the species indicative of dry (Pimpinella saxifraga, Peucedanum officinale, Veronica teucrium, Linaria vulgaris, Plantago media, Filipendula vulgaris, Sanguisorba minor, Galium verum) and wet (Selinum carvifolia, Inula britannica, Pseudolysimachion longifolium, Sanguisorba officinalis, Filipendula ulmaria) habitats, including the water potentials − 0.25 and − 0.5 MPa (excluding Galium palustre). Significance levels: * = P< 0.05, *** = P< 0.001.
Discussion
Germination responses
In accordance with our first hypothesis, the germination of all species was negatively affected by reduced water availability, and almost ceased at a water potential of − 1.5 MPa. This emphasizes the importance of soil moisture as a key requisite for the germination process (Baskin and Baskin, Reference Baskin and Baskin2001) and is in line with other studies in which germination was affected by low water potentials (Evans and Etherington, Reference Evans and Etherington1990; Swagel et al., Reference Swagel, Bernhard and Ellmore1997; Akhalkatsi and Lösch, Reference Akhalkatsi and Lösch2001; Springer, Reference Springer2005; Daws et al., Reference Daws, Crabtree, Dalling, Mullins and Burslem2008). Two of these studies included species that were able to germinate at reduced water potentials (for details see Evans and Etherington, Reference Evans and Etherington1990; Springer, Reference Springer2005). Also in our study it is surprising that germination of four species (Centaurea jaceae, Inula britannica, Sanguisorba minor and S. officinalis) was unaffected by a water potential as low as − 0.5 MPa. A further seven species germinated to an equal percentage at water potential − 0.25 MPa compared to the control conditions (Galium boreale, Inula salicina, Linaria vulgaris, Pimpinella saxifraga, Plantago media, Pseudolysimachion longifolium, Serratula tinctoria). Germination time and synchrony of germination were also influenced by the water potential in our study. This finding corresponds to other studies in which a delayed onset of germination was reported at reduced water potentials (Evans and Etherington, Reference Evans and Etherington1990; Daws et al., Reference Daws, Crabtree, Dalling, Mullins and Burslem2008).
While the three response variables were mainly influenced by species identity (which fits with the results of Burmeier et al., Reference Burmeier, Donath, Otte and Eckstein2010) followed by water potential (see results of the three-way ANOVA, Table 2), it has to be acknowledged that all response variables were affected by plant family. Concerning germination time, an accumulation of species with the ability of very fast germination ( < 24 h) within certain plant families was recently reported in a review on this topic (Parsons, Reference Parsons2012). It seems that fast germination may be especially abundant in high-stress habitats as, for example, functional floodplains (Parsons, Reference Parsons2012). Overall, we can conclude that germination characteristics are species specific and partly phylogenetically determined.
The seeds of most species germinated faster at the lower temperature (15/5°C), therefore we have to reject our second hypothesis. Probably the lower temperature regime corresponds better to the temperature conditions of meadow habitats during spring under temperate climates, the season in which many floodplain meadow species usually germinate (Hölzel and Otte, Reference Hölzel and Otte2004). Additionally, seed germination probably avoids warmer periods when increased evaporation may cause decreased water availability for seedlings. In two studies on Australian plant species, optimum germination temperatures coincided with the average summer or winter temperatures of the local habitats (Jurado and Westoby, Reference Jurado and Westoby1992; Cochrane et al., Reference Cochrane, Daws and Hay2011), but only in one study is the preferred germination seasons in the field suggested to be the winter (Cochrane et al., Reference Cochrane, Daws and Hay2011). Furthermore, studies on species of semi-arid grasslands document that the water potential is less limiting when germination temperature is close to the optimum (Romo et al., Reference Romo, Grilz, Bubar and Young1991; Qi and Redmann, Reference Qi and Redmann1993). In our study on species of temperate grasslands, however, no significant interaction between temperature and water potential was found for the response variable germination time (see Table 2). Concerning potential climate change effects, faster seed germination at lower temperatures could result in an earlier onset of germination, since overall higher future temperatures are projected for Germany (Jacob et al., Reference Jacob, Göttel, Kotlarski, Lorenz and Sieck2008). A shift to earlier germination may ensure moist germination conditions, as increases in precipitation in early spring are projected (Jacob et al., Reference Jacob, Göttel, Kotlarski, Lorenz and Sieck2008). However, this may also lead to new threats for the seedlings, such as an increased mortality risk caused by spring floods during periods of suitable germination temperature.
Germination of seeds from the Rhine and Elbe rivers
The germination of seeds from along the River Elbe was surprisingly similar to the germination of the seeds from along the River Rhine. Differences in germination between species were larger by far than between the populations from the two rivers. This is further evidence that germination characteristics are species specific. Maybe from a seed's perspective the rivers Elbe and Rhine are not so different. Both floodplains are more or less regularly inundated and are dry during summer. These features may be more important than the mean annual precipitation or temperature. Further, higher mean annual temperature at the River Rhine might lead to similar water potentials in the soils due to a higher evapo-transpiration, despite a higher annual amount of precipitation. Due to the great similarity in germination between seeds of the two origins, it can be assumed that our findings for the tested species from floodplains along the River Rhine are transferable to other Central European floodplain ecosystems with similar climatic conditions.
Germination responses of species indicative of wet and dry habitats
We hypothesized that germination of plant species indicative of wet habitats will decrease more at reduced water potentials than that of species indicative of dry habitats, as we expected species from dry habitats to be better able to cope with drier conditions. Strikingly, we found the opposite. Selection pressures for responding to the moisture status of their environment might be low for species indicative of wet habitats. Thus, under experimental conditions, they even germinate under conditions unfavourable for successful establishment, which is a rare situation in their habitat in situ. Another environmental factor (i.e. temperature) might be more important for their germination. In turn, seeds of species indicative of dry habitats are probably capable of sensing the moisture status of their environment, thereby avoiding germination under unfavourable conditions in the field. The results of Evans and Etherington (Reference Evans and Etherington1990) are in contrast to our findings; in their study wetland species did not germinate to a great extent at low water potentials, but some dry habitat species germinated successfully under dry conditions. Still, they concluded that the ‘inability’ to germinate under dry conditions might be a dormancy mechanism to avoid the failure of seedling establishment. In our study, however, all seeds were cold-wet stratified to ensure breaking of dormancy prior to the experiment. Germinating under dry conditions could easily lead to the death of the seedling as the probability that water availability increases again is rather low in dry habitats. Hence, the selection pressure towards moisture-sensing mechanisms might be high in dry habitats, in order to respond to the right window of opportunity for successful germination.
Germination time of species indicative of dry habitats was longer than that of species indicative of wet habitats. We speculate that it takes some time for seeds of species from dry habitats to sense the actual environmental conditions in their surroundings, while seeds of species from wet habitats germinate immediately. This fits with the finding that the seeds of species indicative of wet habitats germinate more synchronously than the seeds of species indicative of dry habitats, although the absolute difference between groups was small and only significant for the water potential − 0.25 MPa. Also, the difference in germination time between the two groups of species is rather small (the seeds of species from wet habitats germinated approximately 2 d earlier than the seeds from species indicative of dry habitats) at the water potential − 0.5 MPa. It remains unclear if this finding is ecologically relevant under field conditions. Nevertheless, especially in the productive wet meadows, early germination could be advantageous for establishment. This corresponds to a study on the germination of 91 species in response to a temperature gradient, where species of productive grassland germinated rapidly (Olff et al., Reference Olff, Pegtel, Vangroenendael and Bakker1994). Therefore, we assume that the species of wet sites follow an all-or-nothing-strategy, with fast and synchronous germination to maximize competitive advantages and betting on a high probability of moist conditions for establishment (‘optimists’). In contrast, species of dry sites follow a bet-hedging strategy with a moisture-sensing mechanism for the right conditions, betting on a high probability for unsuitible conditions (‘pessimists’ sensu Jones, Reference Jones1992, who coined this terminology for strategy types of photosynthetic reactions to drought stress), resulting in a slower and less synchronous germination.
Conclusions
The germination of almost all studied species was decreased by lower water potentials, which strengthens the results of former studies (Evans and Etherington, Reference Evans and Etherington1990; Swagel et al., Reference Swagel, Bernhard and Ellmore1997; Akhalkatsi and Lösch, Reference Akhalkatsi and Lösch2001; Springer, Reference Springer2005; Daws et al., Reference Daws, Crabtree, Dalling, Mullins and Burslem2008) and demonstrates that floodplain meadow species will be negatively influenced in their earliest life stage (i.e. seed germination) by decreasing water availabilities during future climate change. However, the species indicative of wet and of dry habitats of floodplain meadows might be affected differently. Our experimental data suggest that seeds of species indicative of dry habitats show sensitivity to the moisture status of their immediate environment. Their ‘pessimistic’ response (germinating only when they sense sufficient moist conditions) probably enables them to track the time windows with high probability for successful germination and establishment. Seeds of species indicative for wet habitats do not possess such a mechanism since the conditions in their typical habitat are usually sufficiently moist. Their ‘optimistic’ response to this environmental factor probably makes them comparably vulnerable to climate change. Shifts of these species further down the elevation gradient in floodplains (where conditions are still moist enough for successful establishment) might be the consequence. Further, faster seed germination under low temperatures could also lead to a shift to earlier germination, when the soil is still moist from precipitation during winter and spring. Then other factors, such as the occurrence or timing of spring floods or frosts may gain importance. More research is needed concerning the timing of germination under changing climatic conditions.
Acknowledgements
We thank Josef Scholz vom Hofe, Christiane Lenz-Kuhl, Ute Zerahn and Annabell Fraenkel for their assistence in sampling seeds and counting seedlings, and the two anonymous referees for their valuable comments.
Financial support
As part of the KLIWAS project, this study was financed by the Federal Ministry of Transport, Building and Urban Development, Germany.
Conflicts of interest
None.







