INTRODUCTION
It is of fundamental importance to understand the past variability and magnitude of climate change, to predict and prepare for future climatic impacts on human activity. In particular, determining the exact timing of climatic shifts is important for determining the controlling factors and mechanisms. Historical climatic signals have been identified from sedimentary deposits, including lake, wetland, and deep-sea sediments. Recently, these climatic shifts have been shown to have occurred at multi-decadal to centennial timescales, especially in high-resolution proxies that require precise dating results.
Radiocarbon dating is one of the most commonly used methods for determining when sediments were deposited, and for analyzing the climatic indicators in sediments in terms of temporal variability. To obtain reliable age dates, it is important to use appropriate dating materials. 14C dates of plant fragments (PF) and soil organic matter have been obtained previously. Three fractions of soil organic matter are distinguished based on pH solubility: humin (the acid and alkali-insoluble fraction), humic acid (HA; alkali-soluble, acid-insoluble), and fulvic acid (acid and alkali-soluble) (e.g., Campbell et al. Reference Campbell, Paul, Rennie and McCallum1967; Abbott and Stafford Reference Abbott and Stafford1996; Cook et al. Reference Cook, Dugmore and Shore1998; Martin et al. Reference Martin, Goff, Jacopsen and Mooney2019). There have been reports of differences in age among the fractions. The dating of peat has proven problematic due to its complex heterogeneous and heterochronous composition. For example, in an analysis of British Isles peat, Shore et al. (Reference Shore, Bartley and Harkness1995) reported possible age differences between humin and humic fractions of up to 1210 years. It is likely that the ages of HA and humin fractions are influenced by their grain size fractions, with coarse fractions showing older age (Brock et al. Reference Brock, Lee, Housley and Ramsey2011).
Pessenda et al. (Reference Pessenda, Gouveia and Aravena2001) showed that total soil organic matter was significantly younger than humin fractions and charcoal at similar depths, due to contamination by younger carbon. They suggested that the humin fraction is a more reliable material for 14C dating in soils. Xu and Zheng (Reference Xu and Zheng2003) analyzed organic fractions in Erhai Lake sediments, which showed the following order of age: PF < HA ≤ humin < fulvic acid. This suggests that caution is needed when using bulk sediments for radiocarbon dating. Sediments from Lake Rara, Western Nepal, were younger than bulk sediments by ~500 years, with no clear pattern according to depth (Nakamura et al. Reference Nakamura, Yokoyama, Maemoku, Yagi, Okamura, Matsuoka and Dangol2012).
Previous studies have rarely analyzed variability in 14C age differences among soil organic fractions according to possible climate change. Here, we measured 14C age differences among PF, HA, and humin, with the aim of determining whether there is systematic variability in the age difference due to climate change.
METHODS
Study Area and Sampling
The Muljangori-oreum wetland (elevation: 900 m, 33°24'N 126°36'E), one of the crater lakes on Jeju Island, South Korea was registered to the Ramsar Convention in 2008. Since then, development has been prohibited and the area is relatively well preserved (Figure 1). The wetland is located ~8.5 km northeast of the summit of Mt. Halla (elevation: 1950 m), and has a circumference of ~400 m; the present water depth in the central part of the wetland is 0.8–1 m.

Figure 1 Study area and sampling site (core MJO3-4) in the Muljangori-oreum wetland, a volcanic crater of Jeju Island, South Korea, and an example core.
The wetland, located at 900 m in elevation, has an annual mean temperature of 8.7°C and an aquatic area of 13.1 × 103 m2. Vegetation in the wetland consists of both terrestrial (70) and aquatic (7) plant species, including helophytes (5) and hydrophytes (2), while deciduous broadleaved forest surrounds the wetland (Kim et al. Reference Kim, Lee, Jegal and Choi1999). Plant communities found from the wetland area include Trapa incisa community, Scirpus triqueter community, Juncus papillosus-Scirpus tabernaemontani community, Juncus effuses var. decipiens community, and Panicum bisulcatum-Isachne globose community. Forest area of the inner slope of Muljangori-oreum is dominated by Calanthe reflexa-Carpinus laxiflora community (Kim et al. Reference Kim, Lee, Jegal and Choi1999). The inner slope deposits of the Muljangori-oreum are covered by basalt fragments, scoria and volcanic ash soils, and the pH of the wetland sediments is weak acid ranging between 5.24~6.06 (World Heritage and Mt. Hallasan Research Institute 2016).
Coring work was carried out from a barge in the middle of the wetland (July 2017) (Jeju Special Self-Governing Province (World Heritage Office) and Korea Institute of Geoscience and Mineral Resources 2017). A peat core sampler was used for sampling soft upper sediments (0–6 m), and a percussion hammer (Cobra TT; Atlas Copco, Sweden) was used for sampling hard lower sediments and weathered bedrock (6–8 m). An 8-m-long sediment core was recovered without significant loss of material, as shown in Figure 1. The core was stored in a refrigerator during transportation to prevent secondary contamination. Ten pairs of humic acid (HA) and plant fragments (PF) samples, and three pairs of HA and humin samples, from the same depths were prepared to test age differences. All samples are out of the same core. Most of the samples were dark brown in color and there was enough organic matter to produce a sufficient amount of CO2.
14C Dating
Pretreatment for 14C dating was done based on previous studies (e.g., Kigoshi et al. Reference Kigoshi, Suzuki and Shiraki1980; Abbott Reference Abbott and Stafford1996; Kretschmer et al. Reference Kretschmer, Anton, Bergmann, Finckh, Kowalzik, Klein, Leigart, Merz, Morgenroth, Piringer, Küster, Low and Nakamura1997; Pessenda et al. Reference Pessenda, Gouveia and Aravena2001). A schematic diagram of the chemical pretreatment is shown in Figure 2. PF (n = 10) underwent a series of acid–alkali–acid treatments to remove contaminants. HA (n = 19) and humin (n = 4) samples were treated as described in Figure 2. After graphitization of the pretreated HA, humin, and PF samples, radiocarbon dating was performed using the accelerator mass spectrometry facility of the Korea Institute of Geoscience and Mineral Resources (Hong et al. Reference Hong, Park, Kim, Woo, Kim, Choi and Kim2010a, 2010b). 14C ages (conventional radiocarbon dates) were converted to calibrated ages (Cal BP) using the software OxCal 4.3 (Bronk Ramsey Reference Bronk Ramsey2009a, Reference Bronk Ramsey2009b) and IntCal13 calibration curve (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013). Calibrated ages were reported as probability density ranges at the 95.4% confidence level.
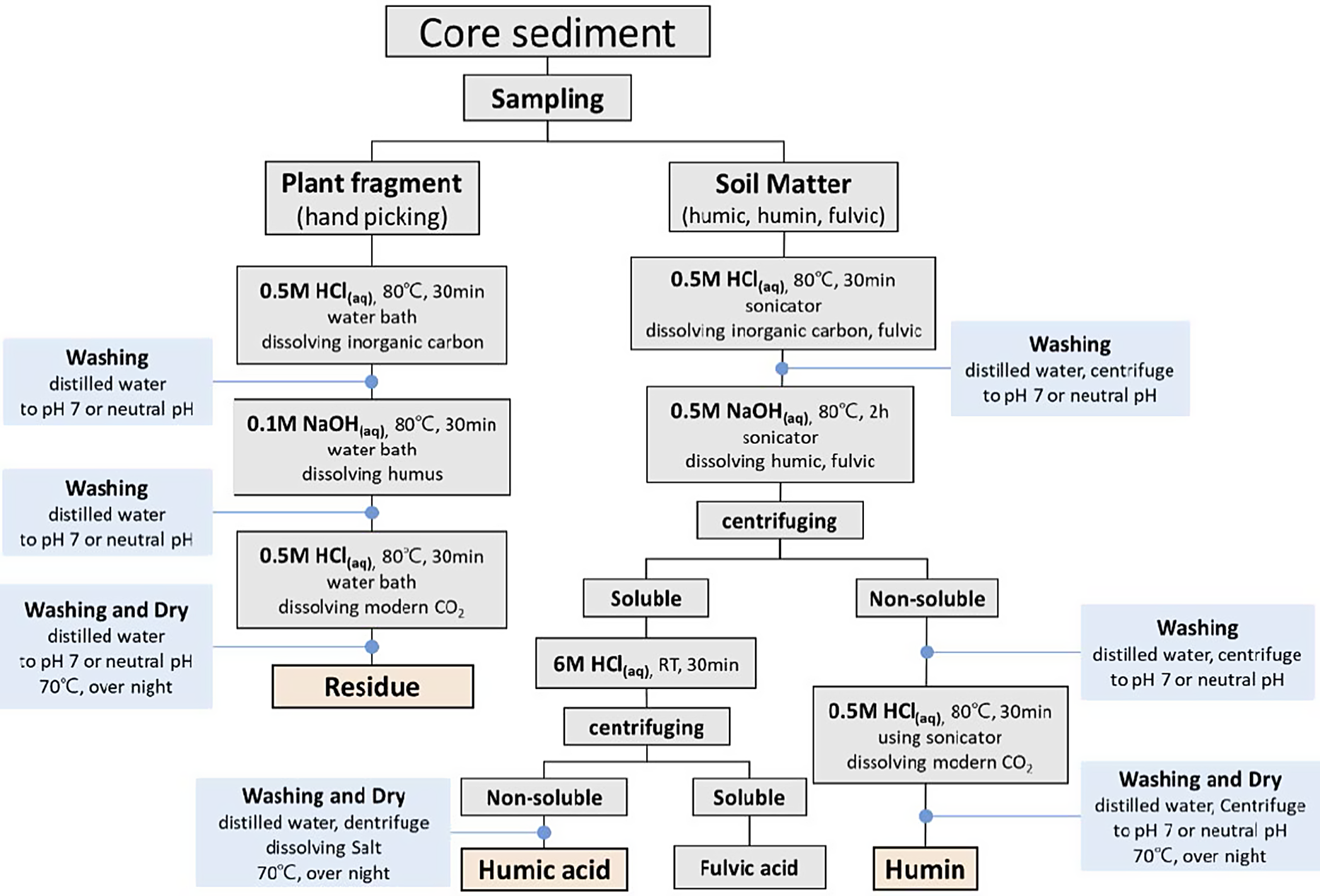
Figure 2 Schematic diagram of the pretreatment procedure for 14C dating materials (modified from Kigoshi et al. Reference Kigoshi, Suzuki and Shiraki1980; Kretschmer et al. Reference Kretschmer, Anton, Bergmann, Finckh, Kowalzik, Klein, Leigart, Merz, Morgenroth, Piringer, Küster, Low and Nakamura1997).
Total Organic Carbon (TOC) and Carbon Isotope (δ13C) Analyses
Bulk subsamples (~500 mg) were treated with 1 N HCl at ~100°C for 1 hr, then rinsed with distilled water. Approximately 3–5 mg of the HCl-treated subsamples was loaded into a tin combustion cup, and the TOC content was determined using a CNS elemental analyzer (vario Micro Cube; Elementar, Langenselbold, Germany). δ13C analyses of the HCl-treated samples were performed using a continuous-flow isotope ratio mass spectrometer (IsoPrime100; GV Instruments, Manchester, UK) coupled with the CNS elemental analyzer. The data are expressed as δ relative to the Vienna Pee Dee Belemnite standard. The reference material used was International Atomic Energy Agency (IAEA)-CH-6 (sucrose, δ13C = −10.45 ± 0.033‰). Standard and sample replications lead for typical error lower than 0.2‰.
Grain-Size Analysis
Approximately 300 mg of dry sample for grain size analysis was treated with 35% H2O2 to decompose organic matter, and then boiled in 1 N HCl for 1 hr to remove carbonates and iron oxides. After rinsing with distilled water and treating with an ultrasonic vibrator to keep the grains in suspension, grain size analysis was then performed using a Mastersizer 2000 laser particle size analyzer (Malvern Instruments, Malvern, UK), which automatically provides grain size percentages (e.g., for clay, silt, and sand) and median grain sizes.
RESULTS AND DISCUSSION
Age Differences among Humic Acid, Plant Fragment, and Humin Samples
The age of each component in the sediments showed an increasing trend with depth, indicating continuous deposition in the Muljangori wetland during the past ca. 8000 years (Table 1 and Figure 3). The cores can be divided into four sedimentary units based on the texture, grain size, and color. Unit 1 is overlaid on the basalt and consists of scoria fragments and bright gray sandy mud, suggesting rapid deposition at ca. 8000 cal BP. Unit 2, corresponding to a core depth of 7.5–4 m, consists of layers of dark gray silty mud. Unit 3 is located between 4 and 1.2 m; it has a different color (a dark chocolate) layer from Unit 2, but a similar grain size of silty mud. Unit 4 is the layer between the surface and a depth of 1.2 m; it is a dark chocolate silty mud layer with some upward coarsening, as shown by an increasing median grain size from < 10 to > 20 µm.
Table 1 Radiocarbon ages from the Muljangori-oreum wetland sediments (core MJO3-4), a volcanic crater of Jeju Island, South Korea and age difference among the dating materials (humic acid (HA), plant fragments (PF) and humin).

a χ2 test between 14C ages of pairs of humic acid (HA) and plant fragment (PF) or of pairs of humic acid (HA) and humin obtained on same depth. χ2 test value is reported with χ2 critical distance for p=0.05.
b Age range with 95.4% probability. 14C ages (conventional radiocarbon dates) were converted to calibrated ages (cal BP) using the software OxCal 4.3 (Bronk Ramsey Reference Bronk Ramsey2009a, Reference Bronk Ramsey2009b) and IntCal13 calibration curve (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013). Median age indicates the median of the probability distribution (the corrected 2-σ age range) calculated by Markov chain Monte-Carlo analysis in the software program.
c DIFHA-PF age means the median age difference between humic acid (HA) and plant fragments (PF). Values in italics are for information only. The 14C ages are not significantly distinct.
d DIFHA-humin age means the median age difference between humic acid (HA) and humin.

Figure 3 14C dating results for humic acid (HA, yellow triangles), plant fragments (PF, light blue squares), and humin (red stars) from wetland sediments in the Muljangori volcanic cone, Jeju Island, South Korea. Radiocarbon dates were provided with each median age with error corresponding to the corrected 2-σ age range which was calculated by Markov chain Monte-Carlo analysis in the OxCal 4.3 program (Bronk Ramsey Reference Bronk Ramsey2009a, Reference Bronk Ramsey2009b; Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013). Age difference (DIFHA-PF, open diamond) was calculated by subtracting the PF age from the HA age as shown in Table 1.
To facilitate comparison of age difference among different dating materials, we used median age for the probability distribution (the corrected 2-σ age range) which was calculated by Markov chain Monte-Carlo analysis in the OxCal 4.3 program (Bronk Ramsey Reference Bronk Ramsey2009a, Reference Bronk Ramsey2009b; Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013) (Table 1). The age data for humic acid (HA), plant fragments (PF), and humin indicate continuous deposition, but with clear age differences among these three chemical fractions.
Based on 10 paired HA and PF samples obtained from the same depths, the difference between 14C ages is not significant for the upper 1.2 m but is significant below. The 14C age difference yields for shift between calibrated range median values increasing with depth reaching a maximum of 380 years range at 2.15 m, slowly decreasing thereafter. Interestingly, the increased DIFHA-PF (300 years) was observed at 7.52 m, corresponding to the bottom part of the sediment cores. Comparison between HA and humin on the 3 samples obtained from the same depth yields for no significant 14C age difference at 1.2 and 2.65 m but significant difference at 2.9 m. This difference results in a 250-year shift between the medians of the calibrated intervals.
Long-Term Changes of DIFHA-PF during the Past 8000 Years
Many previous studies have noted age differences among various organic fractions in lake and peat sediments, but there have been few reports of systematic variation in age among the fractions (e.g., Shore et al. Reference Shore, Bartley and Harkness1995; Abbott and Stafford Reference Abbott and Stafford1996; Xu and Zheng Reference Xu and Zheng2003; Nakamura et al. Reference Nakamura, Yokoyama, Maemoku, Yagi, Okamura, Matsuoka and Dangol2012). However, this study seems to show systematic changes in age difference between HA and PF fractions (Figure 3).
The consistently older age of HA fractions suggests a limited influence of the younger humic fraction (e.g., subsurface roots) in the wetland sediments in this study. Frequent reversals of 14C age in tropical peat deposits have been observed, attributed to the input of younger carbon through the root system (Page et al. Reference Page, Wust, Weiss, Rieley, Shotyk and Limin2004; Wüst et al. Reference Wüst, Jacobsen, von der Gaast and Smith2008). Furthermore, mobile organic matter (e.g., dissolved organic carbon) can infiltrate vertically into sub-sediments, disturbing the initial carbon information (Kaiser et al. Reference Kaiser, Guggenberger and Zech2001; Paul et al. Reference Paul, Balesdent and Hatté2020). In the Muljangori sediments, the influence on the HA fraction of such sub- and inter-sedimentary mechanisms seems to be very weak. Considering that the PF consistently showed a younger age, the influence of HA fractions on the 14C age appears to be exerted through input of depleted 14C from slope deposits. The extent of 14C depletion in the slope deposits may be another factor contributing to the older age of HA fractions.
As factors potentially associated with the age difference among the organic fractions, carbon/nitrogen (C/N) ratios and total organic carbon isotope (δ13CTOC) values were obtained. As shown in Figure 4, the C/N ratios are relatively high in terrestrial plants compared to aquatic plants due to their low cellulose and lignin contents (Meyers Reference Meyers1994). In general, carbon isotope ratios are useful for distinguishing between different types of land plants (Meyers Reference Meyers1994, Reference Meyers1997; Lamb et al. Reference Lamb, Wilson and Leng2006; Lim and Fujiki Reference Lim and Fujiki2011). C3 plants (e.g., trees and shrubs) using the Calvin cycle reportedly led to a shift in δ13C of approximately −20‰, which, combined with that of atmospheric CO2 (δ13C ≈ −7‰), resulted in an average shift of −27‰. Meanwhile, C4 plants (mainly grasses) using the Hatch–Slack pathway reportedly led to a shift in δ13C shift of approximately −7‰, which, combined with that of atmospheric CO2, resulted in an average shift of −14‰ (O’Leary Reference O’Leary1981, Reference O’Leary1988; Farquhar et al. Reference Farquhar, O’Leary and Berry1982; Tieszen Reference Tieszen1991). Freshwater algae have δ13C values similar to those of C3 plants (Meyers Reference Meyers1994, Reference Meyers1997), and C/N ratios in combination with δ13CTOC values can provide information on the organic matter in wetland sediments.

Figure 4 (A) Cross-plot between the carbon/nitrogen (C/N) ratio and total organic carbon isotope (δ13CTOC) values in wetland sediments from the Muljangori volcanic cone of Jeju Island, South Korea. (B) Cross-plot between the C/N ratio and median grain size during Stages 1–3. Shaded areas indicate typical organic δ13C and C/N ratio ranges in freshwater and terrestrial environments (Meyers Reference Meyers1994, Reference Meyers1997; Lamb et al. Reference Lamb, Wilson and Leng2006 and references therein).
Among the TOC (%), C/N ratio, grain size, and δ13CTOC values obtained in this study, the C/N ratios showed the strongest association with the long-term changes in DIFHA-PF (dashed line in Figure 5). The long-term trend in DIFHA-PF was divided into Stages 1–4 according to the average values of DIFHA-PF and C/N ratios. Based on the PF ages, Stage 1 was characterized by rapid deposition, showing fining-upward sequences and one clear peak in the C/N ratio, suggesting high terrestrial plant input. During Stages 2–4, the long-term trend of DIFHA-PF was quite similar to that of the C/N ratios; in Stage 3, the δ13CTOC values also showed a similar trend to DIFHA-PF. These results suggest that the increase in DIFHA-PF may have been caused by the same factors responsible for the simultaneous increases in terrestrial and C4 plant inputs; in other words, the older age of the HA fraction was likely influenced by the previously drier climate. As shown in the cross-plot between median grain size and C/N ratio (Figure 4B), the increase in C/N ratios was correlated with a decrease in sediment grain size. This implies that the older age of HA fractions was influenced by finer grain size and increased terrestrial plant input. This coupling of finer grain size with increased C/N ratios suggests a more arid climate with less precipitation than that seen today. During Stage 4, the DIFHA-PF decreased in accordance with the decrease in C/N ratios. Furthermore, the δ13CTOC reached a minimum value of −28‰ and grain size showed abrupt coarsening during this stage, suggesting a significant increase in aquatic organic matter and coarse grain input into the wetland sediments from slope deposits. This climate change toward more wet condition seems to have influenced on the extent of 14C depletion in the slope deposits, resulting in the decrease in DIFHA-PF.

Figure 5 Total organic carbon (TOC, %), median grain size, and total organic carbon isotope (δ13CTOC) values of wetland sediment samples from the Muljangori volcanic cone on Jeju Island, South Korea. Age difference was calculated by subtracting the plant fragment (PF) age from the humic acid (HA) age. The long-term trend in age difference (DIFHA-PF) was divided into Stages 1–4 based on the average values of DIFHA-PF and C/N ratios.
Regarding possible linkage between climate change and HA behavior in the catchment areas and sinking areas (e.g., lake and wetland), there are limited number of case studies (e.g., Abbott and Stafford Reference Abbott and Stafford1996; Reinikainen and Hyvärinen Reference Reinikainen and Hyvärinen1997). In general, soil organic matter is originated from plant litter and the micro biomass, and plant component consists of aliphatic biopolymers, tannins, polysaccharides and lignin, making complex mixtures of organic matter (Kogel-Knabner Reference Kögel-Knabner2002). Among them, HA which is not soluble in water comprises a mixture of weak aliphatic (carbon chain) and aromatic (carbon rings) organic acids with molecular weight ranging from 10,000 and 100,000 while fulvic acid is a mixture of weak aliphatic and aromatic organic acids which are soluble in water and with molecular weight of 1000 ~ 10,000 (Pettit Reference Pettit2004). Based on studies of soil, peat, and sediment sections, it was suggested that the 14C age of humic substances increases with increasing molecular weight, and that age differences among substances increase over time (Abbott and Stafford Reference Abbott and Stafford1996). Regarding to humic acid behavior, especially, based on the Holocene lake sediments, Reinikainen and Hyvärinen (Reference Reinikainen and Hyvärinen1997) found significant changes in the amount and proportion of HA and FA according to past climate change. For example, decreasing trend in the HA/FA ratio caused by a slight increase in FA since late Holocene (ca. 2 ka BP) was attributed to decreasing humidity. This study suggested that the decrease in HA in the lake sediments might have been influenced by increased input of poorly humified organic matter eroded and washed in from the drier surfaces around the basin coupled to lake-level rise and intensified surface runoff.
Considering these previous results, we posit that, during intensified runoff periods, less humified organic matter from slope deposits to the Muljangori wetland increased, which could in turn have decreased the formation of HA in the slope deposits. By contrast, during dry periods, the content of HA in the slope sediments may have increased, resulting in increased 14C depletion in these sediments until washed into the wetland or lake. Thus, the increase in DIFHA-PF in the wetland sediments seems to have been influenced by the dry climate at that time, characterized by an increase in the rate of formation of HA in the slope sediments. These processes may have led to 14C depletion in the slope sediments, such that the difference in 14C composition between the wetland and slope sediments increased. When the climate was drier, input of allochthonous HA from slope areas to the wetland may have decreased, while the influence of the markedly depleted 14C in slope sediments would have increased, thus exacerbating the difference between HA and PF. However, our interpretations of the DIFHA-PF change based on a single site must be tentative only. This hypothesis regarding a possible association between long-term changes in the DIFHA-PF and climate change, according to HA formation and 14C depletion, is based on a limited number of dating points and should be validated at other sites with additional dating points and comparable geochemical indicators.
CONCLUSIONS
In Muljangori-oreum wetland sediments, HA fractions were older than PF and the age difference between HA and PF was not constant over the past 8000 years: a long-term increasing trend in the age difference seems to have been influenced by a drying climate, as supported by similar increases in C/N ratios and δ13CTOC values. This study demonstrates long-term changes in the 14C age of various organic fractions of wetland sediments that accord with climate change. The extent of 14C depletion in the slope deposits and its influence on the HA age may be tested using geochemical and isotopic data (e.g., C/N ratios and δ13CTOC values).
ACKNOWLEDGMENTS
This research was supported by the Basic Research Project (GP2020-003), entitled “Geological survey in the Korean Peninsula and publication of the geological maps” of the Korea Institute of Geoscience and Mineral Resources funded by the Ministry of Science and ICT of Korea and by a project (IP2019-003), entitled “Survey of Geomorphology, Vegetation, and Climate in the Hallasan Natural Protection Area” funded by Jeju Special Self-Governing Province (World Heritage Office).









