Introduction
Attention deficit hyperactivity disorder (ADHD) is a severe mental disorder affecting 1–4% of the adult population (Faraone & Biederman, Reference Faraone and Biederman2005; Kessler et al. Reference Kessler, Adler, Barkley, Biederman, Conners, Demler, Faraone, Greenhill, Howes, Secnik, Spencer, Ustun, Walters and Zaslavsky2006), with core symptoms of weak impulse control, hyperactivity and attentional deficits. However, deficient emotion processing is being increasingly regarded as another core problem. Emotional deficits in adult ADHD have been addressed with various approaches, comprising physiological (Conzelmann et al. Reference Conzelmann, Mucha, Jacob, Weyers, Romanos, Gerdes, Baehne, Boreatti-Hümmer, Heine, Alpers, Warnke, Fallgatter, Lesch and Pauli2009; Herrmann et al. Reference Herrmann, Schreppel, Biehl, Jacob, Heine, Boreatti-Hümmer, Mühlberger and Fallgatter2009), neuronal (Plichta et al. Reference Plichta, Vasic, Wolf, Lesch, Brummer, Jacob, Fallgatter and Grön2009), behavioural (Miller et al. Reference Miller, Hanford, Fassbender, Duke and Schweitzer2011) and psychometrical studies (Friedman et al. Reference Friedman, Rapport, Lumley, Tzelepis, VanVoorhis, Stettner and Kakaati2003; Reimherr et al. Reference Reimherr, Marchant, Strong, Hedges, Adler, Spencer, West and Soni2005), and also in diagnostic (Wender et al. Reference Wender, Reimherr and Wood1981; Ward et al. Reference Ward, Wender and Reimherr1993; Retz-Junginger et al. Reference Retz-Junginger, Retz, Blocher, Weijers, Trott, Wender and Rossler2002, Reference Retz-Junginger, Retz, Blocher, Stieglitz, Georg, Supprian, Wender and Rosler2003) and therapeutic manuals (Hesslinger et al. 2002a; Philipsen et al. Reference Philipsen, Richter, Peters, Alm, Sobanski, Colla, Münzebrock, Scheel, Jacob, Perlov, Tebartz van Elst and Hesslinger2007; review in Albert et al. Reference Albert, López-Martín, Fernández-Jaén and Carretié2008). Emotional deficits described in ADHD include poor regulation of affect leading to high affective instability (Reimherr et al. Reference Reimherr, Marchant, Strong, Hedges, Adler, Spencer, West and Soni2005; Herrmann et al. Reference Herrmann, Biehl, Jacob and Deckert2010), weak emotion recognition (Rapport et al. Reference Rapport, Friedman, Tzelepis, Van Voorhis and Friedman2002; Friedman et al. Reference Friedman, Rapport, Lumley, Tzelepis, VanVoorhis, Stettner and Kakaati2003; Miller et al. Reference Miller, Hanford, Fassbender, Duke and Schweitzer2011), including poor recognition of fear cues in facial expressions (Miller et al. Reference Miller, Hanford, Fassbender, Duke and Schweitzer2011), and higher anxiety ratings in some studies (Kitchens et al. Reference Kitchens, Rosèn and Braaten1999). Psychophysiological measurements, however, specifically found lower responses to positive but not to neutral or negative stimuli (Conzelmann et al. Reference Conzelmann, Mucha, Jacob, Weyers, Romanos, Gerdes, Baehne, Boreatti-Hümmer, Heine, Alpers, Warnke, Fallgatter, Lesch and Pauli2009; Herrmann et al. Reference Herrmann, Schreppel, Biehl, Jacob, Heine, Boreatti-Hümmer, Mühlberger and Fallgatter2009, Reference Herrmann, Biehl, Jacob and Deckert2010). Both deficient recognition of fear cues and poor regulation of affect might be factors contributing to risk-taking behaviours in ADHD (Matthies et al. Reference Matthies, Philipsen and Svaldi2012), such as substance abuse (Biederman et al. Reference Biederman, Wilens, Mick, Milberger, Spencer and Faraone1995; Wilson & Levin, Reference Wilson and Levin2005; Matthies et al. Reference Matthies, Philipsen and Svaldi2012), risky sexual practices (Flory et al. Reference Flory, Molina, Pelham, Gnagy and Smith2006) and driving (Barkley & Cox, Reference Barkley and Cox2007).
Imaging studies found abnormalities with respect to structure and function in anterior cingulate (Dickstein, 2006; Bush, Reference Bush2011) and ventral prefrontal areas (Hesslinger et al. 2002; Dickstein, 2006), the striatum (Seidman et al. Reference Seidman, Valera and Makris2005; Scheres et al. Reference Scheres, Milham, Knutson and Castellanos2007; Almeida Montes et al. Reference Almeida, Ricardo-Garcell, Barajas De La, Prado, Martínez, Fernández-Bouzas and Avila2010), amygdala and hippocampus (Plessen et al. Reference Plessen, Bansal, Zhu, Whiteman, Amat, Quackenbush, Martin, Durkin, Blair, Royal, Hugdahl and Peterson2006). Konrad et al. (Reference Konrad, Neufang, Hanisch, Fink and Herpertz-Dahlmann2006) found a methylphenidate effect on insula activation in ADHD children. All these regions are involved in emotion processing (Kober et al. Reference Kober, Barrett, Joseph, Bliss-Moreau, Lindquist and Wager2008); however, imaging studies investigating actual emotion processing in ADHD are sparse. Posner et al. (Reference Posner, Nagel, Maia, Mechling, Oh, Wang and Peterson2011) investigated performance in a task involving the subliminal presentation of fearful faces and found higher activity in the right amygdala to fearful faces and greater connectivity between the amygdala and the lateral prefrontal cortex (LPFC), regardless of anxiety ratings. Brotman et al. (Reference Brotman, Rich, Guyer, Lunsford, Horsey, Reising, Thomas, Fromm, Towbin, Pine and Leibenluft2010) compared brain activity towards facial stimuli in patients with ADHD versus other psychiatric disorders and healthy controls, and reported higher amygdala responses while rating fear in neutral faces.
Fear conditioning is of particular interest because other patient groups with impulse control and emotion regulation deficiencies (i.e. which have symptom overlap with ADHD) show abnormalities in fear conditioning compared to healthy controls. Patients with antisocial personality disorder (ASPD), who show high-risk behaviour and emotional blunting, do not show signs of fear conditioning regarding brain activation and psychophysiological patterns in a fear-conditioning paradigm (Birbaumer et al. Reference Birbaumer, Veit, Lotze, Erb, Hermann, Grodd and Flor2005). By contrast, patients with borderline personality disorder (BPD), who display impulsive and high-risk behaviours combined with emotional dysregulation (Lieb et al. Reference Lieb, Zanarini, Schmahl, Linehan and Bohus2004), failed to decrease fear-related amygdala signals [and increase orbitofrontal cortex (OFC) activation] over time in an instructed fear paradigm (Kamphausen et al. Reference Kamphausen, Schröder, Maier, Bader, Feige, Kaller, Glauche, Ohlendorf, van Elst, Klöppel, Jacob, Silbersweig, Lieb and Tüscher2012). Because affect-related deficits in ADHD comprise high emotional reactivity, ‘blunted’ responses when perceiving emotions and high-risk behaviour, the nature of fear processing is of particular interest in this disorder.
Intact fear recognition and regulation are vital for adapting to (changes in) the emotional significance of a stimulus and for making the right decisions when it comes to handling risky situations (Loewenstein et al. Reference Loewenstein, Weber, Hsee and Welch2001; Dahl, Reference Dahl2003). Clinical observations and psychological findings of risk-taking behaviour (Matthies et al. Reference Matthies, Philipsen and Svaldi2012) and excessive emotional reactivity or affective lability (Reimherr et al. Reference Reimherr, Marchant, Strong, Hedges, Adler, Spencer, West and Soni2005) in ADHD can possibly be understood in the context of impaired fear processing.
In the present study we investigated two different fear-learning paradigms: uninstructed fear (UF) and instructed fear (IF). In UF (classical Pavlovian) conditioning, a neutral conditioned stimulus (CS) is associated with an aversive stimulus (unconditioned stimulus, UCS) by paired presentation. This leads to a conditioned response (CR) to the CS (Pavlov, Reference Pavlov1927). By contrast, in IF, CS and UCS are only linked verbally (i.e. subjects are told that CS and UCS will occur together). The CR then occurs even if subjects never in fact experience this co-occurrence. Thus IF uses the aversive–predictive quality of warning, where fear information is passed from one individual to another (Olsson & Phelps, Reference Olsson and Phelps2007). This form of fear transmission prevails in social contexts and can produce fear learning with similar neuronal and behavioural reactions like UF (Olsson & Phelps, Reference Olsson and Phelps2007). In IF, anterior cingulate cortex (ACC) deficits reported in ADHD might diminish or even impede fear learning because social transmission of fear (Olsson & Phelps, Reference Olsson and Phelps2007) requires ACC functioning to allow conscious fear appraisal (Mechias et al. Reference Mechias, Etkin and Kalisch2010).
Neuronal networks related to fear conditioning and instructed fear include the ACC, insular cortex, basal ganglia, dorsolateral PFC (dlPFC), the amygdala, and the dorsomedial (dmPFC) and ventromedial prefrontal cortex (vmPFC) (Etkin & Wager, Reference Etkin and Wager2007; Delgado et al. Reference Delgado, Li, Schiller and Phelps2008a , Reference Delgado, Nearing, Ledoux and Phelps b ; Sehlmeyer et al. Reference Sehlmeyer, Schöning, Zwitserlood, Pfleiderer, Kircher, Arolt and Konrad2009; Mechias et al. Reference Mechias, Etkin and Kalisch2010; Kamphausen et al. Reference Kamphausen, Schröder, Maier, Bader, Feige, Kaller, Glauche, Ohlendorf, van Elst, Klöppel, Jacob, Silbersweig, Lieb and Tüscher2012). The ACC, amygdala, dlPFC and insula have been shown to be implicated in ADHD, in both structural and functional studies (Bush et al. Reference Bush, Frazier, Rauch, Seidman, Whalen, Jenike, Rosen and Biederman1999; Ernst et al. Reference Ernst, Kimes, London, Matochik, Eldreth, Tata, Contoreggi, Leff and Bolla2003; Konrad et al. Reference Konrad, Neufang, Hanisch, Fink and Herpertz-Dahlmann2006; Tian et al. Reference Tian, Jiang, Wang, Zang, He, Liang, Sui, Cao, Hu, Peng and Zhuo2006; Brotman et al. Reference Brotman, Rich, Guyer, Lunsford, Horsey, Reising, Thomas, Fromm, Towbin, Pine and Leibenluft2010). The amygdala serves as a major fear input and output channel and is the site where, during UF, CS and UCS information converge to form fear memories (Olsson & Phelps, Reference Olsson and Phelps2007). The ACC is involved in conscious appraisal (together with the dorsal PFC) and motivational aspects of emotions (Mechias et al. Reference Mechias, Etkin and Kalisch2010). The insula processes affective and autonomic fear information (Sehlmeyer et al. Reference Sehlmeyer, Schöning, Zwitserlood, Pfleiderer, Kircher, Arolt and Konrad2009; Holtz et al. Reference Holtz, Pané-Farré, Wendt, Lotze and Hamm2012) whereas the ventral PFCs deal with emotion regulation and valence coding (Phillips et al. Reference Phillips, Drevets, Rauch and Lane2003). Thus these areas are of particular interest in this study.
We therefore hypothesized that ADHD patients would show abnormal fear reactions and neural activation patterns in the above regions of interest (ROIs) in IF and UF. Considering affect-related deficits in ADHD we stated three hypotheses. First, because of the symptom overlap with BPD (Kamphausen et al. Reference Kamphausen, Schröder, Maier, Bader, Feige, Kaller, Glauche, Ohlendorf, van Elst, Klöppel, Jacob, Silbersweig, Lieb and Tüscher2012), we predicted a lack of down-regulation of the amygdala signal in both IF and UF as a result of an excessive emotional reactivity. Second, considering ‘blunted’ responses in emotion perception and symptom overlap with ASPD patients along with risk-taking behaviour and poor emotion perception, we hypothesized a diminished activation of brain areas involved in appraising harm-predicting stimuli (ACC, dorsal PFC) whereas fear-related physiological responses should be unaffected as seen in ADHD in children (Pliszka et al. Reference Pliszka, Hatch, Borcherding and Rogeness1993). Third, conscious fear appraisal occurs in both UF and IF, but only in IF is it a prerequisite for fear learning. Because the ACC and the dorsal PFC are essential for IF learning and have been shown to be affected in ADHD, we postulated a more pronounced deficit in IF physiological and functional measures compared to UF.
Method
Subject recruitment
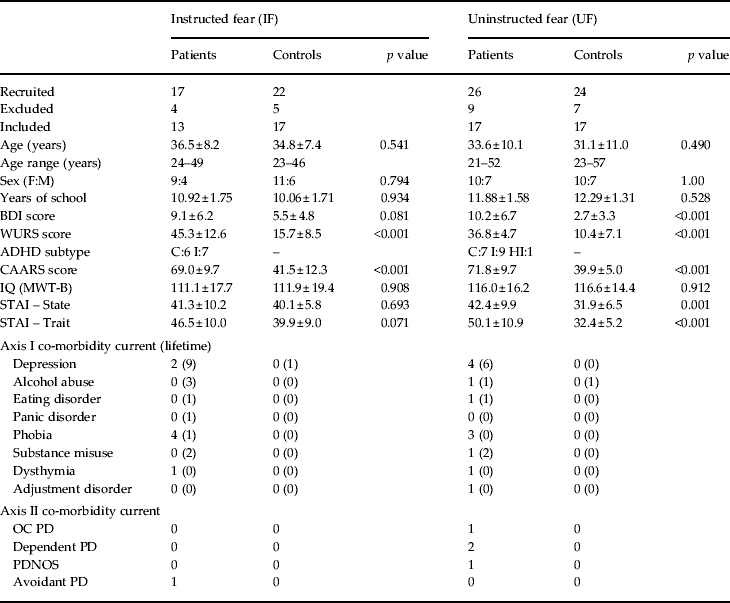
For both studies, IF and UF, conditioning patients were recruited from an ADHD multicentre therapy study. All patients were diagnosed with ADHD by an experienced psychiatrist and exhibited a score >30 on the Wender Utah Rating Scale (WURS; Retz-Junginger et al. Reference Retz-Junginger, Retz, Blocher, Weijers, Trott, Wender and Rossler2002, Reference Retz-Junginger, Retz, Blocher, Stieglitz, Georg, Supprian, Wender and Rosler2003) and a score >65 on the Conner's Adult ADHD Rating Scale (CAARS; Christiansen et al. Reference Christiansen, Hirsch, Philipsen, Oades, Matthies, Hebebrand, Ueckermann, Abdel-Hamid, Kraemer, Wiltfang, Graf, Colla, Sobanski, Alm, Rösler, Jacob, Jans, Huss, Schimmelmann and Kis2012). In patients and healthy control subjects, co-morbid disorders were assessed using the Structured Clinical Interview for DSM-IV Axis I and II Disorders (SCID-I and SCID-II; for an overview of the diagnostic results see Table 1; First et al. Reference First, Spitzer, Gibbon and Williams1996a ,Reference First, Spitzer, Gibbon and Williams b ; German versions: Fydrich et al. Reference Fydrich, Renneberg, Schmitz and Wittchen1997; Wittchen et al. Reference Wittchen, Wunderlich, Gruschwitz and Zaudig1997). Depressive symptoms were assessed with the Beck Depression Inventory (BDI; Beck & Steer, 1987; German version: Hautzinger et al. Reference Hautzinger, Bailer, Worall and Keller1994). Intelligence was measured by the Mehrfachwahl Wortschatz Test (MWT-B; Lehrl et al. Reference Lehrl, Triebig and Fischer1995) and anxiety level with the State–Trait Anxiety Inventory (STAI; Spielberger et al. Reference Spielberger, Sydeman, Owen, Marsh and Maruish1999). State and trait anxiety ratings of one ADHD patient in each study (IF and UF) respectively were missing. Healthy controls were assessed by trained staff and were excluded when fulfilling ADHD criteria in terms of a supra-threshold WURS and CAARS or when meeting any Axis I or II disorder or exceeding 12 points on the BDI. All participants gave informed consent prior to participation. The local ethics committee approved this study.
Table 1. Demographic and psychometric data
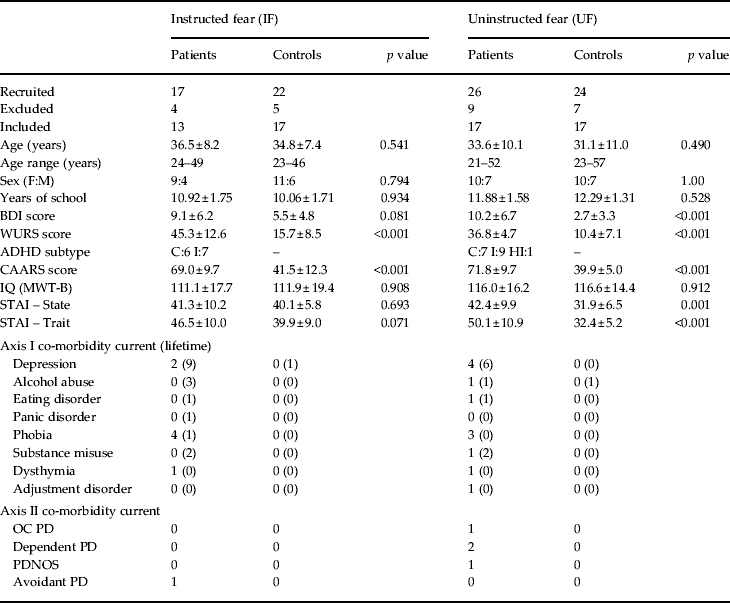
BDI, Beck Depression Inventory; CAARS, Conner's Adult attention deficit hyperactivity disorder (ADHD) Rating Scale; MWT-B, Mehrfachwahl Wortschatz Intelligenz-Test; OC, obsessive compulsive; PD, personality disorder; PDNOS, personality disorder not otherwise specified; STAI, State-Trait Anxiety Scale; WURS-K, Wender Utah Rating Scale.
ADHD subtypes: I = Inattentive, HI = Hyperactive Impulsive, C = Combined Type.
Subjects were matched for age, sex, IQ and education.
Values given as n (%) or mean ± standard deviation.
IF
Seventeen ADHD patients and 22 healthy control subjects were initially scanned. Four patients and five controls were excluded because of excessive head movement, magnetic resonance (MR) or other technical artefacts. Groups were matched for age (t 28 = 0.62, p = 0.541), sex (z = −0.256, p = 0.798), years of school education (t 28 = −0.12, p = 0.902) and intelligence (t 28 = −0.12, p = 0.908) (Table 1). Assignment of the two neutral visual stimuli as either CS+ or CS− was counterbalanced between groups (CS+ in patients: Stim1: 54%, Stim2: 46%; in controls: Stim1: 53%, Stim2: 47%; p = 0.961). Seven ADHD patients were of the inattentive type (ADHD-I) and six patients of the combined type (ADHD-C). ADHD patients and healthy controls differed significantly in the WURS (t 28 = 7.67, p < 0.001) and CAARS (t 28 = 9.65, p < 0.001) scores (Table 1). Group differences did not reach significance level for BDI (t 28 = 1.81, p = 0.081), state (t 28 = 0.40, p = 0.693; data missing for one ADHD patient) and trait anxiety (t 28 = 1.88, p = 0.071) ratings (Table 1).
UF
Twenty-four healthy controls and 26 subjects diagnosed with ADHD were initially scanned. Nine patients and seven controls were excluded because of excessive head movement, MR or other technical artefacts. Groups were matched for age (t 32 = 0.70, p = 0.490), sex (z = 0.00, p = 1.000), years of school education (t 32 = −8.3, p = 0.414) and intelligence (t 32 = −1.11, p = 0.912) (Table 1). Stimuli were counterbalanced between groups (CS+ in patients: Stim1: 53%, Stim2: 47%; in controls: Stim1: 59%, Stim2: 41%; p = 0.730). One patient was of the impulsive hyperactive type (ADHD-HI), nine patients were ADHD-I and seven were ADHD-C. ADHD patients and healthy controls differed significantly in BDI (t 32 = 4.21, p < 0.001), WURS (t 32 = 12.77, p < 0.001), CAARS (t 32 = 12.00, p < 0.001), state anxiety (t 32 = 3.66, p = 0.001) and trait anxiety (t 31 = 6.01, p < 0.001; data missing for one ADHD patient) scores (Table 1).
Experimental procedure
UCS
In both studies, unpleasant electrodermal stimulation was used as the UCS. Unpleasant stimuli were applied through Ag-AgCl electrodes attached to the right wrist using a Digitimer DS7A stimulator (Digitimer, UK). To standardize perceived UCS aversiveness across subjects, prior to scanning, the current of electrodermal stimulation to be received was determined using a standardized dial-up procedure in which stimuli were increased gradually to a level of intensity experienced as ‘uncomfortable but not painful’ (Butler et al. Reference Butler, Pan, Tuescher, Engelien, Goldstein, Epstein, Weisholtz, Root, Protopopescu, Cunningham-Bussel, Chang, Xie, Chen, Phelps, Ledoux, Stern and Silbersweig2007).
IF
This IF study strictly reproduced the experimental procedure of Butler et al. (Reference Butler, Pan, Tuescher, Engelien, Goldstein, Epstein, Weisholtz, Root, Protopopescu, Cunningham-Bussel, Chang, Xie, Chen, Phelps, Ledoux, Stern and Silbersweig2007), who adapted a paradigm initially published by Phelps et al. (Reference Phelps, O'Connor, Gatenby, Gore, Grillon and Davis2001). After the dial-up procedure and before entering the scanner room, subjects viewed the two neutral stimuli (yellow and blue squares) for the purpose of habituation and were then instructed that one of the two stimuli (CS+) might be accompanied by a UCS during the experimental procedure. For the CS+ (threat) condition, subjects were instructed that the UCS ‘stimulation might occur anytime the corresponding coloured square was presented’. For the CS− (safe) condition, participants were informed that ‘no shock would occur at any time the corresponding colour was presented’. In the scanner, before the first run started, subjects were asked to recall during the presentation of which colour they might experience an unpleasant stimulus. Subjects still wore the same stimulation electrodes as during the ‘dial-up’ procedure and were now connected to mock stimulation cables. The scanning experiment consisted of two test runs (IF-Test1 and IF-Test2) of about 5 min each, between which scanning was stopped. Both runs began with a rest period of 20 s, after which each CS was presented five times in pseudo-random order. A CS lasted 12 s and was followed by an 18-s inter-trial interval (ITI) during which a fixation cross was presented. No UCS was given at any time. Subjects were debriefed after the scanning with special regard to their expectancy of a UCS.
UF
Inside the scanner subjects viewed for the purpose of habituation the two neutral stimuli (two Rorschach pictures; Blechert et al. Reference Blechert, Michael, Grossman, Lajtman and Wilhelm2007), which later became the conditioned stimuli CS+ and CS− for the purpose of habituation. Subjects were instructed that the stimuli would be presented in a random order and that electrodermal stimulation might occur. Subjects were left unaware about stimulus contingencies, or time point or frequency of UCS delivery. Stimuli were presented in a pseudo-randomized order. The experimental procedure consisted of two acquisition runs [UF conditioning (UF-Cond)1 and UF-Cond2], each with 12 CS− trials never being paired with the UCS, and 12 CS+ trials, six of which were reinforced with a UCS (50% partial reinforcement). After acquisition, subjects experienced one paired CS+ (‘refresher’ CS) preceding extinction of 12 CS− and 12 unreinforced CS+ trials (UF-Test run). CS were presented for 5 s followed by an ITI of varying duration from 13.5 to 16.5 s in which subjects saw a fixation cross. UCS delivery occurred at the end of the paired CS+ trials. After habituation and before conditioning (baseline) and after each run, subjects rated their UCS expectancy and their perceived CS+ and CS− valences on an 11-point visual analogue scale (expectancy: from ‘absolutely sure no shock will occur’ to ‘absolutely sure a shock will occur’; valence: from pleasant to unpleasant).
Skin conductance
The skin conductance response (SCR) signal was recorded with a BrainAmpsExG MR system (BrainProducts, Germany) at a sampling rate of 5 kHz through Ag-AgCl electrodes attached to the distal phalanges of the second and third digits of the left hand. SCRs were further analysed using in-house software (Avg_q; Feige et al. Reference Feige, Scheffler, Esposito, Di Salle, Hennig and Seifritz2005). Data were filtered for (mainly scanner-induced) high-frequency artefacts with a 0.5-Hz low-pass filter. SCR quantification involved the following steps. First, the SCR waveform was baseline corrected by subtracting the average skin conductance 2 s before the onset of the stimulus. Second, an SCR detection algorithm was applied, classifying an SCR as successful when the waveform reached its half maximum in a time window from 1.5 to 2.5 s after stimulus onset. Third, the amplitude of the SCR was registered as the mean of the corrected SCR waveform during a 2-s time window centred on the local maximum within a 3–8-s window after stimulus onset. To extract the remaining amplitude information available in the signal in blocks where no SCR peak could be detected, we used the mean latency of unequivocally detected peaks to compute the 2-s time window amplitude.
Functional imaging
Functional images were acquired in a Siemens 3-T tim-TRIO magnetom (Erlangen, Germany) equipped with an eight-channel head coil. Blood oxygen level-dependent (BOLD)-sensitive functional images were recorded with a T2*-weighted echo–planar imaging (EPI) sequence [IF: repetition time (TR) = 2 s, echo time (TE) = 30 ms, flip angle = 90°, field of view (FOV) = 192 mm, voxel size = 3 × 3 × 3 mm, water suppression; UF: TR = 2.5 s, TE = 30 ms, flip angle = 90°, FOV = 192 mm, voxel size = 3 × 3 × 3 mm, fat suppression]. In the IF study every run comprised 177 EPI volumes, in the UF 197. Directly after image acquisition, all EPI volumes run through a rigid body transformation to correct for head motion and through a distortion correction algorithm to enhance the signal of orbitofrontal and middle temporal areas, which are distorted due to adjacent air enclosures (Zaitsev et al. Reference Zaitsev, Dold, Sakas, Hennig and Speck2006). After the functional runs a T1-weighted anatomical reference scan was recorded (TR = 2200 ms, TE = 4.11 ms, flip angle = 12°, FOV = 256 mm, voxel size 1 × 1 × 1 mm).
Data preprocessing and statistical analyses were performed using SPM8 (Welcome Trust Centre of Imaging Neuroscience, UK; for details, see www.fil.ion.ucl.ac.uk/spm/software/spm8) running on Matlab R2009b for Linux (The Mathworks Inc., USA). After discarding the first five volumes of every run, the anatomical scan was manually rigid-body transformed to match the first functional volume of the first run. Then, all functional images were realigned to the first remaining functional volume of the first run to correct for head motion. The anatomical scan was co-registered to the first remaining functional volume of the first run. Functional images were spatially normalized (linear and non-linear transformations) into the Montreal Neurological Institute (MNI) reference system (Collins et al. Reference Collins, Zijdenbos, Kollokian, Sled, Kabani, Holmes and Evans1998). A subsequent spatial smoothing step with a three-dimensional isotropic Gaussian kernel (8 × 8 × 8 mm full-width at half-maximum, FWHM) was applied to increase the signal-to-noise ratio and to compensate for inter-individual differences in location of corresponding functional areas. All data were high-pass filtered (128 s) to remove low-frequency noise.
On the single-subject level, separate multiple regression models (general linear model, GLM) for the two studies were fitted voxel-wise to the BOLD signal time courses. In the IF study, the model contained an (unpaired) CS+ and a CS− regressor that were both constructed from 12-s ‘box cars’ at each stimulus onset, plus two constants for each run and one global constant. In the UF study, the model contained one paired CS+, one unpaired CS+ and one CS− regressor for each of the acquisition runs, and one refresher CS+, one unpaired CS+ and one CS− regressor for the test run, all constructed from 5-s box cars. In addition, there were three constants for each of the runs and one global constant. CS regressors were convolved with a canonical haemodynamic response function. The resulting parameter estimate (β) images for the (unpaired) CS+ and CS− regressors were entered in a voxel-wise group-level random effects analysis separately for each study using SPM's ‘full factorial’ model. Specifically, group × condition interactions were modelled in three separate ‘full factorial’ analyses (one for the IF-Test, one for UF-Cond and one for the UF-Test) with the factors group (ADHD and healthy controls) and stimulus (unpaired CS+ and CS−). For comparison with other studies (Kamphausen et al. Reference Kamphausen, Schröder, Maier, Bader, Feige, Kaller, Glauche, Ohlendorf, van Elst, Klöppel, Jacob, Silbersweig, Lieb and Tüscher2012), an additional analysis was calculated for the IF study. At the single-subject level, the model comprised 10 CS+ and 10 CS− box car regressors (one for each trial), plus two constants for each run and one global constant. The group-level analysis of the trial-by-trial CS parameter estimates used a ‘full factorial’ model with factors Group (ADHD and control group), Stimulus (unpaired CS+, CS−) and Time (trial). According to Kamphausen et al. (Reference Kamphausen, Schröder, Maier, Bader, Feige, Kaller, Glauche, Ohlendorf, van Elst, Klöppel, Jacob, Silbersweig, Lieb and Tüscher2012), a planned parametric contrast was used to test for a linear increase in activation over CS− trials and a linear decrease in activation over CS+ trials, contrasting both groups.
In all analyses correction for multiple comparisons was limited to predefined ROIs (small volume correction, SVC) and followed Gaussian random field theory [family-wise error (FWE) rate method at p < 0.05]. Based on our a priori hypotheses for regions shown to be crucial for IF and UF, we defined ROIs in the dorsal anterior cingulate cortex (dACC)/dmPFC, insular cortex, basal ganglia, vmPFC, dlPFC and amygdala (Phelps et al. Reference Phelps, O'Connor, Gatenby, Gore, Grillon and Davis2001; Butler et al. Reference Butler, Pan, Tuescher, Engelien, Goldstein, Epstein, Weisholtz, Root, Protopopescu, Cunningham-Bussel, Chang, Xie, Chen, Phelps, Ledoux, Stern and Silbersweig2007), using the Automated Anatomical Labelling (AAL) set (Tzourio-Mazoyer et al. Reference Tzourio-Mazoyer, Landeau, Papathanassiou, Crivello, Etard, Delcroix, Mazoyer and Joliot2002). For loci outside the a priori areas, the statistical significance threshold for exploratory, descriptive analyses (except regression analyses) was p < 0.001 uncorrected exceeding 10 voxels. All analyses were corrected for effects of depressiveness in terms of BDI scores. Bar graphs of activity were generated by the rfx plot as described by Gläscher (Reference Gläscher2009).
Results
Behavioural data
IF
At debriefing after IF, all subjects indicated that they had expected to receive electrodermal stimulation during the presentation of the threat (CS+) stimulus, until some point in time when expectancy was starting to decrease.
UF
In the UF experiment, UCS expectancy and CS valence were rated formally. Repeated-measures analysis of variance (rm-ANOVA) of expectancy ratings before and after the UF runs showed a clear CS+/CS− discrimination [main effect of Stimulus (CS+, CS−): F 1,31 = 62.62, p < 0.001]. Ratings changed over time [main effect of Time (before UF-Cond1, after UF-Cond1, UF-Cond2 and UF-Test): F 3,93 = 7.90, p < 0.001]. The time effect was influenced by stimulus type (Stimulus × Time interaction: F 3,93 = 22.20, p < 0.001). No group effects were evident (all p > 0.192).
Rm-ANOVA of valence ratings before and after the UF runs showed a CS+/CS− discrimination [main effect of Stimulus (CS+, CS−): F 1,31 = 3.50, p = 0.071] only with ongoing time [main effect of Time (before UF-Cond1, after UF-Cond1, UF-Cond2 and UF-Test): F 3,93 = 1.86, p = 0.141; Stimulus × Time interaction: F 3,93 = 5.42, p = 0.002]. There were no group effects (all p > 0.267).
SCR
IF
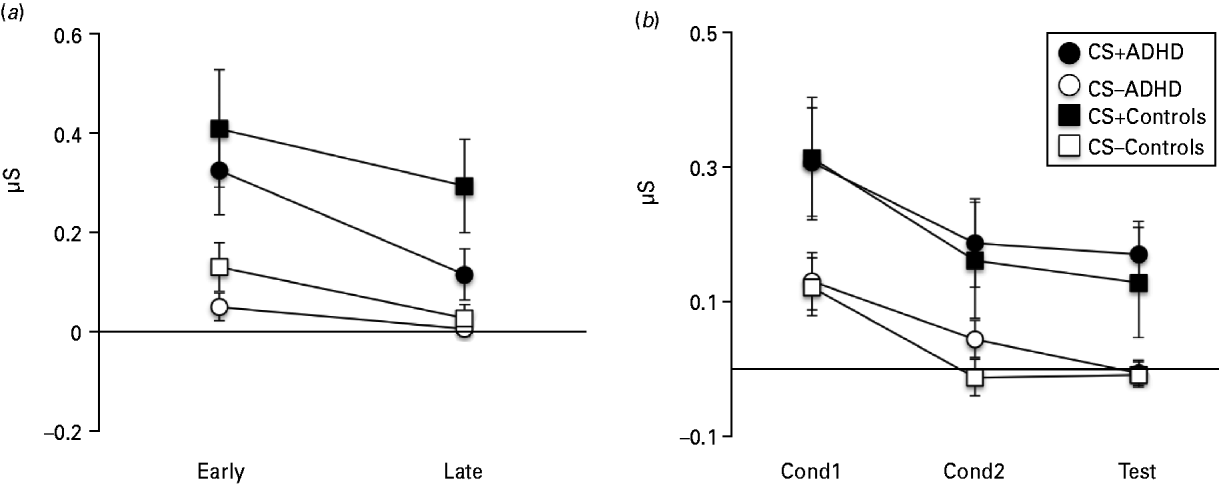
Rm-ANOVA of SCR data showed a clear CS+/CS− discrimination; that is, threat response [main effect of Stimulus (CS+, CS−): F 1,28 = 24.65, p < 0.001]. Responses declined over time [main effect of Time (IF-Test1, IF-Test2): F 1,28 = 15.08, p = 0.001] in a manner that was affected by stimulus identity (Stimulus × Time interaction: F 1,28 = 5.05, p = 0.033). Figure 1 a suggests that this was caused by a steeper decline in CS+ than CS− responses, in line with moderate extinction of CRs probably due to absence of reinforcement by the UCS. Groups did not differ in stimulus (F 1,28 = 0.73, p = 0.402) or time (F 1,28 = 0.09, p = 0.764) effects but there was a trend for extinction to be quicker in patients (Group × Stimulus × Time interaction: F 1,28 = 3.77, p = 0.062).
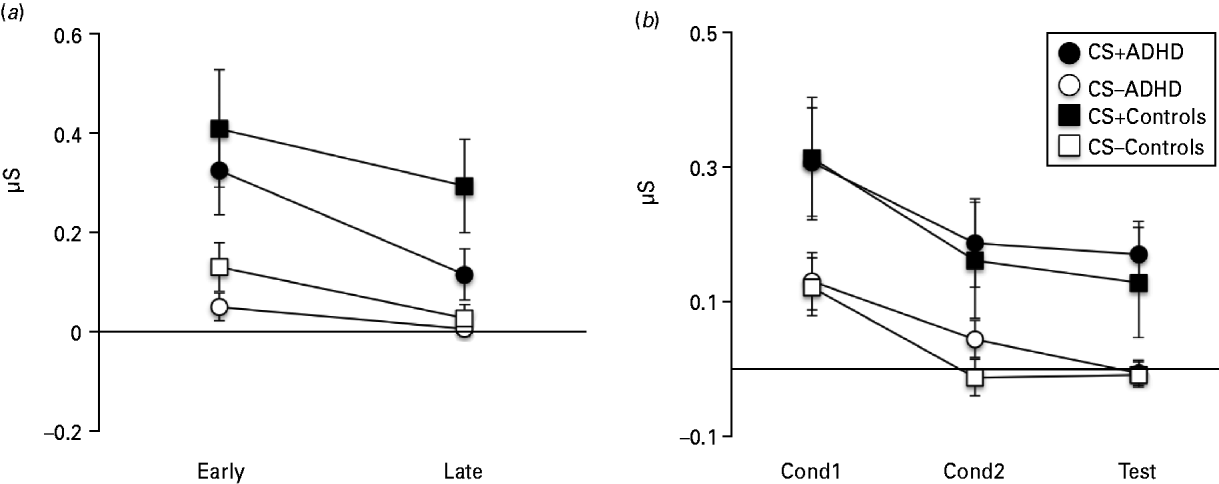
Fig. 1. Skin conductance response (SCR) during the instructed fear test (IF-Test), UF conditioning (UF-Cond) and the uninstructed fear test (UF-Test). (a) SCRs show stable threat responding across the two test runs (IF-Test1, IF-Test2) and no significant difference between the attention deficit hyperactivity disorder (ADHD) group and Controls. (b) SCRs during UF-Cond1, UF-Cond2 and UF-Test were restricted to unpaired conditioned stimulus (CS+) trials and show no significant difference between ADHD and Controls. Error bars show the standard error of the mean (s.e.m.).
UF
Analysis of UF SCR data was restricted to unpaired CSs only. Rm-ANOVA also showed clear CS+/CS− discrimination [main effect of Stimulus (CS+, CS−): F 1,32 = 20.15, p < 0.001]. Responses were modulated by time [main effect of Time (UF-Cond1, UF-Cond2, UF-Test): F 2,64 = 16.45, p < 0.001], an effect that was not affected by stimulus type (Stimulus × Time interaction: F 2,64 = 0.29, p = 0.752) (Fig. 1 b). There were no group effects (Stimulus × Group interaction: F 1,32 = 0.00, p = 0.979; Time × Group: F 2,64 = 0.27, p = 0.768; Stimulus × Time × Group: F 2,64 = 0.41, p = 0.665).
Neuroimaging results
We focused our analysis on networks implicated in fear processing, namely the dACC/dmPFC, insular cortex, basal ganglia, vmPFC, dlPFC and amygdala. All data reported here were FWE corrected for the afore-mentioned a priori ROIs defined by AAL masks at a threshold of p < 0.05.
IF
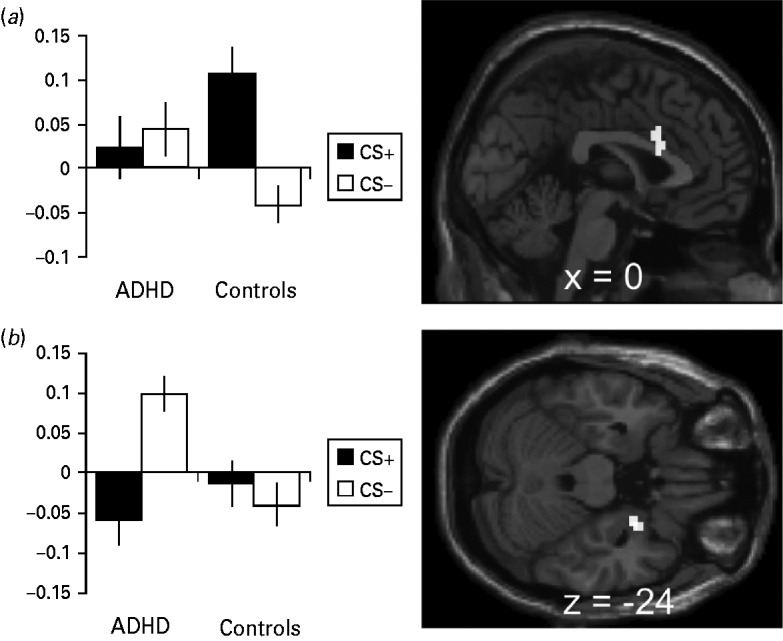
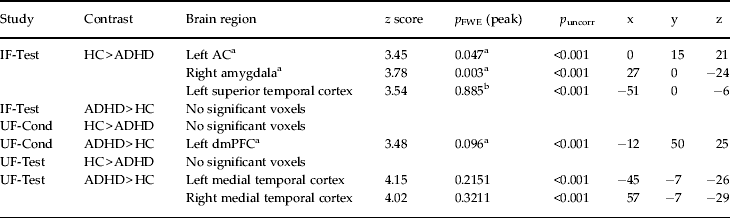
Direct comparison of CS+ v. CS− activation differences between healthy controls and ADHD patients showed significant effects in the ACC and the right amygdala (Table 2; Fig. 2 a, b). Parameter estimates of the identified peak voxels (ACC = 0, 15, 21; amygdala = 27, 0, −24; Fig. 2 a, b) show that, in the ACC, this group difference results from lower responses towards the CS+ in the patient group whereas, in the amygdala, ADHD patients show abnormally enhanced CS− responses (Fig. 2 a, b). These effects persisted despite correction for influences of co-morbidities assessed with SCID-I and SCID-II (ACC = 0, 15, 21: z = 3.45, p FWE = 0.046; amygdala = 27, 0, −24: z = 3.76, p FWE = 0.004) and correction for state and trait anxiety effects assessed with the STAI (ACC = 0, 15, 27: z = 3.52, p FWE = 0.038; amygdala = 27, 0, −24: z = 3.95, p FWE = 0.002). When correcting for STAI influences, an additional a priori area showed significantly elevated activation for CS+ compared to CS− (left insula = −39, 9, −9: z = 3.73, p FWE = 0.025).
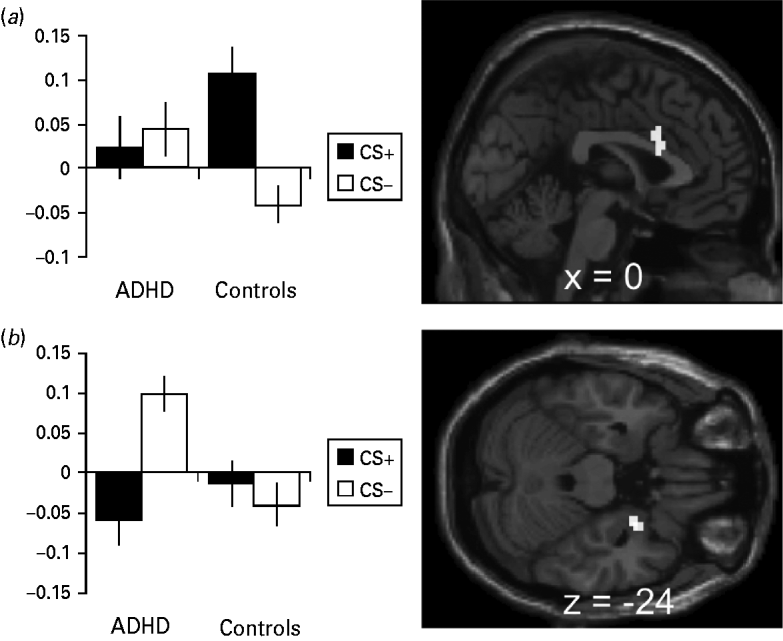
Fig. 2. Activation and β values during the instructed fear test (IF-Test). (a) Anterior cingulate activation during the IF-Test contrasting conditioned stimuli (CS+ > CS−) in healthy controls > attention deficit hyperactivity disorder (ADHD) patients with the β values at the peak voxel (0, 15, 21). (b) Amygdala activation during the IF-Test contrasting CS+ > CS− in healthy controls > ADHD patients with the β values at the peak voxel (27, 0, −24). Error bars show the standard error of the mean (s.e.m.). Activation plotted at p < 0.001, k = 10.
Table 2. Differential activation to CS+ v. CS− in healthy controls minus ADHD patients and ADHD patients minus healthy controls
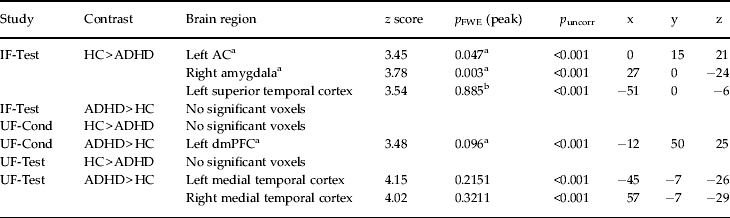
CS+, Excitatory conditioned stimulus; CS−, inhibitory conditioned stimulus; ADHD, attention deficit hyperactivity disorder; IF-Test, test phase of instructed fear; UF-Cond, conditioning phase of uninstructed fear; UF-Test, test phase of uninstructed fear; HC, healthy controls; ACC, anterior cingulate cortex; dmPFC, dorsomedial prefrontal cortex; FWE, family-wise error.
a A priori regions with p FWE values after small volume correction (SVC) at peak level.
b Non-a-priori regions with whole-brain p FWE values at peak level.
Regression models with CAARS subscores resulted in FEW-corrected significant negative correlation of ratings for inattentiveness with the right amygdala (27, 0, −21: z = 3.82, p FWE = 0.003; Fig. 3 a) and ratings for impulsivity with the ACC (Fig. 3 b), bilateral insula and putamen (ACC = 0, 15, 27: z = 3.85, p FWE = 0.014; right insula = 45, 9, −6: z = 3.57, p FWE = 0.045; left insula = −36, 9, 6: z = 3.8, p FWE = 0.023; putamen = 24, 18, −6: z = 3.66, p FWE = 0.019), whereas positive correlations resulted in no significant voxels. Regression models testing CAARS ratings for hyperactivity showed neither a positive nor a negative interaction surviving correction for multiple comparisons.
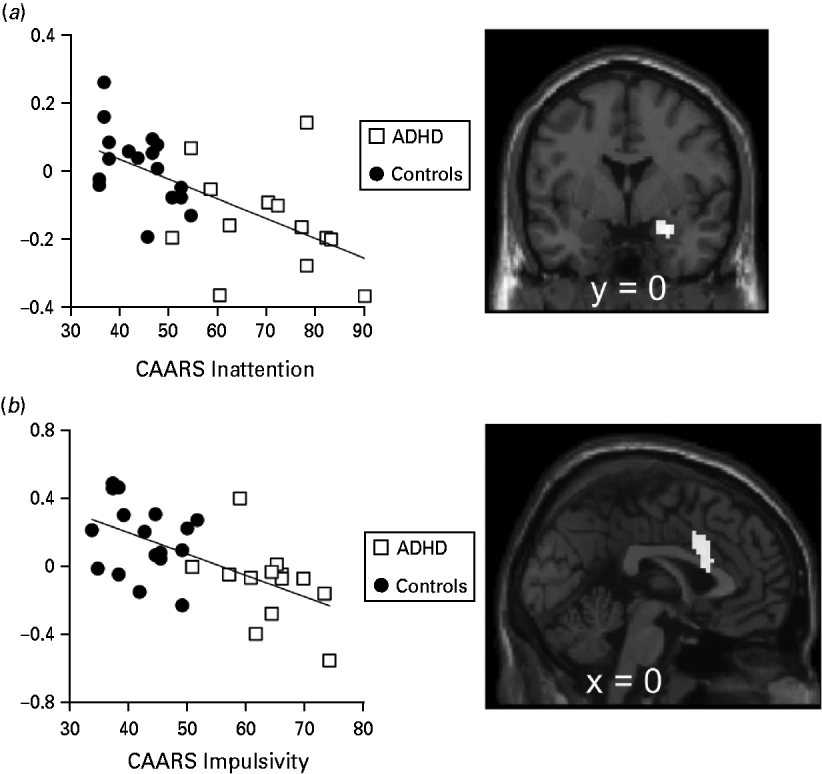
Fig. 3. Regression of Conner's Adult attention deficit hyperactivity disorder (ADHD) Rating Scale (CAARS) scores and β values [excitatory conditioned stimulus (CS+) versus inhibitory CS (CS−)] during the instructed fear test (IF-Test). (a) Amygdala β values at the peak voxel (27, 0, −21) plotted against CAARS inattention scores and t map of the according regression analysis. (b) Anterior cingulate β values at the peak voxel (0, 15, 27) plotted against CAARS impulsivity scores and t map of the according regression analysis. Activation plotted at p < 0.001, k = 10.
A second analysis testing for increases over trials in CS− responses and decreases in CS+ responses that differed between healthy controls and ADHD patients (according to Kamphausen et al. Reference Kamphausen, Schröder, Maier, Bader, Feige, Kaller, Glauche, Ohlendorf, van Elst, Klöppel, Jacob, Silbersweig, Lieb and Tüscher2012) yielded no significant effects.
UF
Comparison of CS+ v. CS− activation differences between healthy controls and ADHD patients yielded no significant effects in either UF-Cond or UF-Test (Table 2), regardless of co-morbidity influences assessed by SCID-I and SCID-II and influences of state and trait anxiety assessed with the STAI.
Discussion
In this study we investigated the neural and psychophysiological response of ADHD patients to fear predictive cues compared to healthy controls. This is the first functional magnetic resonance imaging (fMRI) study to investigate neural responses to threat and safe cues in adult ADHD, and the first functional study using IF and UF to address emotional deficits in this patient group.
For both experiments and both groups, neurophysiological data in terms of SCR resulted in an initially higher response towards threat-predicting stimuli compared to the control stimuli. There were no significant differences in SCR between groups for both studies; this is in line with our second hypothesis and an earlier UF study by Pliszka et al. (Reference Pliszka, Hatch, Borcherding and Rogeness1993) reporting similar SCRs towards CS+ in ADHD and controls. In contrast to our third hypothesis, subject acquired a comparable fear-related SCR in IF. Furthermore, we found no significant functional differences for the contrasts of interest (CS+ > CS−) in the uninstructed fear paradigm. In IF, unlike healthy controls, ADHD patients failed to differentially recruit the caudal part of the dACC in response to the threat-predicting stimulus (CS+) compared to the control stimulus (CS−). The amygdala showed an inverse activation pattern in ADHD patients, reacting more strongly towards CS− than towards CS+ stimuli, whereas control subjects showed similar activation towards both conditions. The IF ACC findings are in line with the second and third hypotheses of dysfunctional high-level processing of fear in ADHD, whereas in UF, patients and controls were comparable.
Our findings regarding the dACC are in line with prior research. The dACC is the most consistently reported region to be dysfunctional in ADHD (Bush, Reference Bush2011), with a crucial physiological role in attention, cognition and emotion processing (Bush et al. Reference Bush, Luu and Posner2000; Dolan, Reference Dolan2002; Milad et al. Reference Milad, Quirk, Pitman, Orr, Fischl and Rauch2007; Pessoa, Reference Pessoa2008; Vogt, Reference Vogt2009). Volumetric differences in the ACC have been reported in children, adolescents (Semrud-Clikeman et al. Reference Semrud-Clikeman, Pliśzka, Lancaster and Liotti2006; Shaw et al. Reference Shaw, Lerch, Greenstein, Sharp, Clasen, Evans, Giedd, Castellanos and Rapoport2006) and adults with ADHD (Seidman et al. Reference Seidman, Valera, Makris, Monuteaux, Boriel, Kelkar, Kennedy, Caviness, Bush, Aleardi, Faraone and Biederman2006, Reference Seidman, Biederman, Liang, Valera, Monuteaux, Brown, Kaiser, Spencer, Faraone and Makris2011; Makris et al. Reference Makris, Biederman, Valera, Bush, Kaiser, Kennedy, Caviness, Faraone and Seidman2007). ADHD patients further showed deficits in ACC activation during attention and executive function tasks (Bush et al. Reference Bush, Frazier, Rauch, Seidman, Whalen, Jenike, Rosen and Biederman1999; Rubia et al. Reference Rubia, Overmeyer, Taylor, Brammer, Williams, Simmons and Bullmore1999; Ernst et al. Reference Ernst, Kimes, London, Matochik, Eldreth, Tata, Contoreggi, Leff and Bolla2003; Konrad et al. Reference Konrad, Neufang, Hanisch, Fink and Herpertz-Dahlmann2006; Pliszka et al. Reference Pliszka, Glahn, Semrud-Clikeman, Franklin, Perez, Xiong and Liotti2006).
In healthy subjects, both IF and UF tasks recruit the dACC. However, the caudal and rostral parts of the dACC and dmPFC seem to be functionally different. The rostral parts are thought to be implicated in the conscious appraisal of fear whereas the caudal areas are associated with sympathetic and (because of its vicinity to the pre-supplementary motor area, pre-SMA) also motor-related fear expression (Meyer et al. Reference Meyer, McElhaney, Winston, McGraw, Laitinen and Livingston1973; Critchley et al. Reference Critchley, Mathias, Josephs, O'Doherty, Zanini, Dewar, Cipolotti, Shallice and Dolan2003; Gentil et al. Reference Gentil, Eskandar, Marci, Evans and Dougherty2009; Mechias et al. Reference Mechias, Etkin and Kalisch2010; Raczka et al. Reference Raczka, Gartmann, Mechias, Reif, Büchel, Deckert and Kalisch2010). We found a trend for quicker extinction of SCRs in patients that might be related to the weaker activation in this group of the caudal dACC. Together, this might indicate that the sympathetic path is affected in ADHD patients. Because motor fear expression in the narrower sense has not been assessed, we cannot draw definite conclusions. Given that behavioural experimental research shows increased risk-taking behaviour reactions in ADHD patients, our ACC results may indicate an impaired fear expression network in ADHD; for example, patients with ADHD may be capable of perceiving fear but are impaired to show an adequate reaction. This is not necessarily in contrast to earlier studies (e.g. Corbett & Glidden, Reference Corbett and Glidden2000) reporting impaired fear recognition for facial fear cues.
The finding of increased amygdala response to CS− stimuli in the ADHD group resembles earlier findings with the same paradigm in patients with panic disorder (Tuescher et al. Reference Tuescher, Protopopescu, Pan, Cloitre, Butler, Goldstein, Root, Engelien, Furman, Silverman, Yang, Gorman, LeDoux, Silbersweig and Stern2011). Furthermore, Plessen et al. (Reference Plessen, Bansal, Zhu, Whiteman, Amat, Quackenbush, Martin, Durkin, Blair, Royal, Hugdahl and Peterson2006) reported altered amygdala shape in ADHD. Volumetric differences have not been shown (Plessen et al. Reference Plessen, Bansal, Zhu, Whiteman, Amat, Quackenbush, Martin, Durkin, Blair, Royal, Hugdahl and Peterson2006; Perlov et al. Reference Perlov, Philipsen, Tebartz van Elst, Ebert, Henning, Maier, Bubl and Hesslinger2008). Of note, Brotman et al. (Reference Brotman, Rich, Guyer, Lunsford, Horsey, Reising, Thomas, Fromm, Towbin, Pine and Leibenluft2010) found increased left amygdala responses in childhood ADHD as compared to healthy controls, contrasting fear and emotionally neutral ratings of neutral facial stimuli. Although these results are not directly comparable to our findings because the rating of fear in neutral facial images differs qualitatively from eliciting an instructed fear response by coloured squares, both studies show an increased amygdala response when subjects perceive an emotionally neutral stimulus (neutral faces and CS− respectively). In addition, amygdala hyperactivation to neutral stimuli is only eminent when conscious emotional appraisal is required because consciously rating the emotional content of neutral faces requires conscious appraisal and instructed fear requires conscious appraisal to link CS+ and UCS as well. On the contrary, Posner et al. (Reference Posner, Nagel, Maia, Mechling, Oh, Wang and Peterson2011) showed greater amygdalar activity towards subliminal presentation of fearful faces in adolescent ADHD patients. As the amygdala is known to be involved in processing fear input and output, our amygdala findings can be interpreted in different ways: (1) in line with the discussion of the ACC results presented earlier, they may hint at impaired expression of fear (output); (2) alternatively, they may be a sign of disturbed saliency detection (input), resulting in deficient appraisal of stimuli as threatening or harmless; (3) a further possible explanation is an increased amygdala response in ADHD as a signal of delay aversion during CS− when no exciting US delivery is to be expected (Plichta et al. Reference Plichta, Vasic, Wolf, Lesch, Brummer, Jacob, Fallgatter and Grön2009; Sonuga-Barke et al. Reference Sonuga-Barke, Wiersema, van der Meere and Roeyers2010). In ADHD patients the amygdala might code the aversion towards a no-task/no-risk condition, such as the CS− stimulus, as more aversive because the imminent threat of a US delivery during CS+.
ADHD patients showed higher self-rated anxiety trait and state measures in the UF study. However, subjective anxiety measures had no effect on the results reported in this study. After controlling for effects of state and trait anxiety and co-morbid disorders, the UF study still exhibited no group effect and the ACC and amygdala findings in the IF study remained comparable. Furthermore, regression analyses of fear activation with inattention and impulsivity scores showed a positive correlation in these areas. These findings support the interpretation of the reported group effects in IF being a result of ADHD traits, rather than of differences in anxiety level or associated co-morbid disorders.
Compared to other often co-morbid disorders with deficits in emotional processing and regulation, our findings in ADHD did not parallel findings of similar studies in ASPD or BPD, thus indicating at least some disease specificity of emotional dysregulation in ADHD. When comparing ASPD (Birbaumer et al. Reference Birbaumer, Veit, Lotze, Erb, Hermann, Grodd and Flor2005) with our ADHD results: (1) in UF, ASPD but not ADHD show altered SCR (i.e. ASPD do not show conditioned SCR), valence ratings and activation in limbic-prefrontal areas; (2) in ASPD (UF), amygdala abnormalities are related to lower activation towards CS+, whereas in ADHD (IF), activation is higher towards CS−; and (3) in ASPD (UF), lower activation in the dACC/dmPFC is bound to the rostral dACC/dmPFC (fear appraisal), but in ADHD (IF) the posterior dACC/dmPFC (fear expression) is dysfunctional.
Regarding BPD, the main finding of a lack of fear habituation reflected by a weaker decrease in SCR and prolonged amygdala activation (Kamphausen et al. Reference Kamphausen, Schröder, Maier, Bader, Feige, Kaller, Glauche, Ohlendorf, van Elst, Klöppel, Jacob, Silbersweig, Lieb and Tüscher2012) to fear cues is clearly different to our ADHD data (compare first hypothesis).
If our findings indeed indicate problems in sustaining fear expression or appraisal in ADHD with regard to instructed fear, they may have therapeutic implications. It may be important for ADHD patients to understand that they have problems adapting behaviourally to threat-predicting stimuli, when threat is only indicated verbally. Although our data do not allow a direct statistical comparison of UF and IF, this study might suggest that ADHD patients have less problems learning adequate fear when they are indirectly exposed to the fear-eliciting stimulus or event (UF). Deficient processing of verbally transmitted fear would affect patients mainly in a social context (Olsson & Phelps Reference Olsson and Phelps2007), such as responding adequately to a verbal warning, and thus might account for impulsive risk-taking behaviour in ADHD. Transferred to psychotherapy, ADHD patients might particularly benefit from therapeutic approaches where emotion-eliciting events or situations can be experienced directly.
This study has several limitations. Because of the relatively small sample size (although in the normal range for functional imaging studies), our findings warrant replication by further investigations; larger group sizes might also allow conclusions regarding ADHD subtypes. The absence of findings in UF does not necessarily imply that ADHD patients do not have any problems in UF learning, especially in fear extinction because this was not formally tested. Both studies are comparable in terms of age, gender, IQ and current ADHD symptomatology according to CAARS. Differing results in IF and UF might be partly due to varying effect sizes in the two studies and different stimulus material, timing and length. Further studies should investigate amygdala activation in ADHD in more detail and further disentangle the differential effects of threat and safe cues in particular. As ACC findings have been reported in a variety of ADHD studies, a closer look at the subregions and their different functions might be desirable.
Summary
Although basic fear-learning mechanisms in terms of UF seem to be unaffected in ADHD, the neural response to IF is altered in brain regions centrally involved in emotion regulation. Further investigation is needed to determine whether ADHD patients show difficulties specifically when responding to emotional cues requiring conscious appraisal and how these difficulties apply to behavioural and therapeutical outcomes.
Acknowledgements
We thank two anonymous reviewers for their valuable comments on an earlier version of this manuscript. This work was funded by the German Federal Ministry of Education and Research (BMBF 01GV0606 to A.P. and L.T.v.E), Freiburg University Medical School (a fellowship grant to O.T.) and the Deutsche Forschungsgemeinschaft (DFG Emmy Noether grant KA 1623/3-1 to R.K).
Declaration of Interest
None.