I. INTRODUCTION
Li-ion batteries (LIBs) have higher-energy density, portative design, and longer lifetime than comparable battery technologies (Tarascon and Armand, Reference Tarascon and Armand2001). Spinel Li4Ti5O12, a so-called zero-strain insertion material, has been commercialized as an anode material because of its exceptionally high rate performance, excellent cycling stability, and Li-insertion electrochemistry with formal potential of 1.55–1.56 V versus Li+/Li (Ohzuku et al., Reference Ohzuku, Ueda and Yamamoto1995; Cho et al., Reference Cho, Kim, Kim and Park2001; Ronci et al., Reference Ronci, Reale, Scrosati, Panero, Rossi Albertini, Perfetti, di Michiel and Merino2002). Generally speaking, Li-(de)intercalation in Li4+zTi5O12 (z = 0–3) proceeds through a two-phase reaction, as given in Eq. (1), resulting in flat plateaus in the charge and discharge curves.
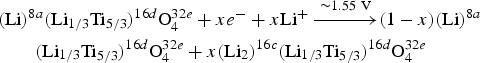 $$\eqalign{& \lpar {\rm Li}\rpar ^{8a} \lpar {\rm Li}_{1/3} {\rm Ti}_{5/3} \rpar ^{16d} {\rm O}_4^{32e} + xe^ - + x{\rm Li}^ + \mathop{-\!\!\!-\!\!\!-\!\!\!-\!\!\!\longrightarrow}\limits^{{ \sim 1.55\, \, {\rm V}}} \lpar 1 - x\rpar \lpar {\rm Li}\rpar ^{8a} \cr &\qquad\lpar {\rm Li}_{1/3} {\rm Ti}_{5/3} \rpar ^{16d} {\rm O}_4^{32e} + x\lpar {\rm Li}_2 \rpar ^{16c} \lpar {\rm Li}_{1/3} {\rm Ti}_{5/3} \rpar ^{16d} {\rm O}_4^{32e}}$$
$$\eqalign{& \lpar {\rm Li}\rpar ^{8a} \lpar {\rm Li}_{1/3} {\rm Ti}_{5/3} \rpar ^{16d} {\rm O}_4^{32e} + xe^ - + x{\rm Li}^ + \mathop{-\!\!\!-\!\!\!-\!\!\!-\!\!\!\longrightarrow}\limits^{{ \sim 1.55\, \, {\rm V}}} \lpar 1 - x\rpar \lpar {\rm Li}\rpar ^{8a} \cr &\qquad\lpar {\rm Li}_{1/3} {\rm Ti}_{5/3} \rpar ^{16d} {\rm O}_4^{32e} + x\lpar {\rm Li}_2 \rpar ^{16c} \lpar {\rm Li}_{1/3} {\rm Ti}_{5/3} \rpar ^{16d} {\rm O}_4^{32e}}$$ (Li)8a(Li1/3Ti5/3)16dO432e and (Li2)16c(Li1/3Ti5/3)16dO432e, where the site multiplicity and Wyckoff letter is shown in superscript, crystallize in the Fd ![]() $\overline 3$m space group. 1/6th of 16d sites are occupied by the “electrochemically inert” Li, with the remainder occupied by Ti (see Figure 1). The “electrochemically active” Li occupies the tetrahedral 8a sites of the Li4Ti5O12 lattice and is re-positioned together with the newly inserted Li at the octahedral 16c sites to form stable Li7Ti5O12 upon Li-intercalation. The Li-(de)intercalation of Li4Ti5O12 is also reported to occur via the following solid-solution reaction [Eq. (2)]:
$\overline 3$m space group. 1/6th of 16d sites are occupied by the “electrochemically inert” Li, with the remainder occupied by Ti (see Figure 1). The “electrochemically active” Li occupies the tetrahedral 8a sites of the Li4Ti5O12 lattice and is re-positioned together with the newly inserted Li at the octahedral 16c sites to form stable Li7Ti5O12 upon Li-intercalation. The Li-(de)intercalation of Li4Ti5O12 is also reported to occur via the following solid-solution reaction [Eq. (2)]:
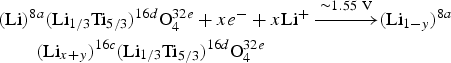 $$\eqalign{& \lpar {\rm Li}\rpar ^{8a} \lpar {\rm Li}_{1/3} {\rm Ti}_{5/3} \rpar ^{16d} {\rm O}_4^{32e}+xe^ - + x{\rm Li}^+ \mathop{-\!\!\!-\!\!\!-\!\!\!-\!\!\!\longrightarrow}\limits^{{ \sim 1.55\, \, {\rm V}}}\lpar {\rm Li}_{1 - y} \rpar ^{8a} \cr & \qquad\lpar {\rm Li}_{x+y} \rpar ^{16c} \lpar {\rm Li}_{1/3} {\rm Ti}_{5/3} \rpar ^{16d} {\rm O}_4^{32e} }$$
$$\eqalign{& \lpar {\rm Li}\rpar ^{8a} \lpar {\rm Li}_{1/3} {\rm Ti}_{5/3} \rpar ^{16d} {\rm O}_4^{32e}+xe^ - + x{\rm Li}^+ \mathop{-\!\!\!-\!\!\!-\!\!\!-\!\!\!\longrightarrow}\limits^{{ \sim 1.55\, \, {\rm V}}}\lpar {\rm Li}_{1 - y} \rpar ^{8a} \cr & \qquad\lpar {\rm Li}_{x+y} \rpar ^{16c} \lpar {\rm Li}_{1/3} {\rm Ti}_{5/3} \rpar ^{16d} {\rm O}_4^{32e} }$$where z, y ≤ 1.

Figure 1. (Color online) (Left) Crystal structure of the as-prepared Li4Ti5O12 as refined using high-resolution NPD data. Li (8a) is shown in green, O in red, and mixed Ti/Li sites in light blue. For clarity, Li and Ti sites are shown as fully occupied. Li at 16c sites (yellow) are also shown in the Li4+zTi5O12 structure (right) following lithiation.
The phase transition between Li4Ti5O12 and Li4+zTi5O12 (Li7Ti5O12 in the two-phase mechanism), as outlined in Eqs. (1)–(2), involves a <0.1% change in the lattice volume. The Li accommodated by the anode results in changes to the Ti oxidation state, affecting the structure of the TiO6 octahedral framework (Figure 1).
Given that the underlying mechanism of the phase transitions upon lithiation remains controversial and, importantly, may control the performance of the Li4Ti5O12 anodes, we have studied the Li4+zTi5O12 structure in detail during battery cycling. Neutron powder-diffraction (NPD) data were collected during the non-equilibrium charge and discharge of a LiFePO4|| Li4Ti5O12 battery within the 1.0–3.0 V window (versus Li4Ti5O12). While the details of the Li diffusion within the Li4+zTi5O12 structure is thought to control the lattice response and is discussed in detail elsewhere in work focusing on particle-size differences (Pang et al., Reference Pang, Peterson, Sharma, Shiu and Wu2014a), here we focus on the structural details and distortion of the TiO6 octahedron. In this work, we extract and discuss the Ti–O bond length and O–Ti–O angle that dictate the size of the TiO6 unit and assist in maintaining the stability of the anode during lithiation and delithiation.
II. EXPERIMENTAL
A. Preparation
LiFePO4 cathode powder was provided by Tatung Fine Chemicals Co., Taiwan. Li4Ti5O12 anode powder was prepared via a sol–gel method using Li acetate (98%, Acros) and titanium butoxide (98.0%, Acros). The stoichiometrically mixed powders were dissolved in an adequate amount of ethanol (99.5%, Shimakyu) and the solution aged for 3 h to form a white-colored gel. The resulting gel was heated at 80 °C to yield an organic precursor with a fine, white powder-product obtained by heat-treating in air at 800 °C for 4 h.
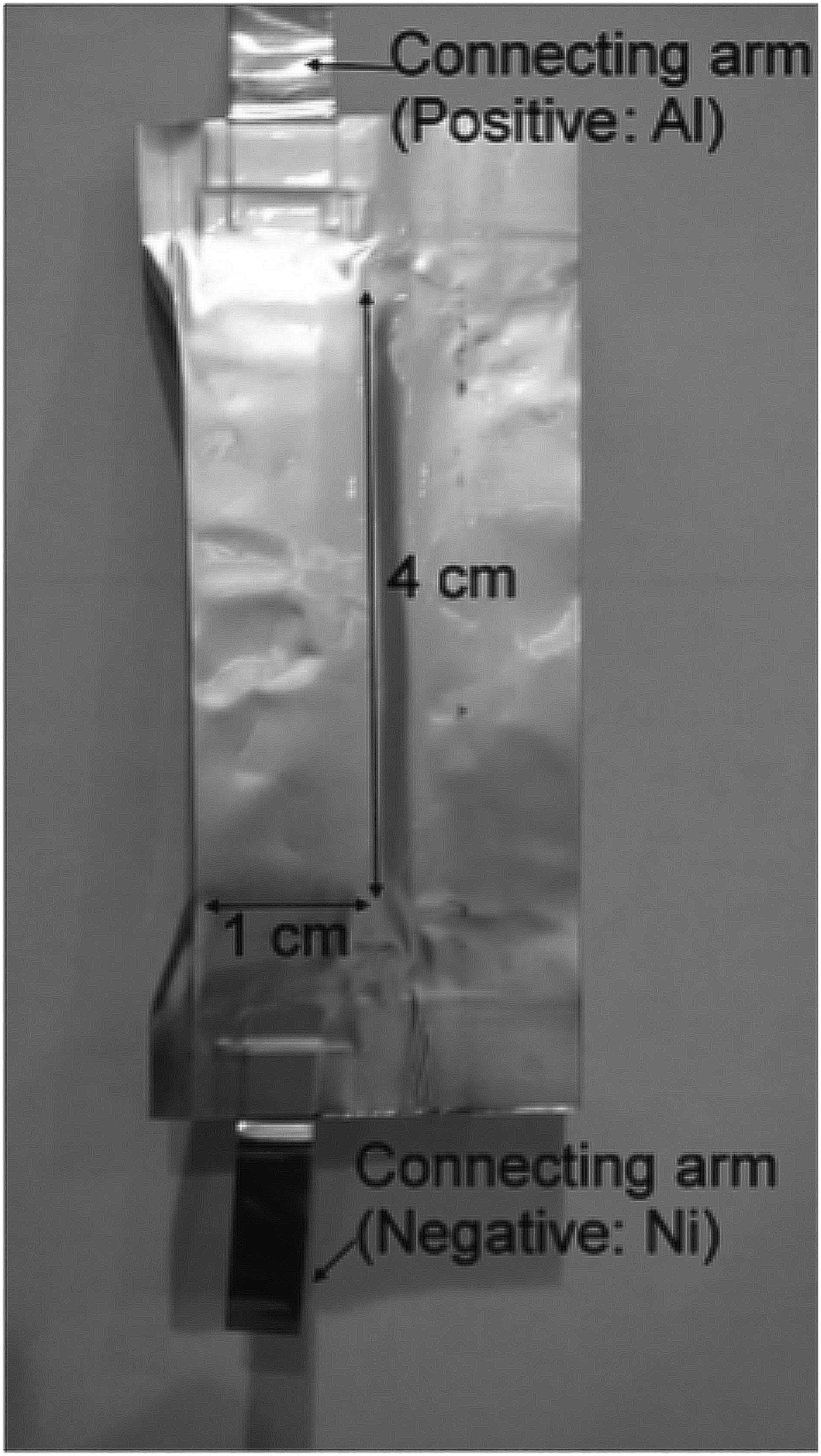
A purpose designed LiFePO4|| Li4Ti5O12 pouch-type battery (Figure 2) was used in the collection of in situ NPD data. The LiFePO4 cathode was prepared by casting a slurry of the active materials (80 wt%), acetylene black (10 wt%), and polyvinylidene difluoride (PVDF) binder (10 wt%) onto an Al foil. The Li4Ti5O12 anode was prepared using the same procedure, but with Li4Ti5O12 powder as the active material. The loading ratio between the anode and cathode was designed to be ~4:6 by weight. The electrodes were cut into 1 × 4 cm strips. Immobilon-P PVDF membrane (Millipore) was used as a separator because of its lower hydrogen content relative to the conventionally used the Celgard membrane, where the strong incoherent neutron-scattering of hydrogen is detrimental to the NPD signal. The LiFePO4||Li4Ti5O12 battery was prepared by stacking 30 anode/separator/cathode assemblies with a parallel connection. The stack was placed in an Ar-filled glove box for 24 h and then wrapped in a polypropylene-coated Al foil to form a pouch. Prior to the in situ NPD experiment, deuterated electrolyte-solution (1 M lithium hexafluorophosphate (99.99%, Sigma-Aldrich) in a 1:1 volume ratio of deuterated dimethyl carbonate (99.5%, Novachem) to deuterated ethylene carbonate (98%, Novachem) was injected into the pouch, which was heat-sealed under Ar. After 1 day of wetting, the battery was used in the in situ NPD experiment. During the in situ NPD experiment the pouch-type battery was cycled galvanostatically using a potentiostat/galvanostat (Autolab PG302N) at currents of 11 mA (theoretically equivalent to 0.1 °C) for 1 cycle between 1.0 and 3.0 V (versus Li4Ti5O12).

Figure 2. Pouch cell used in the in situ NPD experiment.
B. Data collection and analysis
High-resolution NPD data of the as-prepared Li4Ti5O12 sample were collected using ECHIDNA, the high-resolution neutron powder-diffractometer at the Open Pool Australian Light-water (OPAL) research reactor at the Australian Nuclear Science and Technology Organisation (ANSTO; Liss et al., Reference Liss, Hunter, Hagen, Noakes and Kennedy2006). The neutron beam wavelength was 1.6214(4) Å, determined using the La11B6 NIST standard reference material 660b. NPD data were obtained in the 2θ angular range 4–164° with a step size of 0.125°. Rietica ver. 1.77 (Hunter, Reference Hunter1998) was used to perform Rietveld analysis of the high-resolution NPD data. The refinable parameters included the background coefficients, zero-shift, peak shape parameters, lattice parameters, O positional parameter, and isotropic atomic displacement parameters. Micrographs of the as-prepared Li4Ti5O12 were collected using field-emission scanning electron microscopy (SEM) with a SU8000 (Hitachi, Japan). All observations were carried out without a conductive coating under a 10 kV acceleration voltage.
In situ NPD data of the LiFePO4||Li4Ti5O12 battery were collected using WOMBAT (Studer et al., Reference Studer, Hagen and Noakes2006), the high-intensity neutron powder-diffractometer at the OPAL research reactor at ANSTO. WOMBAT features an area detector that continuously covers 120° in 2θ and has a relatively intense neutron beam, allowing the rapid collection of data. A neutron beam wavelength of 2.9592(2) Å was used, determined using the La11B6 NIST standard reference material 660b. The diffractograms were obtained with an exposure time of 5 min in the angular range 16.1–136.9° in 2θ during the charge–discharge cycling of the batteries. Sequential Rietveld refinements were carried out using the NPD data using Fullprof with visualization in WinplotR (Rodríguez-Carvajal, Reference Rodríguez-Carvajal1993; Roisnel and Rodriguez-Carvajal, Reference Roisnel and Rodriguez-Carvajal2000). The refinements were performed using data in the range 60–120° in 2θ.
III. RESULTS AND DISCUSSION
A. Crystallography and microstructure of the Li4Ti5O12 anode
Using high-resolution NPD data, the crystallographic details of the anode was established. As reported by Pang et al. (Reference Pang, Peterson, Sharma, Shiu and Wu2014a), the anode adopts ![]() $Fd\bar{3}m$ space-group symmetry, with a minor amount [1.9(3) wt%] of monoclinic Li2TiO3. The crystallographic details are summarized in Pang et al. (Reference Pang, Peterson, Sharma, Shiu and Wu2014a). The as-prepared anode particles are cube-like with an average particle-size of ~200 nm.
$Fd\bar{3}m$ space-group symmetry, with a minor amount [1.9(3) wt%] of monoclinic Li2TiO3. The crystallographic details are summarized in Pang et al. (Reference Pang, Peterson, Sharma, Shiu and Wu2014a). The as-prepared anode particles are cube-like with an average particle-size of ~200 nm.
B. In situ NPD
In situ NPD data of the battery are shown in Figure 3. In the absence of peak splitting in the NPD pattern for the cathode, we modeled the anode lattice evolution as single-phase Li4+zTi5O12 (solid-solution reaction) after Wagemaker et al. (Reference Wagemaker, Simon, Kelder, Schoonman, Ringpfeil, Haake, Lützenkirchen-Hecht, Frahm and Mulder2006), the details of which are presented in Pang et al. (Reference Pang, Peterson, Sharma, Shiu and Wu2014a). The two-phase reaction between LiFePO4 and FePO4 in cathode is also observed in Figure 3.

Figure 3. (Color online) Waterfall plot (left) of a collection of NPD patterns shown for a restricted 2θ range taken during battery charge and discharge. Labels are phase reflections for LiFePO4 (LFP), FePO4 (FP), Li4+zTi5O12 (LTO), and Al. A typical Rietveld-refinement profile before cycling (right) is also shown. The figures-of-merit for the refinements were in the range χ2 = 3.9–8.95, R wp = 14.3–17.1, and R B = 1.0–3.9%.
To effectively refine the O positional parameter, it was necessary to fix the Li occupancy at 8a and 16c sites in the sequential Rietveld refinement, as per previous work (Pang et al., Reference Pang, Peterson, Sharma, Shiu and Wu2014a, Reference Pang, Sharma, Peterson, Shiu and Wu2014b). Figure 4 summarizes the lattice and crystallographic changes occurring during battery cycling, including the variation of lattice parameter, O positional parameter, O–Ti–O bond angle (α), Ti–O bond length, and the estimated Ti oxidation state.

Figure 4. (Color online) (a) Charge–discharge profile, (b) refined anode lattice-parameter, (c) refined oxygen positional-parameter, (d) α bond-angle, and (e) Ti–O bond length, during battery charge and discharge. The titanium oxidation-state in (f) is estimated using the approximate BVS method.
During charge, the anode lithiates and the lattice undergoes rapid expansion, followed by a gradual contraction. The non-linearity in the lattice response is attributed to the interplay of the amount and site of Li insertion (Sharma et al., Reference Sharma, Yu, Zhu, Wu and Peterson2013; Pang et al., Reference Pang, Peterson, Sharma, Shiu and Wu2014a), with the population of Li at the two crystallographic sites (i.e., 8a and 16c) having a different effect on the lattice. Interestingly, these lattice changes are not reflected in the trend of TiO6 octahedral distortion, with no measureable distortion occurring during the initial lattice-expansion. We find that the gradual lattice-contraction, associated with population of Li at the 16c site in the anode (Pang et al., Reference Pang, Peterson, Sharma, Shiu and Wu2014a), is strongly correlated to the trend of the TiO6 distortion. The repositioning of the O atom in response to lithiation at the 16c site is important in maintaining the stability of the anode, and reflects the trend in the estimated oxidation-state of the Ti. As shown in Figure 4, during battery charge the O atom moves further away from the Ti atom, at (0.5, 0.5, 0.5), resulting in an increase in the length of the Ti–O bond that occurs alongside a decrease in the average Ti valence as estimated by the bond-valence summation (BVS) method (Brown and Altermatt, Reference Brown and Altermatt1985). We note that the BVS method will yield an approximate Ti valence as the Ti shares the 16d site with Li. The ideal Ti4+–O2− bond-length of 1.815 Å and an empirical constant of 0.37 Å (Brown et al., Reference Brown and Altermatt1985) are used in this estimation. The bond angle or α also varies with the oxygen positional-parameter, characterizing the distortion of the TiO6 octahedron (Figure 5). The TiO6 octahedron deformation and distortion result in a stable structure during the lithiation and delithiation processes. Alongside the Li repositioning, it is the oxygen positional-parameter changes during battery charge and discharge that completes the picture of the Li4Ti5O12 structural response and anode function.

Figure 5. (Color online) Overlaid structures of the TiO6 unit in the anode at the charged (O atom in red) and discharged (O atom in green) battery states showing that the O–Ti–O bond-angle (α) is larger at the charged state and smaller at the discharged state. All O atoms are crystallographically equivalent.
IV. CONCLUSION
We have successfully monitored the crystallographic change in the Li4+zTi5O12 anode within in a battery during charge and discharge. We report the details of the change to the TiO6 structural unit occurring during Li diffusion that contributes to the structural stability of this “zero strain” anode. We find that while the initial expansion of the lattice upon lithiation is not reflected in the trend of TiO6 octahedral distortion, the gradual lattice-contraction experienced during further lithiation is strongly correlated to the trend of the TiO6 distortion, and associated with the repopulation of Li at the 16c site in the anode.
ACKNOWLEDGEMENTS
The authors acknowledge the travel support funded by National Synchrotron Radiation Research Center (2013-3-100-1). The research was supported by the Australian Nuclear Science and Technology Organization's (ANSTO) Energy Materials project. The authors are also grateful to Professor Lin, Jeng-Yu of Tatung University for providing Li4Ti5O12 sample, Tatung Fine Chemicals Co., Taiwan for providing LiFePO4 sample, and the staff members at the Bragg Institute, ANSTO for their operations support.