Introduction
Nowadays, global climate change is one of the major problems facing humanity. For crops, this will require the release of new cultivars able to adapt to a changing environment, without reducing quality standards or affecting industrial food production according to the demands of a population highly sensitized to food quality (Godfray et al., Reference Godfray, Beddington, Crute, Haddad, Lawrence, Muir, Pretty, Robinson, Thomas and Toulmin2010). Several different studies have suggested that relatives and wild progenitors of wheat species could be interesting candidates for enlarging the gene pool of cultivated wheats (Hajjar and Hodgkin, Reference Hajjar and Hodgkin2007). At the diploid level, the main species of wild diploid wheat are Triticum monococcum L. ssp. aegilopoides Link em. Thell. (syn. T. boeoticum; 2n = 2x = 14; AmAm) and T. urartu Thum. ex Gandil (2n = 2x = 14; AuAu), the latter species having been identified as the A-genome donor of polyploid wheat (Dvorak et al., Reference Dvorak, Terlizzi, Zhang and Resta1993).
The presence and variability of the endosperm storage proteins are associated with the bread-making quality of wheat. These proteins are divided into two main groups (gliadins and glutenins) according to their molecular characteristics (Payne, Reference Payne1987). Glutenins are also divided into high molecular weight (HMWGs) and low molecular weight (B-LMWGs and C-LMWGs) subunits. The HMWGs, encoded by genes at the Glu-1 loci located on the long arm of group-1 homoeologous chromosomes being the best studied (Payne, Reference Payne1987), have been associated with bread-making quality in common wheat (Cornish et al., Reference Cornish, Békés, Eagles, Payne, Wrigley, Békés and Bushuk2006). Each Glu-1 locus contains two tightly linked genes that encode for two types of HMWGs, called x- and y-type (Harberd et al., Reference Harberd, Bartels and Thompson1986), although some of these genes are not expressed in cultivated wheats. In particular, the Ay subunit is absent in all durum and common wheats, while it is expressed in wild diploid and tetraploid wheats (Waines and Payne, Reference Waines and Payne1987; Ciaffi et al., Reference Ciaffi, Lafiandra, Porceddu and Benedettelli1993), its presence being associated with an increase in bread-making quality in wheat (Ciaffi et al., Reference Ciaffi, Lafiandra, Turchetta, Ravaglia, Bariana, Gupta and MacRitchie1995).
Alvarez et al. (Reference Alvarez, Caballero, Nadal, Ramírez and Martín2009) showed that the introgression of T. urartu genome in durum wheat affects the gluten strength. However, these materials were developed from a single line, whereas Caballero et al. (Reference Caballero, Martín and Alvarez2008) found as many as 17 allelic variants for the Glu-A u1 locus in a large collection of T. urartu. The Ax gene was found to be active in all these alleles, while the Ay subunit was detected in nine of them.
The aim of the present study was the molecular characterization of the allelic variants for the Ay gene detected by Caballero et al. (Reference Caballero, Martín and Alvarez2008) to obtain additional data prior to their potential introgression in wheat.
Materials and methods
Seventeen accessions of T. urartu that have the allelic variants found by Caballero et al. (Reference Caballero, Martín and Alvarez2008) were analysed. DNA isolation was carried out from young leaf tissue using the cetyl trimethyl ammonium bromide method (Stacey and Isaac, Reference Stacey, Isaac and Isaac1994).
The primers reported by D'Ovidio et al. (Reference D'Ovidio, Masci and Porceddu1995) were used to amplify the complete coding sequence of the Ay gene. PCR reactions mixtures were carried out in a final volume of 20 μl composed of 1 × Taq PCR buffer (Promega), 125 ng of template DNA, 0.6 μM of each forward and reverse primer, 1.5 mM MgCl2, 0.2 mM of each deoxyribonucleotide and 1 U of Taq DNA polymerase (Promega). DNA was subjected to an initial denaturation step at 95°C for 5 min, and the amplification conditions were 35 cycles at 95°C for 1 min, 60°C for 1 min and 72°C for 2 min, followed by a final incubation step at 72°C for 8 min.
The amplicons (PCR products) were separated in polyacrylamide gel electrophoresis (PAGE) gel with a discontinuous Tris–HCl buffer system (pH: 6.8/8.8) at a polyacrylamide concentration of 8% (w/v, crosslinker (C): 1.68%). These amplicons were digested using DdeI and PstI endonucleases and separated in PAGE gel with a discontinuous Tris–HCl buffer system (pH: 6.8/8.8) at a polyacrylamide concentration of 10% (w/v, C: 1.68%).
Results and discussion
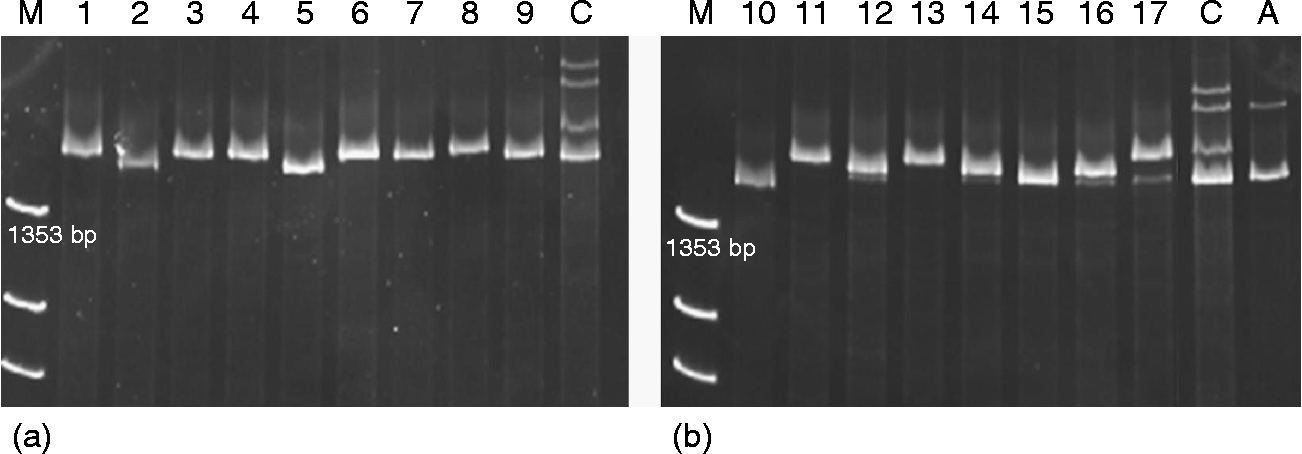
The amplification of the complete coding sequence in the accessions with and without active Ay subunits revealed a single band of around 1500 bp, although with some small differences in size among them, in the accessions with Ay active subunits as well as in others with inactive ones (Fig. 1). This is in agreement with the findings of Caballero et al. (Reference Caballero, Martín and Alvarez2008), who detected four Ay active subunits with differences in their mobility.
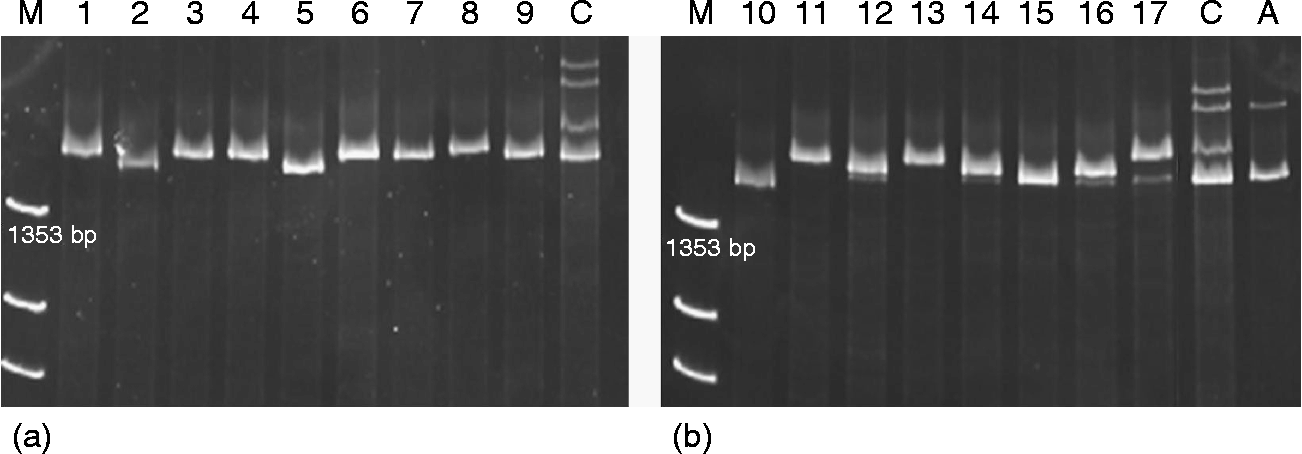
Fig. 1 PAGE separation of PCR products from the Ay genes. (a) active Ay alleles; (b) inactive Ay alleles. M, X174 DNA-Hae III digest; C, cv. Cheyenne; A, cv. Alaga.
Some studies have suggested that digestion with endonucleases could be a useful tool to evaluate the internal differences between these alleles (Lafiandra et al., Reference Lafiandra, Tucci, Pavoni, Turchetta and Margiotta1997; Alvarez et al., Reference Alvarez, D'Ovidio and Lafiandra1998). The amplicon digestion with DdeI (Fig. 2(a) and (b)) showed three restriction patterns between the active Ay alleles, while in the inactive ones, five restriction patterns were identified. In the case of the digestion with PstI (Fig. 2(c) and 2(d)) four patterns were revealed for the active Ay alleles and six for the inactive ones. Although, in general, the restriction patterns of the active alleles were different from those of the inactive alleles, one of the patterns of the active alleles (lanes 2 and 5), together with the pattern of lane 10 (of an inactive allele), showed the same restriction pattern with both digestions (Fig. 2, lanes 2, 5 and 10).

Fig. 2 PAGE separation of PCR products from the Ay genes digested with Dde I and PstI (up and down, respectively). (a and c) digestion patterns of active Ay alleles; (b and d) digestion patterns of inactive Ay alleles. M, X174 DNA-Hae III digest; C, cv. Cheyenne; A, cv. Alaga.
On the other hand, all the restriction patterns found in these T. urartu lines differed from those of the inactive Ay allele present in cv. Cheyenne, while some of them showed similarities with the Ay alleles detected in cv. Alaga (Fig. 2).
In some cases, only the combined use of the restriction patterns from both endonucleases evidenced differences between alleles. The actives alleles shown in lanes 1, 3, 4, 6, 7 and 9 were similar in total size (Fig. 1) and DdeI digestion (Fig. 2); however, the PstI digestion separated these alleles into two groups. The first group (lanes 1, 3, 4 and 7) presented five bands or fragments, whereas the second group (lanes 6 and 9) showed six. The difference was that fragment 2 of group 1 was digested into two fragments for group 2: one fragment comigrated with the band 3 and the other produced a new band of approximately 120 bp that also appeared in the rest of the restriction patterns. The same occurred for DdeI digestion in the inactive alleles, where the alleles showed in lanes 11 and 13 presented similar restriction pattern, but the PstI digestion indicated that the two alleles are different.
Although further research needs to be carried out in the future, such as the sequencing of these Ay alleles, the results of this study demonstrate that the Ay alleles presented in T. urartu, both active and inactive ones, are different from the alleles found in cultivated wheat. For breeding purposes, the variation detected for the active alleles would permit to expand the high-quality gene pool of the cultivated wheats. Consequently, the evaluation and characterization of the genetic resources of this wild species are very important for its conservation and potential use in wheat breeding programmes.
Acknowledgements
This research was supported by grant AGL2007-65685-C02-02 from the Spanish Ministry of Science and Innovation and the European Regional Development Fund (FEDER) from the European Union. C. G. thanks the Spanish Ministry of Education and Science (FPU programme) for a predoctoral fellowship.