Introduction
The Digenea is the most species-rich group of the parasitic worms, however, their diversity is far from being well studied. The main reasons are: (1) lack of faunistic data in many regions; (2) phenotypic variation; (3) the existence of cryptic or just hardly distinguishable species; and (4) the complex life cycles (Cribb, Reference Cribb2016). Happily, an integrative approach incorporating molecular genetics and modern morphological methods applied to different life-cycle stages gives the opportunity to bring the research on the digenean diversity to a new level (Georgieva et al., Reference Georgieva, Selbach, Faltýnková, Soldánová, Sures, Skírnisson and Kostadinova2013; Gilardoni et al., Reference Gilardoni, Etchegoin, Cribb, Pina, Rodrigues, Diez and Cremonte2020; Gonchar and Galaktionov, Reference Gonchar and Galaktionov2021; Huston et al., Reference Huston, Cutmore, Miller, Sasal, Smit and Cribb2021; Faltýnková et al., Reference Faltýnková, Kudlai, Pantoja, Yakovleva and Lebedeva2022). This approach has been barely used for one of the most widespread digenean taxa parasitizing fish – the superfamily Hemiuroidea. Species identification of sexual adults (maritae) in certain hemiuroidean groups is problematic due to ambiguous morphological characteristics, apparently low host specificity and worldwide distribution. These problems are particularly true for the family Derogenidae, subfamily Derogeninae, with Derogenes varicus (Müller, 1784) Looss, 1901 being reported as one of the most widespread marine digenean species (WoRMS, 2022), but suspected to represent a cryptic species complex (Bray et al., Reference Bray, Diaz and Cribb2016). Molecular data on the derogenids are few, and none of them cover the life-cycle stages from the first intermediate hosts.
Hemiuroideans typically possess 3-host life cycles (Hunninen and Cable, Reference Hunninen and Cable1943; Køie, Reference Køie1979, Reference Køie1989, Reference Køie1990a; Køie et al., Reference Køie, Karlsbakk and Nylund2002). Within the first intermediate host they produce a very special type of cercariae – cystophorous ones. The tail of such cercariae is partially transformed into a caudal cyst, and the body of the infective cercaria is withdrawn inside it. The caudal cyst may bear different types of appendages, some of which are used for locomotion (Køie, Reference Køie1979, Reference Køie1990a, Reference Køie1992). The cyst also has a delivery tube – a special structure enabling infection of the next host. The second intermediate hosts of hemiuroideans are small crustaceans, often planktonic copepods. When they bite a caudal cyst, the delivery tube everts and penetrates their foregut, injecting the cercarial body into the haemocoel (Matthews, Reference Matthews1981).
Cystophorous cercariae have been described from marine and freshwater gastropods (Hunninen and Cable, Reference Hunninen and Cable1943; Madhavi, Reference Madhavi1978; Køie, Reference Køie1979, Reference Køie1989; Goater et al., Reference Goater, Browne and Esch1990; Shameem et al., Reference Shameem, Rao and Madhavi1990), and also from bivalves and scaphopods (Wardle, Reference Wardle1975; Køie et al., Reference Køie, Karlsbakk and Nylund2002; Louvard et al., Reference Louvard, Cutmore, Yong, Dang and Cribb2022). Among them are 4 different types of cercariae that have been found in the moonsnails (family Naticidae) from the Northern Atlantic and the adjacent Arctic (Barents and White Seas). The first, Cercaria appendiculata Pelseneer, 1906 has a furcate locomotory appendage, was primarily described from Euspira nitida (Donovan, 1803), and later found in Cryptonatica affinis (Gmelin, 1791) (Chubrik, Reference Chubrik1966; Timofeeva, Reference Timofeeva1976; Køie, Reference Køie1979). Køie (Reference Køie1979) demonstrated experimentally that C. appendiculata is the larva of Derogenes varicus. A similar cercaria was recovered from Euspira pallida (Broderip & G. B. Sowerby I, 1829) by Køie (Reference Køie1990b). At first, she supposed it belonged to Hemiurus levinseni Odhner, Reference Odhner1905, but later reassigned it to the Derogenidae (Køie, Reference Køie1995, Reference Køie2000). Another type of cystophorous cercariae described from the moonsnail C. affinis from the Barents Sea – Cercaria octocauda Tschubrik, 1952 – is characterized by a locomotory appendage with 8 long immotile threads on its end (Chubrik, Reference Chubrik1966; Timofeeva, Reference Timofeeva1976). Finally, Cercaria saccocaudata Tschubrik, 1966 was described from C. affinis at the Barents and White Seas (Chubrik, Reference Chubrik1966; Timofeeva, Reference Timofeeva1976). It lacks a locomotory appendage, and regarding the morphology it was supposed to belong to the family Lecithasteridae (Chubrik, Reference Chubrik1966; Timofeeva, Reference Timofeeva1976), and finally proved to be Lecithaster salmonis (Krupenko et al., Reference Krupenko, Kremnev, Skobkina, Gonchar, Uryadova and Miroliubov2022).
In the present paper we revisit systematics and life cycles of the Derogenidae, subfamily Derogeninae at the White and Barents Seas. Maritae morphologically assigned to D. varicus and Progonus muelleri (Levinsen, Reference Levinsen1881) Looss, 1899 were found to be genetically heterogeneous (in fragments of 28S, 18S, 5.8S rDNA, ITS2 and cox1 gene), thus possibly representing groups of species challenging to identify. Based on previous records of digenean fauna in the White and Barents Seas (Shulman and Shulman-Albova, Reference Shulman and Shulman-Albova1953; Polyansky, Reference Polyansky1955; Chubrik, Reference Chubrik1966), we hypothesized that 3 types of cystophorous cercariae infecting the moonsnails in the White and Barents Seas also belong to the Derogeninae. Cercaria octocauda was proved to be the larva of P. muelleri. Two cercariae with furcate locomotory appendage matched 2 lineages within D. varicus.
Materials and methods
Sampling
Intermediate and definitive hosts were sampled at the White Sea (3 sites: Keret Archipelago, Velikaya Salma Strait and Bolshoy Solovetsky Island) in 2018–2021, and at the Barents Sea (Dalniye Zelentsy) in July–August 2021. Three moonsnail species were checked for the digenean infection: Cryptonatica affinis and Euspira pallida from both the White and Barents Seas, and Amauropsis islandica (Gmelin, 1791) from the White Sea. We searched for maritae of the family Derogenidae in 16 fish species from the White Sea and 4 species from the Barents Sea (Table 1). For other purposes we dissected whelks Buccinum scalariforme Møller, 1842 (N = 31) from the White Sea. A single derogenid marita was found in the stomach of 1 whelk.
Table 1. Fish hosts examined, prevalence (%) and intensity data for derogenid maritae from the White and Barents Seas

Morphological analysis
Rediae and maritae were fixed in 96% ethanol for whole mounts. All specimens of maritae were heat-killed before fixation, except 1 from B. scalariforme. Rediae and maritae were stained with acetocarmine (Sigma Aldrich, Germany) followed by destaining in 0.1 m HCl in 70% ethanol, dehydrated in a graded alcohol series, clarified in xylol, and mounted in Canada balsam. Temporary mounts of cercariae, live and fixed in 2.5% glutaraldehyde in sea water, were used to study their gross morphology. Whole mounts were observed under microscopes Leica DM 500 or DM 2500 (Leica Microsystems, Germany); photos and videos were taken either in bright field, or with phase-contrast microscopy, or with differential interference contrast (DIC) using Nikon DS Fi1, or Sony Alpha 7RII, or smartphone camera. Measurements were made using Fiji software (Schindelin et al., Reference Schindelin, Arganda-Carreras, Frise, Kaynig, Longair, Pietzsch, Preibisch, Rueden, Saalfeld, Schmid, Tinevez, White, Hartenstein, Eliceiri, Tomancak and Cardona2012). Measurements of cercarial body were taken prior to its retraction into the caudal cyst. The delivery tube was measured in live specimens when everted under the cover glass pressure. All measurements are in micrometres.
For scanning electron microscopy (SEM), 2.5% glutaraldehyde-fixed cercariae were rinsed in water, dehydrated in ethanol and acetone, dried in a critical point dryer, coated with platinum, and examined with a Quanta 250 at 15 kV. Visualization of cercarial inner structure was also performed by means of confocal laser scanning microscopy (CLSM). Specimens were fixed in 4% paraformaldehyde in 0.01 m phosphate-buffered saline and stained with tetramethylrhodamine B isothiocyanate (TRITC)-labelled phalloidin and DAPI. The protocol was the same as previously described (Kremnev et al., Reference Kremnev, Gonchar, Krapivin, Knyazeva and Krupenko2020).
Molecular analysis
Ethanol-fixed rediae and maritae were used for molecular studies. To extract DNA, we took 1 redia or fragment of marita (preoral lobe and anterior part of the oral sucker); 32 isolates used for molecular analysis are listed in Table 2. Samples were taken from 96% ethanol and dried completely, incubated in 200 μL of 5% solution of Chelex® 100 resin (Bio-Rad, USA) with 0.2 mg mL−1 of proteinase K (Evrogen, Russia) at 56°C for 4 h, then kept for 8 min at 90°C and centrifuged for 10 min at 16,000 g. The supernatant containing DNA was transferred to a new tube and stored at −20°C.
Table 2. Isolates, their origin and GenBank accession numbers for sequences

We amplified a fragment of the 28S rDNA (domains D1–D3), a fragment containing 5.8S rDNA and ITS2, a fragment of 18S rDNA, and a fragment of the cytochrome oxidase I gene (cox1) with primers and conditions listed in Table 3. The 20 μL reaction mixture contained 4 μL of ScreenMix-HS (Evrogen), 0.5 μL of each primer (10 pmol μL−1), 2 μL of the DNA and 13 μL of PCR grade water (Evrogen). Polymerase chain reactions (PCRs) were run on a Veriti thermal cycler (Applied Biosystems, MA, USA). PCR products were stained with ethidium bromide and visualized through electrophoresis on a 1% agarose gel. Sequencing was performed with PCR primers on an ABI Prism 3500xl genetic analyser (Applied Biosystems, MA, USA). Chromatograms were analysed and edited, and alignments were built in Geneious Prime 2022.0.1 (https://www.geneious.com). Relevant data for alignments were obtained from GenBank (Supplementary Table 1). To annotate 28S and 5.8S + ITS2 regions, we used the sequence of Didymocystis scomberomori (KU341979; Schrandt et al., Reference Schrandt, Andres, Powers and Overstreet2016) as a reference. Pairwise genetic distances between species (as the number of base differences per site) were calculated in MEGA 7 (Kumar et al., Reference Kumar, Stecher and Tamura2016). The maximum likelihood (ML) analysis was conducted at the CIPRES Science Gateway (https://www.phylo.org) using RAxML (Stamatakis, Reference Stamatakis2014) with 1000 bootstrap iterations. The model of nucleotide substitution was estimated as GTR + I for the 28S dataset, TIM2 + G for the 18S dataset, TVM + I for the 5.8S + ITS2 dataset and TIM3 + I + G for the cox1 dataset using JModelTest (Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012) at the CIPRES Science Gateway. A haplotype network for fragments of cox1 was constructed in PopART 1.7 (Leigh and Bryant, Reference Leigh and Bryant2015) by integer neighbour-joining network (reticulation tolerance 0.5).
Table 3. PCR primers and thermocycling conditions; in all reactions initial denaturation was at 95°C for 5 min and final extension was at 72°C for 10 min

Results
At the White Sea, we obtained maritae morphologically identified as Progonus muelleri from Myoxocephalus scorpius, Limanda limanda and Triglops murrayi. The maritae identified as Derogenes varicus were found in 10 fish species from the White Sea and 3 fish species from the Barents Sea (Table 1). A marita found in the stomach of the whelk Buccinum scalariforme was also identified as D. varicus.
Cystophorous cercariae of 4 types were found parasitizing moonsnails. Cercaria saccocaudata = Lecithaster salmonis (family Lecithasteridae) recovered from Cryptonatica affinis is not considered further here. Cercaria appendiculata was found in C. affinis both at the White Sea and Barents Sea. Cercaria octocauda was found in C. affinis from 1 locality at the White Sea (Velikaya Salma Strait). Finally, rediae with cystophorous cercariae of Derogenidae gen. sp. (according to Køie, Reference Køie1995, Reference Køie2000), similar to but much larger than C. appendiculata, were found in Euspira pallida (White and Barents Seas) and Amauropsis islandica (White Sea).
Molecular analysis and linking life-cycle stages
For molecular genetic analysis 32 newly recovered specimens were used: 22 maritae and 10 intramolluscan stages – rediae with cercariae (Table 2). We obtained 1130–1139 bp sequences of the 28S rDNA fragment (domains D1–D3). The following sequences of the representatives of the Derogeninae from GenBank were also used in the analysis: D. varicus AY222189 (Olson et al., Reference Olson, Cribb, Tkach, Bray and Littlewood2003) and MW504598–9 (Sokolov et al., Reference Sokolov, Atopkin and Gordeev2021); D. lacustris LC586089–90 (Tsuchida et al., Reference Tsuchida, Flores, Viozzi, Rauque and Urabe2021); P. muelleri MW507469–71 (Sokolov et al., Reference Sokolov, Atopkin and Gordeev2021). Prosogonotrema bilabiatum (AY222191; Olson et al., Reference Olson, Cribb, Tkach, Bray and Littlewood2003) served as an outgroup. The alignment was 1052 bp long after trimming the ends to match the shortest sequence. The ML tree was built to visualize the results. Our specimens formed 4 distinct groups (Fig. 1, Supplementary Table 2). The first group included all our D. varicus specimens from fish and all rediae of C. appendiculata from Cryptonatica affinis, with no nucleotide substitutions. The second group comprised the marita of D. varicus from the whelk B. scalariforme and derogenid rediae from Euspira pallida and Amauropis islandica; these sequences were identical to one of the D. varicus sequences from GenBank (AY222189) from the North Sea. These 2 groups are later referred to as DV1 and DV2. Divergence between them was 0.0068 ± 0.0053 (17 substitutions). The sequences of D. varicus from the Pacific (MW504598–9) differed from DV1 in 14 and from DV2 in 11 substitutions; they are labelled in Fig. 1 as DV3. The other 2 closely related groups comprised maritae of P. muelleri and rediae of C. octocauda; a single variation was in position 501: 3 maritae and 2 C. octocauda rediae (T) differed from 3 other maritae (C) (distance = 0.0004 ± 0.0009). These 2 groups of specimens are later referred to as PM1 and PM2, respectively. Previously published sequences of P. muelleri from the Pacific and the White Sea (MW507469–71) fell into the PM1 group. Two sequences of D. lacustris formed a separate group with intraspecific pairwise distance 0.0005 ± 0.0008 (Fig. 1, Supplementary Table 2).

Fig. 1. ML phylogenetic tree based on the 28S rDNA sequence data. Bootstrap values are printed in nodes. Scale bar shows the substitution rate. DV1–3 and PM1–2 mark the separate genetic lineages within Derogenes varicus and Progonus muelleri species, respectively. Abbreviations: AI – Amauropsis islandica; AL – Anarhichas lupus; BS – Buccinum scalariforme; CA – Cryptonatica affinis; CS – Caprella septentrionalis; CP – Clupea pallasii marisalbi; EF – Eumicrotremus fedorovi; EN – Eleginus nawaga; EP – Euspira pallida; GM – Gadus morhua; HP – Hippoglossoides platessoides; LL – Limanda limanda; MS – Myoxocephalus scorpius; TM – Triglops murrayi.
We also analysed 525–593 bp fragments including partial 5.8S rDNA (110–150 bp), complete ITS2 and the beginning of 28S (31–74 bp). Accacladocoelium macrocotyle (KF687303; Ahuir-Baraja et al., Reference Ahuir-Baraja, Fraija-Fernández, Raga and Montero2015) was taken as an outgroup. After trimming the ends, the alignment was 531 bp long, including gaps. In the obtained ML tree our specimens grouped exactly the same way as in the 28S rDNA analysis (Fig. 2). Intragroup variation was absent. Divergence between the groups DV1 and DV2 was 0.0374 ± 0.0101 (18 substitutions and 4 indels) (Supplementary Table 3). The groups PM1 and PM2 differed in a single position (260, A/G) (divergence = 0.0021 ± 0.0019).

Fig. 2. ML phylogenetic tree based on the ITS2 rDNA sequence data. Bootstrap values are printed in nodes. Scale bar shows the substitution rate. DV1–2 and PM1–2 mark the separate genetic lineages within Derogenes varicus and Progonus muelleri species, respectively. Abbreviations: AI – Amauropsis islandica; AL – Anarhichas lupus; BS – Buccinum scalariforme; CA – Cryptonatica affinis; CP – Clupea pallasii marisalbi; EN – Eleginus nawaga; EP – Euspira pallida; GM – Gadus morhua; LL – Limanda limanda; MS – Myoxocephalus scorpius; TM – Triglops murrayi.
A fragment of 18S rDNA 775–778 bp long was sequenced for 8 of our specimens, 2 from each of the groups DV1, DV2, PM1 and PM2 (Table 2). The closest BLAST hit was D. varicus (AJ287511; Littlewood and Olson, Reference Littlewood, Olson, Littlewood and Bray2001), which was also included in the analysis. Prosogonotrema bilabiatum (AJ287565; Littlewood and Olson, Reference Littlewood, Olson, Littlewood and Bray2001) served as an outgroup. After trimming the ends, the alignment was 779 bp long, including gaps. Our specimens grouped the same way as in 28S and 5.8S + ITS2 analyses (Fig. 3, Supplementary Table 4). PM1 and PM2 differed from each other in 1 position (599, C/T; distance = 0.0013 ± 0.0012). The difference between DV1 and DV2 was in 11 positions (distance = 0.0143 ± 0.005). Derogenes varicus from GenBank (AJ287511; Cribb et al., Reference Cribb, Bray, Littlewood, Pichelin, Herniou, Littlewood and Bray2001) was closest to DV2, though with 1 substitution and 1 indel (distance = 0.0013 ± 0.0011); this sequence is labelled DV4 on the 18S tree (Fig. 3). We also compared the alignment with another sequence of D. varicus from GenBank – AF029816 (Blair et al., Reference Blair, Bray and Barker1998). It overlapped our alignment by a rather short fragment (213 bp) and was thus not included in the ML analysis. This fragment was identical in AF29816 and our sequences of DV1, and differed from DV2 and DV4 in 9 positions.

Fig. 3. ML phylogenetic tree based on the 18S rDNA sequence data. Bootstrap values are printed in nodes. Scale bar shows the substitution rate. DV1–2, 4 and PM1–2 mark the separate genetic lineages within Derogenes varicus and Progonus muelleri species, respectively. Abbreviations: CA – Cryptonatica affinis; CP – Clupea pallasii marisalbi; EP – Euspira pallida; GM – Gadus morhua; HP – Hippoglossoides platessoides; MS – Myoxocephalus scorpius; TM – Triglops murrayi.
Fragments of cox1 gene were sequenced for 25 specimens (Table 2). After trimming, the alignment was 788 bp long. Didymocystis wedli (AB725624) served as an outgroup. In the obtained ML tree, our specimens grouped the same way as in the rDNA analyses (Fig. 4). Minimal intergroup pairwise distance was between PM1 and PM2 (0.0905 ± 0.041, 61 positions), and maximal intragroup pairwise distance was in DV1 (0.0155 ± 0.0082, 12 positions) (Supplementary Table 5). In DV1 34 polymorphic sites were present; all haplotypes were unique; overall mean distance was 0.0076 ± 0.0014. We built a haplotype network for DV1, because rediae of this species from the White and Barents Seas clearly differed by size (see below), and thus could represent different lineages. However, geographic origin of isolates was not associated with the position of DV1 samples on the haplotype network (Fig. 5). DV2 had 1 polymorphic site with a synonymous substitution. One variable position was also present within PM1.

Fig. 4. ML phylogenetic tree based on partial cox1 gene sequences. Bootstrap values are printed in nodes. Scale bar shows the substitution rate. DV1–2 and PM1–2 mark the separate genetic lineages within Derogenes varicus and Progonus muelleri species, respectively. Branches within the DV1 clade are collapsed. Abbreviations: AI – Amauropsis islandica; AL – Anarhichas lupus; CA – Cryptonatica affinis; CP – Clupea pallasii marisalbi; EN – Eleginus nawaga; EP – Euspira pallida; GM – Gadus morhua; LL – Limanda limanda; MS – Myoxocephalus scorpius.

Fig. 5. Haplotype network for DV1 isolates based on partial cox1 gene sequences. Circle size represents the haplotype frequency. Black dots illustrate missing haplotypes. Number of hatch marks corresponds to the number of substitutions between haplotypes.
Descriptions
All the obtained maritae were identified either as Derogenes varicus or as Progonus muelleri, based on their morphological characteristics. Measurements are given in Table 4. Considering the genetic heterogeneity between all the specimens of D. varicus from fish and the one from Buccinum scalariforme, and between maritae of P. muelleri, the measurements are shown separately – referred to as DV1 and DV2, PM1 and PM2, respectively. Hologenophores for all maritae isolates and 5 paragenophores of DV1 maritae were deposited in the collection of the Department of Invertebrate Zoology of Saint Petersburg University.
Table 4. Measurements of Derogenes varicus (DV1 and DV2) and Progonus muelleri (PM1 and PM2) maritae

FO/BL, forebody to body length ratio; PC/BL, postcaecal region to body length ratio.
1 Body length and oral sucker were measured from some of hologenophores before taking a piece for DNA extraction.
The descriptions of 3 types of intramolluscan stages were made de novo. Measurements from this and previous studies are summarized in Tables 5 and 6.
Table 5. Measurements of C. appendiculata (DV1) and Derogenidae gen. sp. (DV2) cercariae compared with previous descriptions

1 Only narrow distal part width is mentioned – 10 μm.
Table 6. Measurements of Cercaria octocauda (PM1) cercariae compared with previous descriptions

Cercaria appendiculata Pelseneer, 1906 (DV1) (Fig. 6)
Host: Cryptonatica affinis

Fig. 6. Cercaria appendiculata (DV1). Schemes of pre-infective (A), infective (B) cercariae, and caudal cyst with everted delivery tube (C). (D, E) CLSM, TRITC-phalloidin staining; muscular, digestive and excretory systems in pre-infective (D) and infective (E) cercaria. (F) SEM, infective cercaria, lateral view. (G) Bright field, caudal cyst with everted delivery tube. Scale bars – 50 μm. Abbreviations: ap – aperture; c – caecum; cb – cercarial body; cc – caudal cyst; dt – delivery tube; dte – delivery tube extension; oe – oesophagus; ep – excretory pore; ev – excretory vesicle; fc – flame cell; fu – furca; ic – inner cyst layer; la – locomotory appendage; mo – membranous outgrowth; oc – outer cyst layer; os – oral sucker; ph – pharynx; rm – retractor muscles; sph – sphincter; vs – ventral sucker.
Localities: White Sea, Keret Archipelago; Barents Sea, Dalniye Zelentsy.
Sites: Reproductive gland and ducts, partially digestive gland, hypobranchial gland.
Prevalence: 5.2% (14 of 270) at Keret Archipelago, White Sea; 5.8% (6 of 104) at Dalniye Zelentsy, Barents Sea.
Rediae measurements based on 29 ethanol-fixed specimens: 16 from White Sea and 13 from Barents Sea. As rediae from White and Barents Seas differed clearly by size, their measurements are given separately (marked WS and BS). WS rediae elongated, 1848 (608–3411) × 174 (141–221). Pharynx small, 47 (28–32) × 34 (25–30). Caecum 548 (318–939) × 50 (27–73). Birth pore close to mouth opening. BS rediae very long, 4024 (3479–5006) × 154 (123–253). Pharynx small, 42 (37–51) × 30 (25–34). Caecum 929 (566–1536) × 30 (20–40). Birth pore close to mouth opening.
Cercariae measurements based on 12 live and 30 glutaraldehyde-fixed specimens: 29 from the White Sea and 13 from the Barents Sea. Cercariae of cystophorous type, tail consisting of caudal cyst, delivery tube and locomotory appendage. Body 170 (124–249) long, 47 (39–56) wide. Oral sucker 27 (25–31) × 27 (25–30), ventral sucker 28 (24–31) × 30 (27–33) and pharynx 13 (12–16) × 16 (14–17). Intestinal caeca reaching slightly behind the ventral sucker. Excretory vesicle Y-shaped, with widened proximal part; excretory ducts unite dorsally near pharynx. Eight flame cells present within the body. Four more flame cells form compact group within caudal cyst close to its aperture. Muscular sphincter present at cyst aperture; retractor muscles connect body hind end with cyst wall.
In infective cercariae, body and delivery tube pulled into caudal cyst, aperture closed. Body lies with anterior end near folded delivery tube. Caudal cyst roundish, 94 (85–103) × 91 (81–100) in diameter, 61 (57–65) thick, 2-layered, space between layers narrow. One side with depression, and furcate locomotory appendage attached at its bottom; outer layer forms membranous outgrowth. Locomotory appendage 145 (109–178) long, 18 (16–21) × 8 (7–10) in diameter, furca 58 (53–66) long. Everted delivery tube 409 (306–456) long, 15 (12–20) wide in middle region, narrower in distal part, wider at tip; roundish extension present in proximal region.
Derogenidae gen. sp. (DV2) (Fig. 7)
Hosts: Euspira pallida, Amauropsis islandica
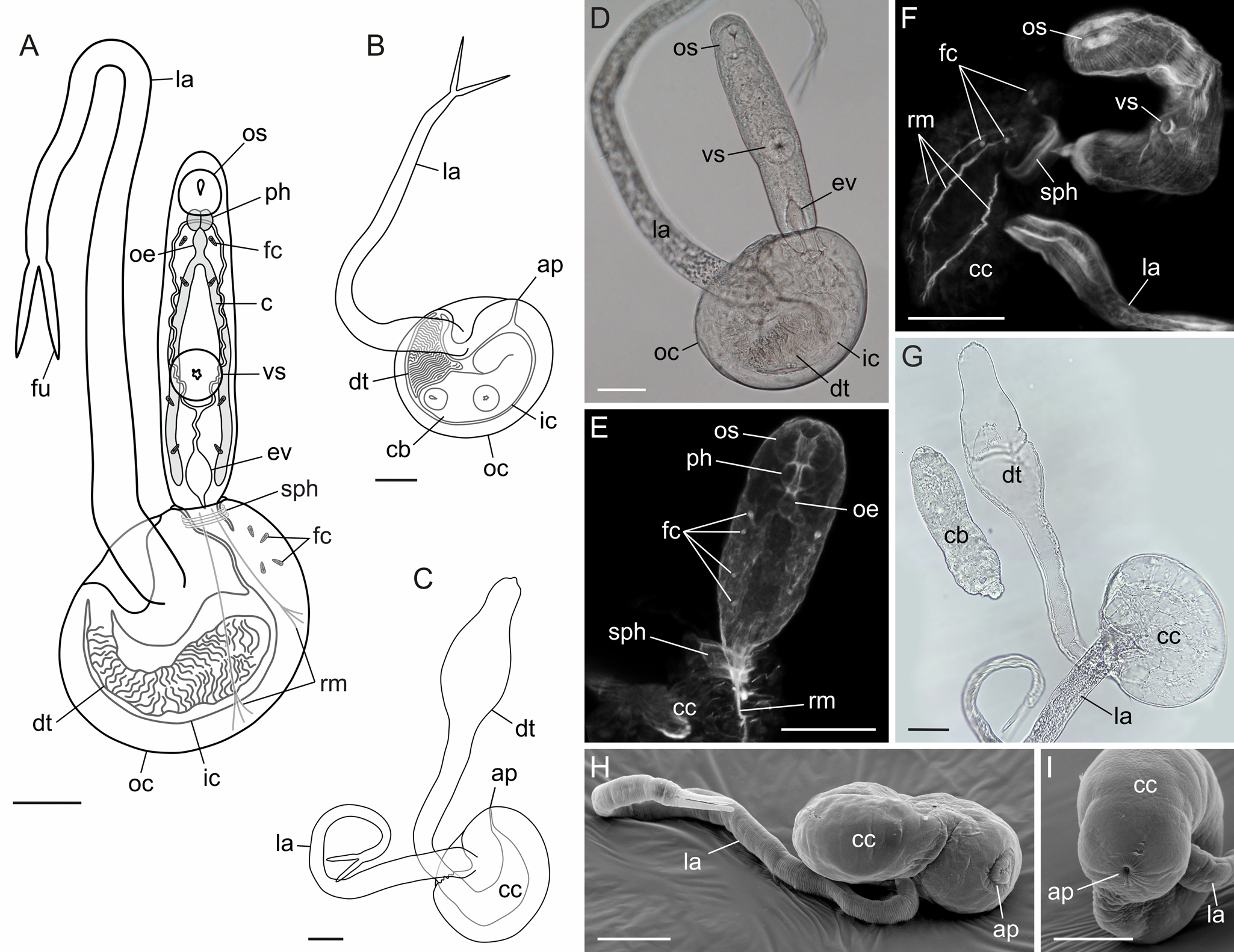
Fig. 7. Derogenidae gen. sp. (DV2). Schemes of pre-infective (A), infective (B) cercaria and caudal cyst with everted delivery tube (C). (D) Bright field, live pre-infective cercaria, general view. (E, F) Pre-infective cercariae, CLSM, TRITC-phalloidin staining; muscular, digestive and excretory systems. (G) Bright field, caudal cyst with everted delivery tube. (H, I) SEM, infective cercaria. Scale bars – 50 μm. Abbreviations: ap – aperture; c – caecum; cb – cercarial body; cc – caudal cyst; dt – delivery tube; oe – oesophagus; ev – excretory vesicle; fc – flame cell; fu – furca; ic – inner cyst layer; la – locomotory appendage; oc – outer cyst layer; os – oral sucker; ph – pharynx; rm – retractor muscles; sph – sphincter; vs – ventral sucker.
Localities: White Sea, Keret Archipelago; Barents Sea, Dalniye Zelentsy.
Sites: Reproductive gland and ducts, partially digestive gland.
Prevalence: 4.2% (1 of 24) E. pallida, 33.4% (1 of 3) A. islandica at Keret Archipelago, White Sea; 12.5% (1 of 8) E. pallida at Dalniye Zelentsy, Barents Sea.
Rediae measurements based on 22 ethanol-fixed specimens: 6 from Barents Sea and 16 from White Sea. Rediae elongated, 2265 (1313–3961) × 219 (145–308). Pharynx small, 55 (42–68) × 42 (31–51). Caecum 1063 (805–1366) × 45 (33–56). Birth pore close to mouth opening.
Cercariae measurements based on 18 live and 9 glutaraldehyde-fixed specimens: 25 from the White Sea and 2 from the Barents Sea. Cercariae of cystophorous type, tail consisting of caudal cyst, delivery tube and locomotory appendage. Body 238 (187–283) long, 77 (59–109) wide. Oral sucker 32 (26–37) × 38 (32–47), ventral sucker 39 (37–45) × 42 (35–53), pharynx 17 (14–21) × 22 (19–24). Intestinal caeca almost reaching posterior body end. Excretory vesicle Y-shaped, with widened proximal part; excretory ducts uniting dorsally near pharynx. Eight flame cells within body, 4 more lying loosely within caudal cyst close to the aperture. Muscular sphincter at cyst aperture; retractor muscles connecting body hind end with the cyst wall.
In infective cercariae, body and delivery tube inside caudal cyst, aperture closed, anterior body end close to tightly packed delivery tube. Caudal cyst oval, 195 (170–211) × 155 (138–169) in diameter, 102 (90–123) thick, 2 layered, space between layers broad. Locomotory appendage 600 (529–688) long, 36 (30–41) × 17 (14–21) in diameter, attached at depression on caudal cyst side; furca 72 (62–85) long. Everted delivery tube 446 (430–471) long, 35 (30–44) wide at base, proximal part inflated, maximal width 79 (71–94).
Cercaria octocauda Tschubrik, 1952 (PM1) (Fig. 8)
Host: Cryptonatica affinis

Fig. 8. Cercaria octocauda (PM1). Schemes of pre-infective (A), infective (B) cercaria and caudal cyst with everted delivery tube (C). (D) Bright field, live pre-infective cercaria, general view. (E, F) CLSM, TRITC-phalloidin staining; muscular, digestive and excretory systems in pre-infective cercaria (E) and in caudal cyst (F). (G) SEM, infective cercaria. (H) Phase-contrast microscopy, cercaria leaving caudal cyst through delivery tube; arrowheads indicate tip of delivery tube. Scale bars – 50 μm. Abbreviations: ap – aperture; c – caecum; cb – cercarial body; cc – caudal cyst; dt – delivery tube; oe – oesophagus; ev – excretory vesicle; fc – flame cell; fi – arrow-shaped fin; ic – inner cyst layer; it – immotile threads; la – locomotory appendage; oc – outer cyst layer; os – oral sucker; ph – pharynx; rm – retractor muscles; sph – sphincter; vs – ventral sucker.
Locality: White Sea, Velikaya Salma Strait.
Sites: Reproductive gland and ducts, partially digestive gland.
Prevalence: 26.7% (4 of 15)
Rediae measurements based on 14 ethanol-fixed specimens. Rediae elongated, 1897 (1018–2906) × 182 (138–228). Pharynx small, 43 (36–48) × 33 (29–38). Caecum 422 (309–512) × 27 (20–32). Birth pore close to mouth opening.
Cercariae measurements based on 24 live and 11 glutaraldehyde-fixed specimens. Cercariae of cystophorous type, tail consisting of caudal cyst, delivery tube and locomotory appendage. Body 282 (205–373) long, 57 (32–84) wide. Oral sucker 29 (23–36) × 33 (21–44), ventral sucker 33 (23–38) × 33 (22–41), pharynx 15 (13–18) × 17 (13–22). Intestinal caeca reaching middle of ventral sucker. Excretory vesicle Y-shaped, with widened proximal part; excretory ducts uniting dorsally near pharynx. Eight flame cells within body, 4 more lying within caudal cyst close to aperture, opposite to locomotory appendage attachment spot. Muscular sphincter at cyst aperture; retractor muscles connecting body hind end with anterior part of cyst wall.
Caudal cyst 311 (277–365) long, rounded in cross section, maximal diameter 81 (67–99), anterior end spherical, opposite end pointed. Cyst 2-layered, space between layers broad; outer layer forming arrow-shaped fin at pointed end, 122 (100–145) long, 77 (67–92) wide. Locomotory appendage attached near fin base, 213 (173–254) long, 17 (14–21) in diameter, with 8 immotile threads 631 (547–734) long. In infective cercariae, delivery tube and body drawn into caudal cyst through aperture at its wide end, aperture closed. Tube everting at pointed end of cyst. Delivery tube 1155 (1045–1272) long, width uniform (27 (25–30) – middle region) except wider tip – 39 (36–41).
Discussion
According to our data based on morphological identification, 2 species of the family Derogenidae, subfamily Derogeninae parasitize fishes in the White Sea: Derogenes varicus and Progonus muelleri. The former was found in a wide range of fishes (10 of 16 examined species), and the latter – predominantly in Myoxocephalus scorpius. Such results are consistent with the published data on White Sea fish parasites (Shulman and Shulman-Albova, Reference Shulman and Shulman-Albova1953). We also recovered 1 marita of D. varicus from the stomach of the whelk Buccinum scalariforme from the White Sea. Such a non-typical host probably received the worm through feeding on an infected dead fish – a post-cyclic transmission event which had been presumed for a long time for various Hemiuroidea (Køie, Reference Køie1979; Gibson and Bray, Reference Gibson and Bray1986) and recently proved to occur in D. lacustris (Tsuchida et al., Reference Tsuchida, Rauque, Viozzi, Flores and Urabe2022). In the Barents Sea we found only D. varicus, obviously due to the small number of fish dissected.
Previously, D. varicus and P. muelleri were recorded as the only derogenid parasites in the Barents Sea (Polyansky, Reference Polyansky1955; Issaitschikov, Reference Issaitschikov1933; Mitenev, Reference Mitenev1993; Køie, Reference Køie2009; Kuklin et al., Reference Kuklin, Kuklina and Kisova2012). Shulman and Shulman-Albova (Reference Shulman and Shulman-Albova1953) claimed to have found 1 more species of the genus Derogenes in the White Sea – D. crassus. The key difference between D. varicus and D. crassus is the egg size: 50–66 × 26–39 (Odhner, Reference Odhner1905; Manter, Reference Manter1926; Lloyd, Reference Lloyd1938) and 63–75 × 33–39 (Manter, Reference Manter1934; Yamaguti, Reference Yamaguti1938), respectively. Shulman and Shulman-Albova (Reference Shulman and Shulman-Albova1953) did not provide morphometric data, and thus there is no convincing evidence that they actually found D. crassus. Worldwide, D. crassus has otherwise been recovered from warm seas (Manter, Reference Manter1934; Yamaguti, Reference Yamaguti1938; McCauley, Reference McCauley1960; Dyer et al., Reference Dyer, Williams and Bunkley-Williams1992; Kuramochi, Reference Kuramochi2014), and this distribution also raises doubts about the presence of this species in the White Sea.
Genetically our specimens of D. varicus split into 2 groups: all the maritae from fish vs the marita from the whelk B. scalariforme. The former matched the D. varicus of Blair et al. (Reference Blair, Bray and Barker1998) (AF029816) from an unspecified locality in 18S rDNA sequence. The latter had the sequence of 28S rDNA fragment identical to the sequence of D. varicus from the North Sea (AY222189; Olson et al., Reference Olson, Cribb, Tkach, Bray and Littlewood2003). Morphological reexamination and morphometry of our specimens identified only 1 difference between maritae from the fish and that from the whelk – average egg size was 51 × 31 and 60 × 35, respectively. However, the ranges overlapped (44–60 × 25–37 and 57–63 × 32–38, respectively), and generally complied with the egg size in the previous descriptions of D. varicus: 50–66 × 26–39 (Odhner, Reference Odhner1905; Manter, Reference Manter1926; Lloyd, Reference Lloyd1938). It should be also taken into account that the marita from B. scalariforme may be morphologically deviant because it was recovered from a non-typical host. Nonetheless, based on the present data and the sequences of D. varicus from GenBank, we conclude that there are 2 clearly distinct lineages of D. varicus which we marked as DV1 and DV2. Unfortunately, we have not found maritae of DV2 in fish to compare with DV1, so we cannot diagnose these groups as 2 species through maritae morphology. We may speculate that DV2 is the species that Shulman and Shulman-Albova (Reference Shulman and Shulman-Albova1953) called D. crassus, but this is yet to be proved. The 18S rDNA sequence AJ287511 of D. varicus (Littlewood and Olson, Reference Littlewood, Olson, Littlewood and Bray2001) from the North Sea differed from both DV1 and DV2, and thus represents another lineage of D. varicus (labelled DV4 in Fig. 3). Finally, one more lineage is represented by 28S rDNA sequences MW504598–9 of D. varicus from the Kuril Islands, Sea of Okhotsk (Sokolov et al., Reference Sokolov, Atopkin and Gordeev2021), labelled DV3 in Fig. 1. As DV3 and DV4 have been recognized by different markers, it cannot be ruled out that they are one and the same lineage. However, the geographic origin (Pacific vs Atlantic) may be an argument that they are not.
In the moonsnails from the White and Barents Seas we found digenean life cycle stages which matched maritae of DV1 and DV2 in the analysed rDNA fragments. Thus, DV1 utilizes Cryptonatica affinis as the first intermediate host, and its rediae and cercariae were described as Cercaria appendiculata (Chubrik, Reference Chubrik1966; Timofeeva, Reference Timofeeva1976; Køie, Reference Køie1979). The first intermediate hosts of DV2 are Euspira pallida and Amauropsis islandica, and its rediae and cercariae were described by Køie (Reference Køie1990b). Cercariae of DV1 and DV2 have similar furcate locomotory appendages, but clearly differ in size and shape of the caudal cyst and delivery tube. Isolates from the first intermediate hosts allowed us to analyse variability in cox1 gene for DV1 and DV2. The monophyly of these groups was supported, and the gap between intra- and intergroup variation was evident. It is curious that DV1 rediae from the White and Barents Seas differed dramatically in length, but these differences were not consistent with molecular diversity in cox1. Such phenotypic variation may arise from different environmental conditions, but currently there are not enough data to discuss it.
To sum up, DV1 and DV2 differ in several genetic markers, both rather conservative (rRNA) and variable (cox1), as well as in the first intermediate hosts and in cercaria structure. Thus, the current criteria of species delineation are accomplished (Huston et al., Reference Huston, Cutmore, Miller, Sasal, Smit and Cribb2021; Bray et al., Reference Bray, Cutmore and Cribb2022) and DV1 and DV2 deserve to be recognized as 2 species.
The maritae of P. muelleri were genetically heterogeneous, too. Three substitutions were present in the analysed fragments of rDNA: 1 in 28S rDNA, 1 in ITS2 and 1 in 18S rDNA. The divergence in cox1 gene between these groups was quite high (61–62 substitutions). As genetically different lineages occurred in sympatry, a question on their conspecificity came up, so we refer to them as PM1 and PM2. However, the genetic distance between these lineages is much smaller than that between DV1 and DV2, and in 28S it is similar to the distance between 2 sequences of D. lacustris from Tsuchida et al. (Reference Tsuchida, Flores, Viozzi, Rauque and Urabe2021). No morphological differences were found between maritae of P. muelleri. Cercaria octocauda from C. affinis matched 3 maritae of PM1 by all the fragments of rDNA analysed and by cox1. Unfortunately, we did not find cercariae matching PM2 to compare them with C. octocauda. Therefore, for now we lack morphological or biological evidence to recognize PM1 and PM2 as 2 species.
Redescription of ‘true’ D. varicus and P. muelleri also cannot be provided as long as there is no molecular data from the type localities and type hosts. For D. varicus these are Danish Exclusive Economic Zone and Salmo salar Linnaeus, 1758 (Müller, 1784). Type locality of P. muelleri is West Greenland Shelf, but type host is not specified, that is either Myoxocephalus scorpius or Gadus macrocephalus Tilesius, 1810 (Levinsen, Reference Levinsen1881).
Our results for the life cycles of DV1, DV2 and PM1 comply with the previous data on the life cycles of the Hemiuroidea in general. Moonsnails serve as the first intermediate hosts for all these 3 species. This may be characteristic of the subfamily Derogeninae, but not the Derogenidae as a whole: representatives of the other subfamilies infect pulmonates and different groups of Littorinimorpha (Madhavi, Reference Madhavi1978; Goater et al., Reference Goater, Browne and Esch1990; Køie and Gibson, Reference Køie and Gibson1991). Metacercariae of D. varicus had been described from various planktonic copepods (Wright, Reference Wright1907; Weinstein, Reference Weinstein1972). They probably serve as the most common second intermediate hosts, at least for DV1, according to the experiments of Køie (Reference Køie1979). Experimental data on DV2 cercariae are ambiguous: Køie (Reference Køie1990b) succeeded in infecting 3 copepod species by cystophorous cercariae from E. pallida, but those copepods did not survive for more than several weeks. Metacercariae identified as D. varicus also had been found multiple times in Chaetognatha (Kulachkova, Reference Kulachkova1972; Weinstein, Reference Weinstein1972; Øresland, Reference Øresland1986; Rolbiecki and Waskusz, Reference Rolbiecki and Waskusz2005), and it is probable that they were infected by eating copepods (Dollfus, Reference Dollfus1960; Køie, Reference Køie1979). Unlike D. varicus, P. muelleri had never been recorded in the second intermediate host until recently when its metacercaria was found in a skeleton shrimp Caprella septentrionalis Krøyer, 1838 (Caprellidae, Amphipoda) from the White Sea (Sokolov et al., Reference Sokolov, Atopkin and Gordeev2021). The 28S rDNA sequence of this metacercaria fell into the PM1 group in our analysis. Thus, all the 3 hosts of PM1 are revealed now.
The hemiuroidean cercariae are quite diverse in tail structure, though some patterns with respect to phylogeny may be derived. For example, the vast majority of the Hemiuridae with known larval stages are characterized by cercariae with an oar-shaped locomotory appendage of the tail (Køie, Reference Køie1990a, Reference Køie1990b, Reference Køie1992, Reference Køie1995; Krupenko et al., Reference Krupenko, Gonchar, Kremnev and Uryadova2020). Cercariae of the Lecithasteridae are immotile and possess roundish caudal cyst (Hunninen and Cable, Reference Hunninen and Cable1943; Køie, Reference Køie1989; Køie et al., Reference Køie, Karlsbakk and Nylund2002; Krupenko et al., Reference Krupenko, Kremnev, Skobkina, Gonchar, Uryadova and Miroliubov2022). Within the family Derogenidae cercariae are not uniform (Madhavi, Reference Madhavi1978; Køie, Reference Køie1979; Goater et al., Reference Goater, Browne and Esch1990; Køie and Gibson, Reference Køie and Gibson1991). Our data showed that cercariae noticeably differ even within the subfamily Derogeninae. Both Derogenes and Progonus have locomotory appendages, but in the former it is furcate and in the latter it has 8 immotile threads. Differences in the cystophorous cercariae structure must be strongly dependent on the second intermediate host biology. A case of Halipegus occidualis Stafford, 1905 is particularly illustrative in this respect: evolutionary switch to ostracod second intermediate hosts resulted in the modification of cercariae tail and infection mechanism (Zelmer and Esch, Reference Zelmer and Esch1998). Close phylogenetic relationship of Derogenes and Progonus contrasts with the difference in their tail morphology, and this brings us back to their life cycles. Structure of Progonus cercariae may be interpreted as adaptive to infect C. septentrionalis which is a filter-feeder (Legeżyńska et al., Reference Legeżyńska, Kędra and Walkusz2012): threads on the locomotory appendage of cercaria let it be easily trapped in the setose antennae 2 of C. septentrionalis. Switch to skeleton shrimps also might have resulted in narrowing the definitive host range of Progonus: demersal fish are favoured, and the highest infection rates are registered for the European sculpin (Shulman and Shulman-Albova, Reference Shulman and Shulman-Albova1953; Polyansky, Reference Polyansky1955; our data). Thus, the life cycle of Progonus seems to be closely associated with benthic communities, rather than with the planktonic ones as in Derogenes.
Conclusion
Marine digeneans with wide geographical distribution and low specificity towards the definitive host are obvious candidates to be species complexes. Derogenes varicus was proved to be the one, and Progonus muelleri is under suspicion now. To untangle these complexes, a worldwide sampling is needed, and primarily – specimens from the type hosts and type localities. At the same time, quite a number of marine digeneans had been described based on a single or few specimens, and do not have reliable diagnostic features. For instance, it is doubtful that D. robustus Brinkmann, 1967, D. chelidonichthydis Shen, 1989, D. minoi Shen, 1990, D. magnus Wang, 1991, D. bohaiensis Qiu & Liang in Shen & Qiu, 1995 can be distinguished from D. varicus. Molecular methods are essential to check whether these species are valid, and life-cycle studies are likely to be equally valuable.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003118202200110X.
Data availability
The aligned 28S, 18S, 5.8 rDNA, ITS2 and cox1 datasets used in phylogenetic analysis are freely accessible at http://dx.doi.org/10.13140/RG.2.2.19523.27683/1.
Acknowledgements
The research study was carried out using the resources and with the help of the staff of the Educational and Research Station ‘Belomorskaia’ of Saint Petersburg University (SPbU), the White Sea Biological Station (WSBS) ‘Kartesh’ of the Zoological Institute of the Russian Academy of Sciences (ZIN RAS), the N. A. Pertsov White Sea Biological Station of Lomonosov Moscow State University, and Biological Research Station of Murmansk Marine Biological Institute of RAS in Dalnie Zelentsy. We thank all our colleagues who helped with sampling, especially Professor George Slyusarev, Sergei Bagrov, Dr Mikhail Makarov and Alexander Semenov. The molecular studies were carried out in the Research Resource Center ‘Molecular and Cell Technologies’ of SPbU, the SEM studies – in the ‘Taxon’ Research Resource Center (http://www.ckp-rf.ru/ckp/3038/) (ZIN, RAS), and the CLSM studies – in the Resource Center for Microscopy and Microanalysis of SPbU. We are grateful to Dr Karin Tsuchida who provided us with relevant literature on derogenid taxonomy. We also thank 2 anonymous reviewers for their helpful comments and efforts to improve the manuscript.
Author's contributions
DK conceived and designed the study. DK, GK, AU, VK, OS, AGu and OK conducted data gathering. DK, GK and AGo performed molecular analyses. AM, DK and GK performed morphological analyses. DK, GK and AGo wrote the article.
Financial support
This study was supported by the Russian Science Foundation (Russia), grant no. 19-74-10029. Work at the WSBS ‘Kartesh’ was supported by a research programme of ZIN RAS (project no. 122031100260-0).
Conflicts of interest
The authors declare there are no conflicts of interest.


















