INTRODUCTION
RNA interference (RNAi) is triggered by double-stranded RNA (dsRNA) (Fire et al. Reference Fire, Xu, Montgomery, Kostas, Driver and Mello1998). This dsRNA is processed into single-stranded small interfering RNAs (siRNAs) that act as guide sequences to target homologous mRNAs and nascent transcripts for post-transcriptional gene silencing (PTGS) (Chekulaeva and Filipowicz, Reference Chekulaeva and Filipowicz2009) and transcriptional gene silencing (TGS) (Moazed, Reference Moazed2009), respectively. A broad array of endogenous RNAi-related mechanisms is used to control gene expression (White and Allshire, Reference White and Allshire2008; Mochizuki, Reference Mochizuki2010; Teixeira and Colot, Reference Teixeira and Colot2010). Likely because it accesses these endogenous gene activities, experimentally induced RNAi is potent and specific (Sharp, Reference Sharp1999), leading to its popular and wide use as a genetic tool (Sioud, Reference Sioud2011). However, many challenges remain. For many organisms, intracellular delivery of dsRNA presents a significant experimental obstacle; coupled to this is variable or low potency. In contrast, RNAi works very well in C. elegans because of ease of delivery coupled to efficient RNA-directed RNA polymerase (RdRP) amplification of effector siRNAs. Here we review what is known about RNA delivery and genetic control of RNAi efficacy in C. elegans with the goal of using this knowledge to enable RNAi in other organisms.
Reverse genetics via RNAi has become extremely popular over the past decade (Silva et al. Reference Silva, Chang, Hannon and Rivas2004). This is particularly true in organisms like C. elegans, Planaria and Apidae that readily take up and apparently spread the triggering dsRNA and/or derived silencing signals. However, biological and methodological diversity in dsRNA delivery can lead to variability in RNAi efficacy (Echeverri et al. Reference Echeverri, Beachy, Baum, Boutros, Buchholz, Chanda, Downward, Ellenberg, Fraser, Hacohen, Hahn, Jackson, Kiger, Linsley, Lum, Ma, Mathey-Prevot, Root, Sabatini, Taipale, Perrimon and Bernards2006). Therefore, to maximize RNAi silencing, it is important to understand the organism-specific limitations as well as advantages of dsRNA uptake (Geldhof et al. Reference Geldhof, Visser, Clark, Saunders, Britton, Gilleard, Berriman and Knox2007; Knox et al. Reference Knox, Geldhof, Visser and Britton2007). The ease of both classic genetics and RNAi has made C. elegans the exemplary model organism for this analysis.
The discovery and subsequent in-depth mechanistic characterization of RNAi in C. elegans helped established the entire RNAi field (Hannon, Reference Hannon2002). RNAi in C. elegans is both easy and remarkably potent. The ease of dsRNA delivery is unmatched, including most notably by ingestion – so called environmental RNAi. However, a variety of enhanced RNAi (Eri) mutants show that even in C. elegans, RNAi can become even more potent (Kennedy et al. Reference Kennedy, Wang and Ruvkun2004). Although not all members of the nematode genus Caenorhabditis are equally accessible for dsRNA delivery, most are capable of RNAi (Felix, Reference Felix2008). Interestingly, the identified eri genes are conserved across Caenorhabditis and, in many instances, widely conserved across evolution. This indicates independent selection for delivery and regulation of potency. Similar observations have been made more broadly in the phylum Nematoda, as in the genera Haemonchus, Heterohabditis, Ostertagia, Heterodera, Globodera, Meloidogyne, Panagrolaimus and Brugia which have all been shown to respond to at least some forms of RNAi delivery, though with different apparent potencies (Felix, Reference Felix2008). These observations indicate that comparative analysis of RNAi in nematodes is likely to reveal much about the selective pressures that modify small RNA pathways. In this review of C. elegans RNAi genetics and methods, we pay particular attention to conserved genetic networks with the goal of leveraging the wealth of mechanistic information available in C. elegans to the application of RNAi in less accessible nematodes.
METHODS
The robustness of RNAi in C. elegans is likely due to both RdRP activity that amplifies silencing signals and the systemic nature of C. elegans RNAi that enables silencing signals to move between cells, tissues and generations. Thus, small amounts of locally delivered dsRNA can cause robust silencing in any tissue in the treated animal as well as its progeny. Here, we compare the relative silencing potency of the three principal dsRNA delivery methods: microinjection, ingestion, and transgene expression (Fig. 1).

Fig. 1. Double-stranded RNA delivery in C. elegans. Microinjection of concentrated dsRNA (red) into the large gut cells (yellow), the syncytial germline (blue), or the body cavity (white) affords the greatest control over delivery and the most potent response; however, throughput is limited. Throughput is improved by soaking whole animals in dsRNA, or feeding worms bacteria engineered to express dsRNA. Both soaking and feeding results in ingested dsRNA that requires the intestinal transmembrane protein SID-2 (green) for delivery into the animal. Finally, transgenic expression of double-stranded RNA or hairpin constructs can target dsRNA delivery to specific cell types not accessible by microinjection, and in sid-1 mutant backgrounds, can limit the RNAi knock-down effect to the targeted cells.
Microinjection is the most direct and potent way to introduce RNAi triggers. Microinjection also provides control over dsRNA concentration and the cell or tissue to score for knockdown. Control of concentration is critical to maximize the effective dose while simultaneously avoiding non-specific toxicity or off-target effects. The concentration of dsRNA to inject will vary from organism to organism (Kuwabara and Coulson, Reference Kuwabara and Coulson2000; Nasevicius and Ekker, Reference Nasevicius and Ekker2000; Svoboda and Stein, Reference Svoboda and Stein2009), from cell type to cell type (Grishok and Mello, Reference Grishok and Mello2002; Wang et al. Reference Wang, Kennedy, Conte, Kim, Gabel, Kamath, Mello and Ruvkun2005), and even from gene target to gene target (Krueger et al. Reference Krueger, Bergauer, Kaufmann, Wolter, Pilk, Heider-Fabian, Kirch, Artz-Oppitz, Isselhorst and Konrad2007). Although in C. elegans silencing signals can spread from the injected cell or tissue, this is not true of other, even closely related species (Winston et al. Reference Winston, Sutherlin, Wright, Feinberg and Hunter2007). Therefore, initial analysis of RNAi effectiveness should be limited to scoring the injected cell or syncytial tissue.
In some organisms, long dsRNA is toxic. For example, in vertebrates, long dsRNA triggers a non-sequence specific interferon response that leads to cell death (Cullen, Reference Cullen2006). Whether long dsRNA is toxic to invertebrates is largely unexplored. These toxic effects are avoided in mammalian cells by using siRNA to trigger RNAi; siRNAs are too short to trigger the non-specific effect (Mittal, Reference Mittal2004). Microinjection of siRNAs is effective in C. elegans, but the response is attenuated compared to long dsRNA (Yang et al. Reference Yang, Lu and Erickson2000; Carpenter and Sabatini, Reference Carpenter and Sabatini2004).
Transgene-expressed dsRNA can also initiate RNAi and allows introduction of dsRNA into cells and tissues that are not accessible to microinjection, including neurons and muscle cells (Schepers, Reference Schepers2005). Another advantage is that transgenic lines can be maintained indefinitely and expanded to large populations that are not accessible by microinjection. In C. elegans, RNAi can be effectively triggered by either expressed hairpin RNA constructs or co-expressed sense and antisense RNA. However, the production of dsRNA-expressing transgenic animals is more complicated and less controllable than injecting dsRNA. First, it is difficult to avoid non-specific expression of the transgene; the promoter may be active in unintended cells (Grove et al. Reference Grove, De Masi, Barrasa, Newburger, Alkema, Bulyk and Walhout2009). Second, it is difficult to assess the quality or quantity of RNAi trigger; unlike loading a microinjection needle with known concentrations of precisely defined dsRNA, endogenously expressed dsRNA does not come with easily quantifiable measures. As a consequence, RNAi potency can vary between independent lines (Praitis et al. Reference Praitis, Casey, Collar and Austin2001) and from simple structural changes to the same hairpin construct (Boudreau et al. Reference Boudreau, Monteys and Davidson2008). Third, transgenes in C. elegans are subject to spontaneous silencing via a mechanism that is at least in part dependent on RNAi-silencing genes. Since RNAi silencing is saturable, expressed dsRNA may interfere with such silencing in a dose-dependent and variable way (Kim et al. Reference Kim, Gabel, Kamath, Tewari, Pasquinelli, Rual, Kennedy, Dybbs, Bertin, Kaplan, Vidal and Ruvkun2005b) which adds a confounding factor when evaluating the presence, absence or penetrance of RNAi silencing.
Ingestion of dsRNA is the third principal means of introducing RNAi triggers into C. elegans. Ingestion can be accomplished either by soaking worms in a concentrated solution of purified dsRNA (Maeda et al. Reference Maeda, Kohara, Yamamoto and Sugimoto2001) or more simply by feeding worms bacteria engineered to expressed dsRNA (Timmons and Fire, Reference Timmons and Fire1998; Timmons et al. Reference Timmons, Court and Fire2001). This mechanism of inducing effective RNAi is entirely dependent on systemic RNAi. However, systemic RNAi is not sufficient as specialized dsRNA uptake machinery is also required (Winston et al. Reference Winston, Sutherlin, Wright, Feinberg and Hunter2007). In C. elegans, the transmembrane proteins SID-1 and SID-2 are required independently for ingestion-mediated RNAi. SID-1 is required for the uptake of silencing signals into all cells, while SID-2 is required only for silencing initiated by ingested dsRNA. SID-2 is expressed exclusively in the intestine and localizes primarily to the apical membrane, suggesting that SID-2 may directly interact with ingested dsRNA for internalization (Winston et al. Reference Winston, Sutherlin, Wright, Feinberg and Hunter2007).
SID-2 homologues are highly divergent, recognizable in only Caenorhabditis nematodes, and even among these, ingested dsRNA induces RNAi in only a few species (Winston et al. Reference Winston, Sutherlin, Wright, Feinberg and Hunter2007). This molecular and functional divergence is consistent with the unpredictable distribution of organisms that are susceptible to ingested dsRNA-mediated RNAi (Whangbo and Hunter, Reference Whangbo and Hunter2008). Consequently, absence of ingestion-mediated RNAi should not be interpreted as absence of RNAi or even systemic RNAi.
In organisms that are susceptible to ingestion-mediated RNAi, the ability to easily subject animals to a large variety of dsRNA sequences has many advantages. In C. elegans, the construction and availability of libraries of engineered ‘RNAi foods’ targeting the entire genome has made “feeding RNAi” an extremely powerful genetic tool (Kamath and Ahringer, Reference Kamath and Ahringer2003; Kamath et al. Reference Kamath, Fraser, Dong, Poulin, Durbin, Gotta, Kanapin, Le Bot, Moreno, Sohrmann, Welchman, Zipperlen and Ahringer2003; Rual et al. Reference Rual, Ceron, Koreth, Hao, Nicot, Hirozane-Kishikawa, Vandenhaute, Orkin, Hill, van den Heuvel and Vidal2004). Furthermore, feeding worms dsRNA-expressing bacteria, like transgene-expressed dsRNA, enables large numbers of RNAi knock-down worms to be produced for genetic screens or biochemical assays; however, this conditional feeding RNAi has an advantage over transgene-expressed dsRNA when targeting genes important for growth, fertility and viability. The apparent delivered dose of ingested dsRNA, however, is less than is achieved by microinjection, causing less penetrant phenotypes, which makes it often necessary to expose animals to ingested dsRNA for multiple generations (Timmons and Fire, Reference Timmons and Fire1998). Furthermore, different tissues respond differently to RNAi triggers, making it difficult to score the relative efficacy of RNAi (Calixto et al. Reference Calixto, Chelur, Topalidou, Chen and Chalfie2010).
Other less frequently used means to introduce RNAi triggers into small metazoans include electroporation transfection, and soaking in liposome-encapsulated dsRNA (Issa et al. Reference Issa, Grant, Stasiuk and Shoemaker2005; Geldhof et al. Reference Geldhof, Murray, Couthier, Gilleard, McLauchlan, Knox and Britton2006; Krautz-Peterson et al. Reference Krautz-Peterson, Radwanska, Ndegwa, Shoemaker and Skelly2007). These methods are not used in C. elegans.
MECHANISMS OF dsRNA TRANSPORT BY SID-1
Intercellular transport of dsRNA-silencing signals in C. elegans requires the highly conserved dsRNA channel SID-1 (Jose and Hunter, Reference Jose and Hunter2007). SID-1 is a transmembrane protein with 11 predicted transmembrane domains, a 400+ amino acid extracellular N-terminal domain and a short cytosolic C-terminal domain (Feinberg and Hunter, Reference Feinberg and Hunter2003). Many recovered sid-1 mutants have missense mutations in the transmembrane domains, suggesting that these sequences are essential for function. SID-1 is autonomously required for the import but not the export of RNAi triggers (Jose et al. Reference Jose, Smith and Hunter2009). A sid-1 promoter gfp construct was found to be expressed from the late embryo throughout adulthood in all non-neuronal tissues (Winston et al. Reference Winston, Molodowitch and Hunter2002). Interestingly, neuronal cells are resistant to RNAi triggered by ingested or injected dsRNA, but sensitive to neuronally expressed dsRNA, indicating the defect is in delivery of dsRNA to neurons, not RNAi effectiveness in neurons; consistent with this, transgenic expression of SID-1 in neurons enables efficient systemic RNAi (Calixto et al. Reference Calixto, Chelur, Topalidou, Chen and Chalfie2010). Furthermore, such expression enhances RNAi efficacy in these cells at the expense of wild-type cells (Calixto et al. Reference Calixto, Chelur, Topalidou, Chen and Chalfie2010). These results suggest that SID-1 expression is limiting for systemic RNAi in C. elegans.
Mechanistic studies performed in Drosophila S2 cells indicate that SID-1 functions as a dsRNA-gated channel. Drosophila lacks a SID-1 homologue and endogenous mechanisms of dsRNA uptake in S2 cells are relatively inefficient, making these cells an ideal ‘blank slate’ system to investigate SID-1 dsRNA transport properties. SID-1 activity in S2 cells has been primarily measured by uptake of radio-labeled dsRNA and by RNAi silencing of reporter genes. Recent studies have also used whole-cell patch-clamp analysis to characterize SID-1 channel properties. 32P-labeled dsRNA added to the culture media of SID-1-expressing S2 cells is rapidly taken up, showing that SID-1 enables dsRNA transport (Feinberg and Hunter, Reference Feinberg and Hunter2003; Shih et al. Reference Shih, Fitzgerald, Sutherlin and Hunter2009). To distinguish between active transport mechanisms that require continuous energy input (ATP) for dsRNA transport – i.e. pumps or receptors that require vesicle transport – versus passive transport mechanisms that could transport dsRNA without additional energy input – i.e. channels or pores – the uptake assays were repeated in either ATP-depleted cells or in cells maintained at 4°C. For both treatments, the endogenous S2 cell RNA uptake was eliminated, while SID-1-dependent uptake was still very productive (Feinberg and Hunter, Reference Feinberg and Hunter2003). These results indicate that SID-1 acts as a passive transporter, likely a channel or pore. Consistent with SID-1 functioning as a channel, whole-cell patch-clamp analysis showed that adding dsRNA to the cell media increased the conductance (opened channels) of SID-1-expressing cells and that washing the dsRNA away led to a return to baseline conductance (Shih and Hunter, Reference Shih and Hunter2011). Together these results indicate that SID-1 is a dsRNA-gated channel.
These same transport and activity assays indicate that SID-1 nucleic acid transport is efficient, specific and selective for dsRNA. SID-1 expression in S2 cells enabled detectable RNAi silencing at a 107-fold lower dsRNA concentration than in control cells (Feinberg and Hunter, Reference Feinberg and Hunter2003; Shih et al. Reference Shih, Fitzgerald, Sutherlin and Hunter2009); this translates into less than one molecule of dsRNA per cell, indicating very efficient uptake. Similar results were obtained with cultured C. elegans cells (Shih et al. Reference Shih, Fitzgerald, Sutherlin and Hunter2009). Although initial studies using RNAi silencing of luciferase reporters indicated that sid-1-dependent uptake efficiency is sensitive to dsRNA length (Feinberg and Hunter, Reference Feinberg and Hunter2003), subsequent studies using radio-labeled 50 bp, 100 bp and 500 bp dsRNAs showed indistinguishable results (Shih et al. Reference Shih, Fitzgerald, Sutherlin and Hunter2009). Similarly sized dsRNAs also indistinguishably open channels on whole-cell patched SID-1-expressing cells (Shih and Hunter, Reference Shih and Hunter2011). Since size does not affect activation or transport, it is thought that longer dsRNA, when delivered systemically, is a more efficient silencing trigger. The whole-cell patch-clamp analysis also indicates that nucleic acid transport by SID-1 is specific to dsRNA-containing molecules. First, neither dsDNA nor a DNA-RNA heteroduplex can activate SID-1 expressing cells. Nucleotide substitution experiments indicate a requirement for the ribose 2′-OH. Although dsRNA is required for transport, molecules that contain single-stranded regions can be transported. Transport of hairpin molecules containing greater than 300 nucleotide single-stranded loops as well as pre-microRNA precursors were also detected. These results dramatically expand the possible repertoire of molecules transported by SID-1.
SID-1 homologues are present in nematodes, diverse invertebrate phyla and all sequenced vertebrate genomes (Altschul et al. Reference Altschul, Madden, Schaffer, Zhang, Zhang, Miller and Lipman1997; Grimson et al. Reference Grimson, Srivastava, Fahey, Woodcroft, Chiang, King, Degnan, Rokhsar and Bartel2008) (Fig. 2). These proteins are highly conserved, which indicates a strongly selected function. Although C. elegans SID-1 has a demonstrated long-dsRNA transport activity, an activity or function remains unknown for all other homologues. C. elegans contains five SID-1 homologues (Fig. 3) (Gille, Reference Gille2006), some of which are more similar to vertebrate homologues than SID-1. However, alleles for none of these were recovered in the Sid screen. There are many reasons why mutations in these were not recovered in the Sid screen: these genes may not function in dsRNA transport, their dsRNA transport function may be redundant with another gene(s), or they may have additional essential functions such that mutations that disrupted dsRNA transport may be lethal. Because mutations in these genes have not yet been recovered, only RNAi is available to study their possible role in systemic RNAi or other functions. A serious limitation of this approach is illustrated by our analysis of sid-1 by RNAi: repeated early attempts to produce an RNAi defect by sid-1(RNAi) failed. However, our certainty of the phenotype led us to continue pursuing RNAi of sid-1 by injection of dsRNA, which ultimately caused a reduction in RNAi sensitivity in up to 50% of the progeny of an injected animal. Our difficulty producing a sid-1(RNAi) Sid phenotype likely reflects the tremendous efficiency of dsRNA transport by SID-1 (Shih et al. Reference Shih, Fitzgerald, Sutherlin and Hunter2009), thus animals that retain even a modicum of SID-1 will be capable of a potent systemic RNAi response.
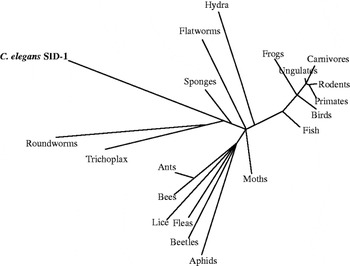
Fig. 2. C. elegans SID-1 is widely conserved. SID-1 homologues are present in many taxonomic groups, suggesting widespread conservation of a protein, which may support systemic RNAi in these other species. The taxonomic tree of C. elegans SID-1 was created using Grishin (protein) distance, with a max sequence difference of 0 85, a fast minimum evolution parameter, and with radial display representing inferred evolutionary distance.

Fig. 3. SID-1 has five homologues in C. elegans. Amino acid alignment of the six C. elegans genes homologous to human SidT2. sid-1, tag-130, C08A9.3 and Y37H2C.1 are similar in size and structure, while C30E1.3 and C30E1.4 are much more divergent. Yellow and orange indicate hydrophobic amino acids, green and purple indicate polar amino acids, red indicates acidic amino acids, cyan indicates basic amino acids, and brown indicates aromatic amino acids. Alignments are generated by Structure based Sequence Alignment Program (STRAP)'s built-in parameters (Gille, Reference Gille2006).
The vertebrate SID-1 homologues are unlikely to transport long dsRNA due to the interferon response (Bridge et al. Reference Bridge, Pebernard, Ducraux, Nicoulaz and Iggo2003). This raises the possibility that because the C. elegans SID-1 paralogues are more similar to the vertebrate proteins than is SID-1, they may share a function and/or nucleic acid specificity different than that of SID-1. These considerations, along with the possibility of functional redundancy, challenge the mirror assumptions that the presence of a SID-1 homologue is evidence for systemic RNAi capacity and the absence of systemic defect when a SID-1 homologue is knocked-down or knocked-out demonstrates lack of dsRNA transport activity for that homologue.
ROLE OF SID-2 IN ENVIRONMENTAL RNAi
For ingested dsRNA to initiate RNAi, it must first be transported into the gut cell cytoplasm. Because SID-1 expressed in Drosophila S2 cells is sufficient to enable uptake, one possibility is that SID-1 functions at the luminal membrane to transport ingested dsRNA across this membrane. However, in the worm, SID-1 is not sufficient, because sid-2 mutants are specifically defective for environmental RNAi. Interestingly, SID-2 alone is also not sufficient, as sid-1 mutants exposed to dsRNA fail to show silencing in gut cells. This indicates that these two proteins function together, either cooperatively or sequentially, to import ingested dsRNA (Winston et al. Reference Winston, Sutherlin, Wright, Feinberg and Hunter2007).
SID-2 is a 311 amino acid single-pass transmembrane protein that is expressed in all gut cells and localizes strongly to the apical/luminal membrane. This indicates that SID-2 may be specialized to interact with ingested dsRNA. Curiously, the presumed dsRNA-interacting extracellular domain is much less conserved that the intracellular domain. The C. briggsae species is unable to initiate RNAi from ingested dsRNA. However, C. briggsae expresses and localizes Cb-SID-2 indistinguishably from Ce-SID-2. Transgenic expression of Ce-SID-2 in C. briggsae enables environmental RNAi, suggesting either expression and/or functional differences between these two genes homologues. In contrast, expressing Cb-SID-2 in a sid-2 mutant C. elegans strain failed to rescue environmental RNAi. The functional difference between the two SID-2 proteins has been mapped by domain swap experiments to the extracellular domain (McEwan and Hunter, unpublished data). The C. elegans extracellular domain attached to the C. briggsae transmembrane and cytoplasmic domains functionally rescue sid-2 mutants. The distribution of environmental RNAi-capable species within the known Caenorhabditis phylogeny is not consistent with either a simple loss or gain of ability. Furthermore, the linkage of environmental RNAi ability with SID-2 function has only been established for C. elegans, C. briggsae, and C. remanei (M. Felix, personal communication). These observations, combined with the non-predictable nature of which species are capable of taking up ingested dsRNA, suggest that gain and loss of this ability is rapid and likely encompasses many different proteins that can perform SID-2's function.
AUTONOMOUS VERSUS SYSTEMIC RNA INTERFERENCE
Systemic RNAi is the organism-wide spread of silencing either via distribution of the initial RNAi trigger or its effectors (Jose and Hunter, Reference Jose and Hunter2007). In contrast, cell autonomous RNAi silencing is restricted to the cells and their descendants that directly encounter dsRNA by injection, infection, transfection or expression. In C. elegans, cell autonomous RNAi is the activity that remains in a sid-1 mutant. In sid-1 mutants, transgene-expressed dsRNA and dsRNA injected directly into the syncytial germline or into single gut cells causes efficient silencing in the germline and injected cell respectively, but no detectable silencing in other cells.
The RNAi silencing machinery is highly conserved, yet not all organisms have been shown to be RNAi-capable. One explanation may be that the machinery is used for TGS or RNA directed DNA elimination (Pal-Bhadra et al. Reference Pal-Bhadra, Bhadra and Birchler2002; Mochizuki, Reference Mochizuki2010). However, the lack of systemic RNAi may impede the detection of experimentally induced silencing phenotypes in many situations (Roignant et al. Reference Roignant, Carre, Mugat, Szymczak, Lepesant and Antoniewski2003). For Caenorhabditis spp. that have systemic RNAi, either injection of dsRNA or transgenc expression of Ce-SID-2 enables whole-animal experimental RNAi and even transgenerational silencing. At least one species of Caenorhabditis, C. brenneri (Caenorhabditis sp. CB5161), apparently lacks systemic RNAi. This was discovered when dsRNA targeting the large subunit of RNA polymerase caused the expected early embryonic lethal phenotype when injected directly into the syncytial germline, but in contrast to all other tested species, failed to cause any detectable phenotype when injected into intestinal cells in C. brenneri (Winston et al. Reference Winston, Sutherlin, Wright, Feinberg and Hunter2007); thus C. brenneri appears to be naturally systemic-RNAi-defective. Interestingly, the C. brenneri genomic sequence indicates that SID-1 is intact, indicating that addtional components required for systemic RNAi may be disabled or missing in this species. The apparent selection for an intact SID-1 in the absence of systemic RNAi indicates that SID-1 may have an additional function(s). While an ecologically important function for systemic RNAi in animals has not yet been reported, systemic RNAi appears to provide protection against viral spread in plants (Mourrain et al. Reference Mourrain, Beclin, Elmayan, Feuerbach, Godon, Morel, Jouette, Lacombe, Nikic, Picault, Remoue, Sanial, Vo and Vaucheret2000), but this will remain speculative until a mutant that specifically disrupts systemic RNAi is recovered.
The presence or absence of systemic RNAi in the target organism can have profound effects on both the determination of whether RNAi works in that organism and how dsRNA can be delivered effectively (Tomoyasu et al. Reference Tomoyasu, Miller, Tomita, Schoppmeier, Grossmann and Bucher2008). The wide-spread use of RNAi in Drosophila is illustrative of the challenges and solutions. Microinjection of dsRNA into early syncytial Drosophila embryos provides access to all nuclei and their trancripts, but the lack of a robust RdRP-based amplification coupled with cellularization restricts effective RNAi to genes that function in the early embryo. This can be overcome by transgene-expressed dsRNA, which bypasses the complete lack of systemic RNAi in this organism (Perrimon et al. Reference Perrimon, Ni and Perkins2010). The lack of systemic RNAi is likely due to lack of a sid-1 homologue as well as other components required for systemic RNAi (SID-1 expression in Drosophila has not yet been reported to enable systemic RNAi, despite many groups attempting this approach (personal communications). Complementing the in vivo approach, RNAi screens have been applied to a variety of Drosophila-derived cultured cell lines, like S2 cells, where dsRNA added to the culture medium is taken up via endogenous scavenging receptors that rely on the endocytosis machinery (Saleh et al. Reference Saleh, van Rij, Hekele, Gillis, Foley, O'Farrell and Andino2006; Ulvila et al. Reference Ulvila, Parikka, Kleino, Sormunen, Ezekowitz, Kocks and Rämet2006) or via transgenic expression of C. elegans SID-1 (Bartscherer et al. Reference Bartscherer, Pelte, Ingelfinger and Boutros2006).
Organisms in which RNAi works very well have both systemic RNAi and RdRP-enabled amplification of RNAi triggers, leading to speculation that they may be mutually dependent. In some organisms, like Arabidopsis, it is these amplified products that become systemically mobile (Fagard and Vaucheret, Reference Fagard and Vaucheret2000).
Viral defence has been proposed as an evolutionary explanation for systemic RNAi. In Drosophila and C. elegans, some RNAi-related genes have antiviral roles, reducing viral titres in infected cells and animals (Lu et al. 2005; Schott et al. Reference Schott, Cureton, Whelan and Hunter2005; Wilkins et al. Reference Wilkins, Dishongh, Moore, Whitt, Chow and Machaca2005; Saleh et al. Reference Saleh, Tassetto, van Rij, Goic, Gausson, Berry, Jacquier, Antoniewski and Andino2009; Ding, Reference Ding2010). However, cultured sid-1 mutant C. elegans cells were not more susceptible to viral infections than wild-type cells (Schott et al. Reference Schott, Cureton, Whelan and Hunter2005), suggesting that systemic RNAi may not play a vital role in viral defence. The recent identification of viruses that can naturally infect whole worms will provide an opportunity to test this hypothesis properly (Felix et al. Reference Felix, Ashe, Piffaretti, Wu, Nuez, Belicard, Jiang, Zhao, Franz, Goldstein, Sanroman, Miska and Wang2011). However, the systemic antiviral interferon response in mammals, which is triggered in response to long dsRNA (Sledz et al. Reference Sledz, Holko, de Veer, Silverman and Williams2003), provides a contrapositive argument to this hypothesis. In plants, viral infection induces a strong anti-viral RNAi response, which includes RdRP amplification of RNAi triggers, which then spread systemically to provide viral immunity to as yet uninfected cells and tissues (Vance and Vaucheret, Reference Vance and Vaucheret2001).
Whatever the evolutionary roles of systemic RNAi may be, it is widely regarded as a powerful addition to cell-autonomous RNAi. As stated previously, the absence of systemic RNAi is not evidence for the absence of RNAi in the organism. RNAi may simply be more difficult to trigger and therefore detect in the absence of efficient delivery to all cells. In the next section, in which we describe the mechanism of cell autonomous RNAi, we make particular note of how understanding such mechanisms can help researchers enhance cell autonomous RNAi, and therefore increase the potency of experimentally-induced RNAi.
MECHANISM OF AUTONOMOUS RNA INTERFERENCE
Mechanism of exogenous RNAi processing
In C. elegans, when exogenously introduced long dsRNA (>100 basepairs) is introduced into a cell, it is bound by a protein complex that contains RDE-4 and DCR-1. RDE-4 contains two copies of a conserved dsRNA-binding motif and binds as a dimer to dsRNA (Knight and Bass, Reference Knight and Bass2001; Tabara et al. Reference Tabara, Yigit, Siomi and Mello2002). DCR-1 is a well-conserved RNase III endoribonuclease that cleaves dsRNA into short (∼22 nucleotide) interfering RNAs (siRNAs) (Zamore et al. Reference Zamore, Tuschl, Sharp and Bartel2000; Knight and Bass, Reference Knight and Bass2001; Pak and Fire, Reference Pak and Fire2007; Habig et al. Reference Habig, Aruscavage and Bass2008). Biochemically, these double-stranded siRNAs have on each strand a 5′ monophosphate, a free 3′ hydroxyl group and 2 nucleotides of overhang at the 3′ end (Macrae et al. Reference Macrae, Zhou, Li, Repic, Brooks, Cande, Adams and Doudna2006).
The RDE-4/DCR-1 complex also includes two Dicer-related helicases of unknown function (DRH-1 and 2) (Duchaine et al. Reference Duchaine, Wohlschlegel, Kennedy, Bei, Conte, Pang, Brownell, Harding, Mitani, Ruvkun, Yates and Mello2006) as well as various members of the large Argonaute (AGO) family, defined by signature PAZ and PIWI domains (Song et al. Reference Song, Smith, Hannon and Joshua-Tor2004). The AGO proteins are thought to be the catalytic machinery of RNAi-based silencing (Czech and Hannon, Reference Czech and Hannon2011). The PAZ domain is hypothesized to interface with DCR-1 (Paddison and Vogt, Reference Paddison and Vogt2008).
In C. elegans, the Ago protein RDE-1 binds to double-strand siRNA produced by the DCR-1 complex and cleaves the passenger strand to produce a single-stranded guide siRNA (Parrish and Fire, Reference Parrish and Fire2001; Tomari et al. Reference Tomari, Matranga, Haley, Martinez and Zamore2004; Steiner et al. Reference Steiner, Okihara, Hoogstrate, Sijen and Ketting2009). In most species, the primary Ago protein – like RDE-1 – uses the guide strand to identify cognate mRNAs and, once bound, the slicer activity cleaves the mRNA between the 10th and 11th positions of the siRNA-mRNA complementary region via the activity of the Ago's RNase H catalytic domain (Hall, Reference Hall2005); this particular event seems to be absent in C. elegans (Steiner et al. Reference Steiner, Okihara, Hoogstrate, Sijen and Ketting2009). In C. elegans, single-strand siRNA produced by the sequential action of DCR-1 and RDE-1 on the long triggering dsRNA is referred to as a primary siRNA. Through still mysterious processes, an RdRP produces from the siRNA-mRNA complex many copies of so-called secondary siRNAs, that are principally anti-sense to, and distributed towards, the 5′ end of the cognate mRNA (Alder et al. Reference Alder, Dames, Gaudet and Mango2003; Pak and Fire, Reference Pak and Fire2007). In C. elegans somatic cells, the primary RdRP is RRF-1, while in the germline the primary RdRP appears to be EGO-1. These RdRPs are at least partially functionally redundant (Smardon et al. Reference Smardon, Spoerke, Stacey, Klein, Mackin and Maine2000). The 5′ end of these RdRP dependent secondary siRNAs contain triphosphate residues, indicating that they represent primary synthesis products; that is, they are not produced by DCR-1 cleavage reactions. The secondary siRNAs are both more abundant than primary siRNAs and target an expanded sequence region on the cognate mRNA. These abundant secondary siRNAs interact with so-called secondary Argonautes (SAGOs) (Yigit et al. Reference Yigit, Batista, Bei, Pang, Chen, Tolia, Joshua-Tor, Mitani, Simard and Mello2006). These secondary siRNA-SAGO complexes appear to be directly involved in sequence-dependent mRNA degradation. Since many SAGOs lack an active RNase H domain, precisely how they degrade mRNA remains unclear. It has been suggested that in C. elegans, mRNA targeted for PTGS are preferentially transported to P bodies or GW bodies (Ding et al. Reference Ding, Spencer, Morita and Han2005; Jakymiw et al. Reference Jakymiw, Lian, Eystathioy, Li, Satoh, Hamel, Fritzler and Chan2005; Liu et al. Reference Liu, Valencia-Sanchez, Hannon and Parker2005). It has been recently shown that these SAGOs, which seem to be responsible for the bulk of the silencing, are poorly conserved compared to the other RNAi components, possibly providing another reason why C. elegans RNAi is so efficient compared to that of other species (Dalzell et al. Reference Dalzell, McVeigh, Warnock, Mitreva, Bird, Abad, Fleming, Day, Mousley, Marks and Maule2011).
While RDE-1 and most SAGOs function in the cytoplasm, recent work has shown that one of the SAGOs, NRDE-3, shuttles secondary siRNAs into the nucleus. NRDE-3 has the signature PIWI and PAZ domains of an Ago protein, but also contains a nuclear localization signal required for its function (Guang et al. Reference Guang, Bochner, Pavelec, Burkhart, Harding, Lachowiec and Kennedy2008). Once inside the nucleus, NRDE-3 interacts with a complex of nuclear RNAi-silencing factors, including the well conserved novel protein NRDE-2 (Guang et al. Reference Guang, Bochner, Burkhart, Burton, Pavelec and Kennedy2010). The nuclear RNAi complex is guided by the siRNA to nascent transcripts and effects transcriptional silencing by impeding RNA polymerase elongation and recruiting histone methyltransferase activity (Guang et al. Reference Guang, Bochner, Burkhart, Burton, Pavelec and Kennedy2010). This mechanism is likely the basis for heterochromatin modifications and other transcriptional gene-silencing phenomena phenotypically linked to RNAi (Motamedi et al. Reference Motamedi, Verdel, Colmenares, Gerber, Gygi and Moazed2004; Grishok et al. Reference Grishok, Sinskey and Sharp2005; Claycomb et al. Reference Claycomb, Batista, Pang, Gu, Vasale, van Wolfswinkel, Chaves, Shirayama, Mitani, Ketting, Conte and Mello2009). The synergistic PTGS and TGS mechanisms are summarized in Fig. 4.
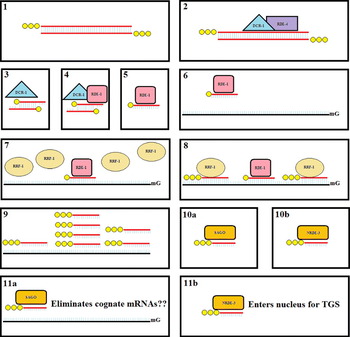
Fig. 4. Summary of the exogenous RNAi pathway in C. elegans. (1) Post delivery in vitro synthesized long (>100 bp) dsRNA (red) with 5′ triphosphate (yellow) ends is (2) bound by the RDE-4 (purple) and DCR-1 (cyan) complex. (3) The endonuclease DCR-1 dices the long dsRNA into of ∼20 bp ds-siRNAs with two nucleotide single stranded 3′ ends. The dicer products have 5′ monophosphate and 3′ hydroxyl ends. (4) Interaction with the Argonaute RDE-1 (pink) leads to slicing of the passenger strange producing (5) a single-stranded ∼22 nucleotide guide siRNA bound to RDE-1. (6) This primary ss-siRNA guides RDE-1 to its cognate mRNA (black). (7) In a mechanistically unclear step, the RdRP RRF-1 (coffee) is recruited to the RDE-1-siRNA-mRNA complex (8) leading to the production of many unprimed secondary siRNAs with 5′triphosphate ends. (9) Most of these secondary siRNAs match the originally targeted region, but secondary siRNAs anti-sense to regions both 5′ and 3′ to the originally introduced long dsRNA are also produced. (10a) In a second mechanistically unclear step, these secondary siRNAs become associated with cytoplasmic secondary Argonautes (SAGOs – orange) or (10b) the nuclear localized Argonaute NRDE-3. (11a) The secondary siRNAs then guide the cytoplasmic SAGOs to cognate mRNAs and via yet another mechanistically unclear step lead to the elimination of the mRNAs. (11b) NRDE-3 shuttles the secondary siRNAs into the nucleus where they guide transcriptional gene silencing processes.
Regulators of exogenous RNAi
Mutations that enhance RNAi silencing have been identified by various means. Mutations in genes required for production of endogenous siRNA-silencing pathways were identified in screens for enhanced neuronal RNAi (Eri mutants) and discovered serendipitously when analyzing the phenotype of worms deleted for the RdRP rrf-3 (Simmer et al. Reference Simmer, Tijsterman, Parrish, Koushika, Nonet, Fire, Ahringer and Plasterk2002; Kennedy et al. Reference Kennedy, Wang and Ruvkun2004). Another large class of mutants is in the worm Rb Tumor suppressor pathway, which appears to enhance RNAi by partial soma to germline transformation (Wang et al. Reference Wang, Kennedy, Conte, Kim, Gabel, Kamath, Mello and Ruvkun2005). It is not clear if this transformation replaces somatic RNAi with germline RNAi, which is particularly robust, or adds additional capacity to the somatic RNAi pathway. Eri mutants were initially sought for their ability to increase the discovery of RNAi phenotypes in large-scale feeding RNAi screens. For example, feeding wild-type worms 447 different RNAi foods resulted in only 307 expected loss-of-function phenotypes, while performing the same screen in the rrf-3 mutant background resulted in 436 loss-of-function phenotypes (Simmer et al. Reference Simmer, Moorman, van der Linden, Kuijk, van den Berghe, Kamath, Fraser, Ahringer and Plasterk2003). Because these mutants are enhanced for RNAi, it indicates that the wild-type eri genes function directly or indirectly to inhibit RNAi. Mechanistic investigations to date indicate that the enhanced RNAi phenotypes reflect indirect effects rather than the action of direct negative regulators.
The Eri class of enhancers are related by their facultative association with DCR-1 (Duchaine et al. Reference Duchaine, Wohlschlegel, Kennedy, Bei, Conte, Pang, Brownell, Harding, Mitani, Ruvkun, Yates and Mello2006; Gent et al. Reference Gent, Schvarzstein, Villeneuve, Gu, Jantsch, Fire and Baudrimont2009; Pavelec et al. Reference Pavelec, Lachowiec, Duchaine, Smith and Kennedy2009). To date, nine Eri loci have been described (Table 1), including five in widely conserved genes (Simmer et al. Reference Simmer, Tijsterman, Parrish, Koushika, Nonet, Fire, Ahringer and Plasterk2002; Kennedy et al. Reference Kennedy, Wang and Ruvkun2004; Duchaine et al. Reference Duchaine, Wohlschlegel, Kennedy, Bei, Conte, Pang, Brownell, Harding, Mitani, Ruvkun, Yates and Mello2006; Fischer et al. Reference Fischer, Butler, Pan and Ruvkun2008; Pavelec et al. Reference Pavelec, Lachowiec, Duchaine, Smith and Kennedy2009). These genes are required for the production or stability of endogenous siRNAs (Asikainen et al. Reference Asikainen, Storvik, Lakso and Wong2007). The current model for this eri-class is that the relatively abundant endogenous siRNAs compete with siRNAs produced from experimentally introduced dsRNA for limiting effector molecules, for example the SAGO proteins (Lee et al. Reference Lee, Hammell and Ambros2006; Yigit et al. Reference Yigit, Batista, Bei, Pang, Chen, Tolia, Joshua-Tor, Mitani, Simard and Mello2006). Thus mutations in the Eri genes reduce the number of endogenous siRNAs and indirectly increase access to limiting components of silencing pathway(s). These limiting RNAi resources have been proposed to be secondary AGOs (Yigit et al. Reference Yigit, Batista, Bei, Pang, Chen, Tolia, Joshua-Tor, Mitani, Simard and Mello2006), DICER (Mikuma et al. Reference Mikuma, Kawasaki, Yamamoto and Taira2004), and even the dsRNA channel SID-1 (Winston et al. Reference Winston, Molodowitch and Hunter2002; Calixto et al. Reference Calixto, Chelur, Topalidou, Chen and Chalfie2010); in each case, over-expression increases RNAi efficacy.
Table 1. Negative regulators of RNA interference in C. elegans

Tissue-specific differences in RNAi sensitivity among the Eri mutants provides additional support for the competition model, and further suggest that the extent of competition differs among tissues (Zhuang and Hunter, Reference Zhuang and Hunter2011). The tissue-specific differences can be explained by tissue-specific components of a competing small RNA pathway, by relative tissue-specific activities of multiple competing pathways, and even by multiple limiting resources, which may show tissue-specific biases. Interestingly, all nine Eri mutants showed robust maternal rescue and enhanced RNAi in the germline. These observations indicate that not only are these Eri genes expressed and active in the germline, but that maternally synthesized product or the product(s) of their activity is apparently well distributed to somatic tissues in the progeny. This also suggests that the maternal contribution to the embryo directly or indirectly includes small RNAs (Zhuang and Hunter, Reference Zhuang and Hunter2011).
These data indicate that exogenous RNAi capacity is regulated by or is responsive to endogenous small RNA-silencing activity levels. Thus the sensitivity of the animal to exogenous dsRNA, whether experimentally introduced, the outcome of a viral infection, or other environmental or genomic stresses, may be tuned by intrinsic or extrinsic events (e.g. pathogens, DNA damage); for instance, systemic RNAi appears to be enhanced by starvation (Winston et al. Reference Winston, Molodowitch and Hunter2002). This could reflect increased dsRNA transport or enhanced RNAi responsiveness mediated by changes in the level of endogenous siRNA levels. Analysis of the Eri class of genes indicates that the endogenous siRNA pathways are important for maturation of sperm (Gent et al. Reference Gent, Schvarzstein, Villeneuve, Gu, Jantsch, Fire and Baudrimont2009; Pavelec et al. Reference Pavelec, Lachowiec, Duchaine, Smith and Kennedy2009), and proper chromosomal segregation cannot take place without the secondary Ago csr-1 (Claycomb et al. Reference Claycomb, Batista, Pang, Gu, Vasale, van Wolfswinkel, Chaves, Shirayama, Mitani, Ketting, Conte and Mello2009). In contrast, rde-4 and rde-1 mutants, which appear to be specific to exogenous RNAi, do not seem to have any non-RNAi phenotypes (Tabara et al. Reference Tabara, Sarkissian, Kelly, Fleenor, Grishok, Timmons, Fire and Mello1999).
The nature of conservation among the eri genes should also be of interest in studying RNAi in other organisms (Altschul et al. Reference Altschul, Madden, Schaffer, Zhang, Zhang, Miller and Lipman1997) (Fig. 5, Table 1). ERI-1 is a well conserved nuclease with siRNase activity (Kennedy et al. Reference Kennedy, Wang and Ruvkun2004); RRF-3 is a well conserved RdRP (Sijen et al. Reference Sijen, Fleenor, Simmer, Thijssen, Parrish, Timmons, Plasterk and Fire2001; Crombach and Hogeweg, Reference Crombach and Hogeweg2011); the dcr-1/eri-4(mg375) mutant is a point mutation in the helicase domain of the well conserved DICER protein (Macrae et al. Reference Macrae, Zhou, Li, Repic, Brooks, Cande, Adams and Doudna2006; Pavelec et al. Reference Pavelec, Lachowiec, Duchaine, Smith and Kennedy2009); ERI-6/7 is a conserved helicase domain (Fischer et al. Reference Fischer, Butler, Pan and Ruvkun2008); and ERGO-1/ERI-8 (Pavelec et al. Reference Pavelec, Lachowiec, Duchaine, Smith and Kennedy2009) is a well conserved Ago protein. Mutations to these conserved genes in other organisms have been shown to have some similar endogenous defects, such as general RNA processing defects (Ansel et al. Reference Ansel, Pastor, Rath, Lapan, Glasmacher, Wolf, Smith, Papadopoulou, Lamperti, Tahiliani, Ellwart, Shi, Kremmer, Rao and Heissmeyer2008), but assays in RNAi efficacy have not been thoroughly performed. This area of research holds vast potential for dramatically increasing RNAi applicability and technology. Even more interesting are the potential roles played by the non-conserved eri genes specific to C. elegans or Caenorhabditis; their predicted molecular identities (Kelley and Sternberg, Reference Kelley and Sternberg2009) suggest that hydrolyases and transferases play a large role in small RNA production in C. elegans (Table 1). Perhaps such class-specific genes in other organisms hold the key to decreasing the competitive regulation of RNAi. Moreover, mutations to novel or non-conserved genes are less likely to have wide-ranging impacts, while maintaining similar degrees of RNAi hypersensitivity. Therefore, studying organism-specific eri genes through genetic screens, if possible, holds tremendous promise for understanding (and pragmatically overcoming) RNAi regulation.

Fig. 5. C. elegans ERI-1 is widely conserved. ERI-1, which is important for the production or stability of endogenous siRNA in C. elegans, has homologues in many taxonomic groups. Since it is likely that endogenous RNAi processes will compete with exogenous RNAi processes in these species, researchers should not only consider the possibility of enhancing RNAi, but the possibility that exogenous RNAi will interfere with essential endogenous processes (e.g. eri-1 and rrf-3 are required for sperm function). The taxonomic tree of C. elegans ERI-1 was created using Grishin (protein) distance, with a max sequence difference of 0 85, a fast minimum evolution parameter, and with a radial display representing inferred evolutionary distance.
Finally, given that the products of eri pathway are effective competitors of the rde pathway, it is worthwhile to examine the chemical structures of small RNAs produced by the eri pathway. The eri gene products produce endogenous siRNAs of 22 or 26 nucleotides that usually begin with a G (22 G or 26 G siRNAs) and contain a 5′ triphosphates (Conine et al. Reference Conine, Batista, Gu, Claycomb, Chaves, Shirayama and Mello2010; Gent et al. Reference Gent, Lamm, Pavelec, Maniar, Parameswaran, Tao, Kennedy and Fire2010; Vasale et al. Reference Vasale, Gu, Thivierge, Batista, Claycomb, Youngman, Duchaine, Mello and Conte2010; Welker et al. Reference Welker, Pavelec, Nix, Duchaine, Kennedy and Bass2010). Perhaps unknown chemical properties of these siRNAs are important for their relative enhanced activity. Attempting to introduce experimental siRNAs which share such properties may thus enhance RNAi efficacy as well (Kim et al. Reference Kim, Behlke, Rose, Chang, Choi and Rossi2005a).
In C. elegans, the core of the RNAi machinery that interacts with experimentally introduced RNAi signals (whether long dsRNAs or siRNAs) is a relatively well-understood framework. Recent advances in deep sequencing revealed more and more of the intricacy and potency of the endogenous small RNA network, as well as its competitive regulation of the exogenous RNAi pathway. Researchers frustrated by the limited utility of RNAi in other species should examine the RNAi regulation perspective to perhaps overcome this seeming impasse. Once the RNAi silencing signal is inside the cell, most organisms from protists to fungi, and from plants to animals, all have some part of the conserved RNAi processing machinery, whether cytoplasmic PTGS or nuclear TGS (Shabalina and Koonin, Reference Shabalina and Koonin2008). It is the relative effectiveness of RNAi that vastly differs (Maida and Masutomi, Reference Maida and Masutomi2011) and is possibly thwarting broader use of RNAi as a technological resource.
IMPLEMENTATION
The hallmarks of RNAi are specificity and potency. In C. elegans, dsRNA is not toxic and studies indicate that increasing dsRNA concentration can increase RNAi potency (Rea et al. Reference Rea, Ventura and Johnson2007; Zhuang and Hunter, Reference Zhuang and Hunter2011). Similarly, mutations in the Eri genes that reduce competition for limiting small RNA resources (Lee et al. Reference Lee, Hammell and Ambros2006) and overexpression of these limiting resources can also increase RNAi potency (Mikuma et al. Reference Mikuma, Kawasaki, Yamamoto and Taira2004; Yigit et al. Reference Yigit, Batista, Bei, Pang, Chen, Tolia, Joshua-Tor, Mitani, Simard and Mello2006; Calixto et al. Reference Calixto, Chelur, Topalidou, Chen and Chalfie2010). However, there is some possibility that these measures reduce specificity (Pavelec et al. Reference Pavelec, Lachowiec, Duchaine, Smith and Kennedy2009). Furthermore, tissues differ in their relative sensitivity: for example, neurons are fairly refractory of RNAi (Kamath and Ahringer, Reference Kamath and Ahringer2003; Kamath et al. Reference Kamath, Fraser, Dong, Poulin, Durbin, Gotta, Kanapin, Le Bot, Moreno, Sohrmann, Welchman, Zipperlen and Ahringer2003) whereas the germline is hypersensitive to RNAi (Sijen and Plasterk, Reference Sijen and Plasterk2003). Consequently, mutations that transform somatic cells towards germline can increase RNAi potency (Wang et al. Reference Wang, Kennedy, Conte, Kim, Gabel, Kamath, Mello and Ruvkun2005). Similarly, developmental stages or environmental conditions can also influence RNAi sensitivity: starved worms are slightly more sensitive to RNAi (Jose and Hunter, Reference Jose and Hunter2007). Therefore, in assaying for RNAi efficacy, the gene target expression profile, both temporal and spatial, as well as environmental conditions need to be considered for optimal phenotypic output (Visser et al. Reference Visser, Geldhof, de Maere, Knox, Vercruysse and Claerebout2006). These C. elegans tissue-specific and developmental sensitivities may be paralleled in other species and should be optimized when implementing RNAi. In summary, to determine whether RNAi is effective in a species one should first not assume that RNAi is systemic. Consequently gene-specific dsRNA must to be delivered directly to the target cells either by microinjection into syncytial tissues, for example into the germline, or transgenically expressed using either general or tissue specific promoters. Second, the expression or function of the targeted gene must be unambiguously assayed either by directly examining RNA levels (e.g. RNA in situ hybridization) or gene function. For example, for Caenorhabditis species, we injected a high concentration of species-specific RNA polymerase II subunit dsRNA directly into the germ line to phenocopy application of the RNA polyermase inhibiting toxin alpha-amanitin to embryos (Winston et al. Reference Winston, Sutherlin, Wright, Feinberg and Hunter2007).
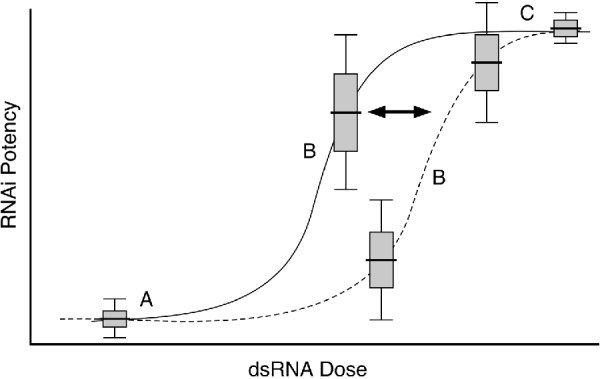
Although RNAi potency increases with dsRNA dose, it is a common misperception that this relationship is linear. When RNAi potency (phenotypic penetrance) is plotted versus dsRNA dose, one clearly observes a sigmoidal curve (Fig. 6). Curiously, for most phenotypes, the expressivity (the severity of the phenotype) is nearly constant, thus the great variability at the empirically determined intermediate dose range reflects a mixture of strongly affected and non-affected individuals. This dose sensitivity likely underlies much of variation in reported RNAi effects, which in some cases are even contradictory (Rea et al. Reference Rea, Ventura and Johnson2007). It is obviously best to use the maximum possible dose, but for potent foods, a simple dose response curve can determine an effective range with minimal variability.

Fig. 6. RNAi phenotypic penetrance is sensitive to dsRNA dose. Measurements of RNAi potency (penetrance) versus dsRNA dose show a sigmoidal relationship with high variability surround the inflection point (B). At low dsRNA dose (A) most worms do not respond, while at sufficiently high dsRNA dose (C) most or all worms do respond. However, slight variations in delivered dsRNA dose at intermediate concentrations can have dramatic effects on perceived phenotypes. Mutations that enhance RNAi tend to shift such dose-response curves toward lower dsRNA dose (solid line) without noticeably affecting the shape of the curve.
Finally, the majority of the studies on C. elegans RNAi, including those referenced in this text, specifically refer to the N2 Bristol strain – the commonly used ‘wild type’ strain for C. elegans research. However, there are variations in RNAi efficacy among wild isolates of C. elegans (Felix et al. Reference Felix, Ashe, Piffaretti, Wu, Nuez, Belicard, Jiang, Zhao, Franz, Goldstein, Sanroman, Miska and Wang2011). While the genetic basis for some of these variations is known, such as a polymorphism in a specific RNAi gene (Tijsterman et al. Reference Tijsterman, Okihara, Thijssen and Plasterk2002), other sources for such differences remain to be identified. Future research into these population-specific RNAi efficacy differences for C. elegans and other species will be extremely relevant because it provides a clue as to the evolutionary scale at which changes in RNAi pathways may occur.
It seems reasonable to apply the lessons of the deep mechanistic and phenomenological observation made in C. elegans, as a first step towards enabling the highest probability of optimizing RNAi in other species. There will inevitably be species in which RNAi does not work, but the conservation of basal RNAi machinery suggests that more often than not, RNAi will function in most species.