Introduction
Parasites are a major problem, both in terms of their influence on human health and their economic significance in animal husbandry (Petney, Reference Petney1997). Over 100 species of food-borne trematodes are known to parasitize humans, many of which also infect domestic animals (Keiser and Utzinger, Reference Keiser and Utzinger2009). At least 750 million people are at risk of infection by food-borne trematodes and more than 40 million people are infected (Hotez et al., Reference Hotez, Brindley, Bethony, King, Pearce and Jacobson2008; Keiser and Utzinger, Reference Keiser and Utzinger2009). All these trematodes use gastropods as intermediate hosts in their life cycles and many employ freshwater fish or crustaceans as second intermediate hosts. Second intermediate host species are often used in aquaculture, a valuable source of nutrition, employment and export trade, especially in developing countries. Infection of these hosts by metacercariae of food-borne trematodes is a cause of considerable economic losses in aquaculture (Keiser and Utzinger, Reference Keiser and Utzinger2005; Clausen et al., Reference Clausen, Madsen, Murrell, Phan Thi, Nguyen Manh, Viet and Dalsgaard2012).
In order to incorporate the parasite ecology into the epidemiology of infections and to increase efficient control and prevention measures in the future, the correct identification of trematodes at all life-cycle stages is important. The transmission of digenetic trematodes from the snail to the next host in the life cycle depends largely on the proportion and density of snails that release cercariae, as well as the number of cercariae released from each infected snail (Anderson and May, Reference Anderson and May1991; Petney et al., Reference Petney, Sithithaworn, Andrews, Kiatsopit, Tesana, Grundy-Warr and Ziegler2012; Kiatsopit et al., Reference Kiatsopit, Sithithaworn, Kopolrat, Namsanor, Andrews and Petney2015). In Thailand, the family Bithyniidae is represented by three genera. However, only snails of the genus Bithynia are of medical importance (Brandt, Reference Brandt1974). There are three currently recognized taxa in this genus that have been reported as intermediate hosts of Opisthorchis viverrini. These taxa are widely distributed in continental Southeast Asia and appear to be extremely susceptible to diverse trematode infections (Brandt, Reference Brandt1974; Lohachit, Reference Lohachit2004–2005; Ngern-klun et al., Reference Ngern-klun, Sukontason, Tesana, Sripakdee, Irvine and Sukontason2006; Sri-Aroon et al., Reference Sri-Aroon, Butraporn, Limsoomboon, Kaewpoolsri, Chusongsang, Charoenjai, Chusongsang, Numnuan and Kiatsiri2007; Chontananarth et al., Reference Chontananarth, Wongsawad and Chai2013; Tesana et al., Reference Tesana, Thapsripair, Suwannatrai, Harauy, Piratae, Khampoosa, Thammasiri, Prasopdee, Kulsantiwong, Chalorkpunrut and Jones2014). Our recent report revealed that as many as 20 different types of trematode cercariae are found in Bithynia siamensis goniomphalos (Kiatsopit et al., Reference Kiatsopit, Sithithaworn, Kopolrat, Namsanor, Andrews and Petney2015). In addition to O. viverrini, several groups of trematodes can be found concurrently in B. s. goniomphalos, including virgulate xiphidiocercariae. In endemic areas of O. viverrini, B. s. goniomphalos have varying trematode prevalence levels that are associated with seasonal and host factors (Kiatsopit et al., Reference Kiatsopit, Sithithaworn, Saijuntha, Boonmars, Tesana, Sithithaworn, Petney and Andrews2012; Namsanor et al., Reference Namsanor, Sithithaworn, Kopolrat, Kiatsopit, Pitaksakulrat, Tesana, Andrews and Petney2015). We recently reported the influence of water irrigation schemes and seasonality on transmission dynamics of O. viverrini in B. s. goniomphalos in the rice paddy fields in northeast Thailand (Kopolrat et al., Reference Kopolrat, Sithithaworn, Kiatsopit, Namsanor, Laoprom, Tesana, Andrews and Petney2020). In addition to O. viverrini, this study also reported several other trematode cercariae found in B. s. goniomphalos, but how the environmental conditions, i.e. irrigation and seasonality affect the transmission of these trematodes is not clear. Furthermore, data on host–parasite interactions between B. s. goniomphalos and their multi-trematode parasites are limited, with most coming from cross-sectional surveys of the prevalence of infections. Long-term studies to capture yearly variation, the effect of seasonality and human land use have not been reported. We hypothesize that seasonality and land use for agriculture have a profound impact on transmission dynamics of trematode cercariae in B. s. goniomphalos similar to that observed in the case of O. viverrini cercariae previously reported (Kopolrat et al., Reference Kopolrat, Sithithaworn, Kiatsopit, Namsanor, Laoprom, Tesana, Andrews and Petney2020).
To understand the transmission dynamics of trematode parasites in their snail hosts, we investigated the impact of season, year-to-year variation, amount of rainfall and snail host factors, as well as fluctuations in the release of irrigation water, on the prevalence and diversity of trematode infection in B. s. goniomphalos.
Materials and methods
Study area and sampling periods
The study area was a rice paddy-field habitat of B. s. goniomphalos in Sakon Nakhon Province, northeast Thailand, the same area as discussed in our previous report where detailed ecology and land-use practices in paddy rice cultivation were described (Kopolrat et al., Reference Kopolrat, Sithithaworn, Kiatsopit, Namsanor, Laoprom, Tesana, Andrews and Petney2020). The area has a tropical monsoon climate with the year being divided into three seasons. The hot-dry season is from March to May, the rainy season and cool-dry season occur from June to October and November to February, respectively (Namsanor et al., Reference Namsanor, Sithithaworn, Kopolrat, Kiatsopit, Pitaksakulrat, Tesana, Andrews and Petney2015).
Snail collection and cercarial shedding
To measure the prevalence of trematode infection in naturally occurring snail populations, monthly collections were made at ten sampling sites in an irrigated area of approximately 4879 m2 from January 2010 to December 2013. Snails were collected by handpicking from objects and solid surfaces and by dredging the sediment with a scoop from shallow water for 10 min/site by two collectors. The snails were then cleaned, blotted dry and placed into plastic bags, and transported to the laboratory where they were identified using standard morphological criteria (Brandt, Reference Brandt1974; Upatham et al., Reference Upatham, Sornmani, Kitikoon, Lohachit and Bruch1983; Chitramvong, Reference Chitramvong1992). We obtained monthly data on the rainfall (mm), and release of irrigation water for the study area (×105 m3) during the sampling period from the Thai Meteorological Office and the Royal Irrigation Department.
Trematode infection in B. s. goniomphalos was examined by cercarial shedding (Kiatsopit et al., Reference Kiatsopit, Sithithaworn, Saijuntha, Boonmars, Tesana, Sithithaworn, Petney and Andrews2012). Individual snails were placed separately into a plastic container (3 cm in diameter by 2.5 cm high) filled with dechlorinated tap water. The containers were covered with a lid studded with pins to prevent the snail from escaping. The snails were then exposed to a light intensity of 1200 lux, with the lamp placed 30 cm above the container, for 5 hours during the daytime at room temperature (25 ± 2°C). The presence of cercariae in water was observed under a stereoscopic microscope. Trematode cercariae were identified morphologically under a high-magnification compound microscope. Cercariae were fixed with 1% iodine and were photographed using software DP2-BSW by Olympus (Olympus DP 25; Olympus, Tokyo, Japan) fitted to an Olympus BX 51 microscope. Cercariae were identified using keys or other information as in Ditrich et al. (Reference Ditrich, Schoiz, Aguiree-Macedo and Vargas-Vazquez1997); Ito et al. (Reference Ito, Papasarathorn and Tongkoom1962); Schell (Reference Schell1970) and Yamaguti (Reference Yamaguti1975).
From the initial shedding, those snails infected with either O. viverrini or with virgulate or those with mixed infections of the two were allowed to stay in the dark for 12 h under laboratory conditions before being exposed to light from 6.00 am to 6.00 pm, as above. The cercariae were counted under a stereomicroscope after staining with 1% Lugol's iodine solution.
To investigate the influence of snail size on prevalence, the shell length was measured using digital vernier calipers. The length of a snail shell was measured between the tip of the apex and the lower edge of the lip.
Diversity index calculation
The following parameters and indices were used to describe species abundance and diversity (Spellerberg and Fedor, Reference Spellerberg and Fedor2003; Tabbabi et al., Reference Tabbabi, Ghrab, Aoun, Ready and Bouratbine2011). Relative abundance, defined as the mean proportion of cercariae of each species contributed by a single snail individual, was calculated and expressed as a percentage to assess dominance. Species richness was defined as the total number of trematode species in sampled snails. Shannon's diversity index (H) was computed to evaluate the trematode diversity across infected snails with the following formula:
where pi is the proportion of snails infected with the ith trematode species (i.e. a relative abundance of species i). A higher value indicates a large number of species with similar abundances, whereas a lower value indicates low diversity dominated by one or a few species (Hill, Reference Hill2005). Species evenness (E), describing equality of the prevalence of each trematode species, was computed with the following formula:
where S is the total number of species/types (i.e. species richness) and H refers to Shannon's diversity index. The values of E range from 0 to 1; values closer to zero represent communities that are dominated by one species, while values closer to 1 represent communities comprised of several species with similar abundances (Hillebrand, Reference Hillebrand and Fath2008).
Statistical analyses
Data were analysed using SPSS version 21.0 software (IBM Software Company, Chicago, IL) and GraphPad Prism 5 (La Jolla, CA). Environmental profile data and prevalence of all trematode infections combined in B. s. goniomphalos were obtained at monthly intervals. By using individual snails as a study unit, odds ratios (OR) with 95% confidence intervals (CI) from logistic regression analyses were calculated to investigate the association between environmental variables (rainfall and irrigation water) and the prevalence of all trematode infections combined. Factors associated with the prevalence of trematode infections were first analysed using univariate analysis, and covariates were considered for further analysis using a mixed-effects multivariable logistic regression model. To avoid collinearity in the multivariable models, the covariance of the selected variables was investigated pairwise to determine the correlation among the variables. Since the patency of trematodes takes around 4 weeks in a snail (Sorensen and Minchella, Reference Sorensen and Minchella2001; Mohammed et al., Reference Mohammed, Madsen and Ahmed2016), a lag period of 4 weeks is included in data analysis in the logistic regression analyses. The prevalence of all trematode cercariae in B. s. goniomphalos was calculated as a percentage for each cercarial type. χ 2 tests were performed to compare the prevalence of all trematode infections combined between seasons in different study years. Independent t-tests and analysis of variance (ANOVA) were used for normally distributed data to assess size differences between groups of infected and uninfected snails. The correlation of cercarial emergences/snail/day between single and mixed infections was evaluated by linear regression and Pearson's correlation coefficient. Kruskal–Wallis tests were used to compare cercarial emergences/snail/day between single and mixed infections. A P value of <0.05 was considered statistically significant.
Results
Profiles of trematode infection, environmental and irrigation factors
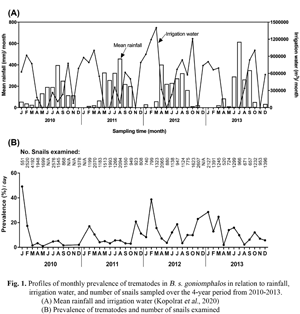
The patterns of rainfall were consistent between years while the duration of irrigation and amount of irrigated water varied during the study period as previously reported (Kopolrat et al., Reference Kopolrat, Sithithaworn, Kiatsopit, Namsanor, Laoprom, Tesana, Andrews and Petney2020) (Fig. 1). Rainfall averages about 120–170 mm per month and falls mostly between April and the end of October. The amount of irrigated water in the whole study area increased during 2011 and again at the start and towards the end of 2012 and remained high until mid-2013. The abundance of snails varied widely over the sampling period, but there was no relationship with the season. The total number of snails collected was 59 727 and the highest number collected on a single occasion was 4192 in March 2010.

Fig. 1. Profiles of monthly prevalence of trematodes in Bithynia siamensis goniomphalos in relation to rainfall, irrigation water and number of snails sampled over the 4-year period from 2010 to 2013. (A) Mean rainfall and irrigation water (Kopolrat et al., Reference Kopolrat, Sithithaworn, Kiatsopit, Namsanor, Laoprom, Tesana, Andrews and Petney2020) and (B) prevalence of trematodes and number of snails examined.
As reported previously (Kopolrat et al., Reference Kopolrat, Sithithaworn, Kiatsopit, Namsanor, Laoprom, Tesana, Andrews and Petney2020), 17 types of trematodes cercariae belonging to eight taxonomic groups were found in B. s. goniomphalos snails (Fig. S1). The details of snail samples categorized by infection status and sampling times by season and year are shown in Table 1. The monthly prevalence of all trematode cercariae was variable over season and study years (Fig. 1).
Table 1. Numbers and percent of Bithynia siamensis goniomphalos samples categorized by the status of trematode infection and sampling years and seasons

Association between the prevalence of trematode infection and environmental variables
The results by multivariable logistic regression model with a lag period of 4 weeks to allow cercarial development in the snail host shown as crude (cOR) and adjusted (aOR) odd ratios (i.e. by year, season, rainfall, irrigated water and snail size) are shown in Table 2. There was an association between the prevalence of all trematode infections combined in B. s. goniomphalos and with reference to 2010, the aOR was significant for 2012 and 2013 (P < 0.001). Considering the seasonal factor in using the hot-dry season as the reference, there was a significant association of all trematode prevalence with the cool-dry season (aOR = 2.779, P < 0.001). The prevalence of trematode infections was found to be negatively associated with the amount of rainfall, i.e. increased rainfall volume reduced the risk of trematode infection in B. s. goniomphalos (P < 0.001). There was a significant association between the prevalence of trematode infections and levels of irrigation water, in comparison with the baseline level, the aORs being 1.579 and 1.395 for medium and high irrigation water categories, respectively (P < 0.001). In addition, the prevalence of trematode infections was significantly associated with the snail size (shell length) when adjusted by year and season (P < 0.001), i.e. larger snail size had a higher risk of trematode infection.
Table 2. Associations between prevalence of combined trematode infection and environmental (season, rainfall and irrigated water) and biological factors (size of snail)

Data presented were analysed by logistic regression model showing cOR and aOR with 95% CI and P values.
aOR by a: season, b: year, c: year, season and irrigated water, d: year, season and rainfall, e: year and season.
**, *** indicate cORs with a significance level of P < 0.01 and P < 0.001, respectively.
Profiles of trematode infection and seasonality
The prevalence of all cercarial infections in B. s. goniomphalos for each year and season is shown in Fig. 2. Overall, the prevalence varied significantly between years (P < 0.001) and season (P < 0.001). In 2010, the highest prevalence occurred in the cool-dry season, followed by the rainy season and the lowest prevalence was in the hot-dry season (P < 0.001). In 2011, the prevalence peaked in the cool-dry season and was low in the hot-dry and rainy seasons (P < 0.001). In 2012, the greatest prevalence was in the cool-dry season being significantly higher than in the hot-dry and rainy seasons (P < 0.001). In 2013, peak prevalence occurred in hot-dry, followed by the rainy season and the lowest prevalence was in the cool-dry season (P < 0.001).

Fig. 2. Prevalence of single and multiple trematode infections in B. s. goniomphalos at different seasons and years. Single trematode infections (A, C, E and G). Data shown are percent positive of infection in the snails and number positive over the number of snails examined. Multiple trematode infections (B, D, F and H). *P < 0.05; **P < 0.01; ***P < 0.001.
Only 80 snails (0.14%) were parasitized with more than one type of trematode larvae. Table 3 details the single infections with each trematode type. Almost all the multiple infections were double infections with virgulate cercariae being one of the groups involved (Table S1). Only in a single snail was echinostome cercariae 1 and 2 found together. Within six multiply infected snails, O. viverrini cercariae existed together with virgulate cercariae. As shown in Fig. 3, there was a significant correlation between the prevalence of single and multiple trematode infections (R 2 = 0.77, P < 0.001).

Fig. 3. Relationship between monthly prevalence of single and multiple (double) trematode infections in B. s. goniomphalos (dots) during the 4-year study period.
Table 3. Seasonal and yearly prevalence of trematode diversity in B. s. goniomphalos in Sakon Nakhon Province, Thailand

Data shown are number and percent positive snails.
The diversity of trematodes across year and season
Details of species richness, abundance, diversity and evenness separated by year and season are shown in Table S2. The species richness peaked in the cool-dry season except in 2012–2013 when it peaked in the hot-dry season. Similarly, abundances were highest in the cool-dry season in 2010–2012 but the hot-dry season in 2013. Shannon index and species evenness are variable and inconsistent with no clear pattern.
Snail size distribution in different seasons
A total of 6222 snails were included for size comparisons from 2011 to 2013. Of these, 2433 snails were infected by virgulate cercariae combined, and 381 snails were infected by O. viverrini cercariae. The frequency distributions of snail size (shell length) between uninfected compared with infected snails by virgulate and O. viverrini cercariae during the different seasons of each year are shown in Fig. 4. The shell length was significantly influenced by season and infection status (ANOVA, P < 0.001). Over 3 years, snails infected with virgulate and O. viverrini cercariae were significantly larger than uninfected snails in all seasons (t-test, P < 0.001) but were similar in the cool-dry season in 2012. The average shell length ranged from 8.76 to 10.69 mm. Small snails (<8 mm) made up 6.12% of those measured, 48.34% were pre-reproductive and of medium size (8–10 mm) and 45.54% were reproductive, large snails (>10 mm).

Fig. 4. Comparison of snail size distributions in different seasons between the snails with virgulate (xiphidiocercaria) and Opisthorchis viverrini cercariae in comparison with uninfected snails (vertical dotted line represents the mean shell length of uninfected snails). (A) 2011, (B) 2012 and (C) 2013.
Cercarial emergence of single and double trematode infection
In the March 2013 sample, there were 51 snails infected with O. viverrini cercariae and 188 virgulate cercariae-infected snails. There were five snails with mixed infections which were used for cercarial emergence examinations (Fig. 5). Opisthorchis viverrini cercarial emergence was similar in single and in double infections. Significantly more virgulate cercarial emerged in single infections compared with double infections (P < 0.05).

Fig. 5. Effects of mixed infection on the cercarial emergence of O. viverrini (A) and virgulate 1 (B) in B. s. goniomphalos.
Discussion
One of the main findings in this study is the discovery of up to 17 types of cercariae, including O. viverrini, in B. s. goniomphalos from a single locality compared with 20 types previously reported across several localities (Kiatsopit et al., Reference Kiatsopit, Sithithaworn, Kopolrat, Namsanor, Andrews and Petney2015), indicating the rich trematode fauna in the rice-paddy habitat which receives regular irrigation water. Our data also confirm the high trematode diversity and susceptibility to infection in B. s. goniomphalos. All trematode cercariae were found in 9.95% of the snails sampled, with the highest prevalence (49.18%) in the cool-dry season in January 2010. The 17 morphologically identified larval trematodes included types similar to those found in other studies (Ito et al., Reference Ito, Papasarathorn and Tongkoom1962; Wykoff et al., Reference Wykoff, Harinasuta, Juttijudata and Winn1965; Ditrich et al., Reference Ditrich, Scholz and Giboda1990, Reference Ditrich, Nasincova, Scholz and Giboda1992; Giboda et al., Reference Giboda, Ditrich, Scholz, Viengsay and Bouaphanh1991; Adam et al., Reference Adam, Arnold, Pipitgool, Sithithaworn, Hinz and Storch1993; Nithiuthai et al., Reference Nithiuthai, Wiwanitkit, Suwansaksri and Chaengphukeaw2002; Lohachit, Reference Lohachit2004–2005; Sri-Aroon et al., Reference Sri-Aroon, Butraporn, Limsomboon, Kerdpuech, Kaewpoolsri and Kiatsiri2005, Reference Sri-Aroon, Butraporn, Limsoomboon, Kaewpoolsri, Chusongsang, Charoenjai, Chusongsang, Numnuan and Kiatsiri2007; Tesana et al., Reference Tesana, Thapsripair, Suwannatrai, Harauy, Piratae, Khampoosa, Thammasiri, Prasopdee, Kulsantiwong, Chalorkpunrut and Jones2014; Kiatsopit et al., Reference Kiatsopit, Sithithaworn, Kopolrat, Namsanor, Andrews and Petney2015). Previous studies have also shown that individual species of snails can act as intermediate hosts for several trematode species (Sousa, Reference Sousa1993; Esch et al., Reference Esch, Curtis and Barger2001; Loy and Haas, Reference Loy and Haas2001).
Natural infections of O. viverrini in B. s. goniomphalos were few. The monthly infection rates in our study ranged from 0.01 to 4.10%, and the average infection rate was 0.71% (Kopolrat et al., Reference Kopolrat, Sithithaworn, Kiatsopit, Namsanor, Laoprom, Tesana, Andrews and Petney2020). These results were similar to Brockelman et al. (Reference Brockelman, Upatham, Viyanant, Ardsungnoen and Chantanawat1986) and Lohachit (Reference Lohachit2004,–2005) who reported infection rates of 0.10–0.36%. In contrast, the virgulate group was present in 7.84% of all snails that we examined. Virgulate cercaria is a stage in the life-cycle of a very complex group of trematodes with many different subgroups (Schell, Reference Schell1970). Kiatsopit et al. (Reference Kiatsopit, Sithithaworn, Kopolrat, Namsanor, Andrews and Petney2015) and Namsanor et al. (Reference Namsanor, Sithithaworn, Kopolrat, Kiatsopit, Pitaksakulrat, Tesana, Andrews and Petney2015) recently reported that B. s. goniomphalos shows particularly high susceptibility to infection by virgulate cercariae, making them the dominant cercarial fauna. Moreover, this particular group of cercariae can be found in other snails, such as Melanoides tuberculata (Krailas et al., Reference Krailas, Namchote, Koonchornboon, Dechruksa and Boonmekam2014), Thiara scabra (Ukong et al., Reference Ukong, Krailas, Dangprasert and Channgarm2007) in Thailand and Biomphalaria tenagophila from South America (Moraes et al., Reference Moraes, Silva, Ohlweiler and Kawano2009).
The strongest factor relating to high all trematode prevalence rates and double infection was the season, with a high prevalence in the cool-dry season in 2010–2012. However, this is not consistent as prevalence peaked in the hot-dry season in 2013. Seasonal variations are the major extrinsic factor associated with prevalence, particularly the amount of rainfall plays an important role in the complex interplay between host and parasite (Brockelman et al., Reference Brockelman, Upatham, Viyanant, Ardsungnoen and Chantanawat1986). Seasonal changes in rainfall cause marked fluctuations in the transmission rates of diseases and parasites (Mouritsen and Poulin, Reference Mouritsen and Poulin2002; Cattadori et al., Reference Cattadori, Haydon and Hudson2005; Kim et al., Reference Kim, Dobson, Gulland, Harvell, Norse and Crowde2005; Altizer et al., Reference Altizer, Dobson, Hosseini, Hudson, Pascual and Rohani2006).
In addition to environmental factors, our study found that irrigation intensity in 2012 also influenced all trematode prevalence rates in the snails. In this study, we showed an increase in the prevalence of all trematode infections combined in B. s. goniomphalos with an increasing quantity of irrigation water (45.36–64.67%) in 2012 compared with 2011 and 2013. Irrigation in paddy-field agriculture makes a second crop of rice in the cool-dry season possible. This practice can alter the natural cycle of snails by increasing the snail population density in rice fields, resulting in a higher prevalence in the hot-dry season in 2013 as opposed to the conventional peak prevalence in the cool-dry seasons in 2010–2012. The changes in the seasonal pattern are probably due to irrigation ditches being functionally connected to domestic and wild animals, human and snail hosts through the transport of feces from households and villages. In areas where sanitary facilities are underdeveloped, human or animal reservoirs host feces containing trematode eggs that can be flushed into water bodies directly or through irrigation ditches in the monsoon season. Trematode eggs from animal and human hosts are likely to sediment quickly and may not disperse over a large distance unless substantial water flow occurs, however, infected snails may travel with water flow to the paddy fields. Thus, the biological characteristics of the cercariae, the snail host population together with the environmental conditions, play important roles in trematode transmission (Haas, Reference Haas1994; Petney et al., Reference Petney, Sithithaworn, Andrews, Kiatsopit, Tesana, Grundy-Warr and Ziegler2012; Ziegler et al., Reference Ziegler, Petney, Grundy-Warr, Andrews, Baird, Wasson and Sithithaworn2013; Wang et al., Reference Wang, Ho, Feng, Namsanor and Sithithaworn2015).
Interestingly, the virgulate type, being the most common class of cercariae in B. s. goniomphalos, was a major type found in double infections. Nevertheless, only 0.14% of the infected snails harboured more than one larval trematode species, which is not unexpected based on the low percentages reported in previous studies (Sousa, Reference Sousa, Esch, Bush and Aho1990, Reference Sousa1993; Lafferty et al., Reference Lafferty, Sammond and Kuris1994). This finding may represent inter-specific competition among trematode parasites, including O. viverrini, occurring in the natural environment (Bayne and Loker, Reference Bayne, Loker, Rollinson and Simpson1987; Jourdane and Theron, Reference Jourdane, Theron, Rollinson and Simpson1987). Some trematode parasites, particularly the virgulate group, may have evolved their competitive ability by becoming more efficient resource users and by developing interference mechanisms that keep competing species from using scarce resources. Thus, virgulate cercariae are the most successful group parasitizing the field population of B. s. goniomphalos, whereas cercariae of O. viverrini are only of moderate prevalence in the paddy-field habitat.
How the virgulate trematode interacts with O. viverrini is not known. Nevertheless, the fact that they are smaller in body size compared with O. viverrini (Schell, Reference Schell1970) and appear to have lower rates of cercarial emergence in mixed infections, suggests that they are unlikely to exert a substantial negative effect on O. viverrini. Laboratory studies of freshwater snail–trematode associations have demonstrated the presence of strong antagonistic interactions between the intra-molluscan of redia and sporocyst stages of species that infect the same host individual and that double infections are more pathogenic to the snails when compared to infections of only one trematode species (Lim and Heyneman, Reference Lim and Heyneman1972; Sousa, Reference Sousa1992; Lafferty et al., Reference Lafferty, Sammond and Kuris1994). The competition among larvae from different trematode species inside mollusks can reduce both parasite number and the snail population (Basch et al., Reference Basch, Lie and Heyneman1969; Lim and Heyneman, Reference Lim and Heyneman1972; Frandsen, Reference Frandsen1987). Larvae of Schistosoma mansoni do not develop in B. tenagophila previously infected by longifurcate cercariae with or without an eyespot (Machado et al., Reference Machado, Magalhaes, de Artigas, da Cordeiro and de Carvalho1988). These authors observed that mollusks infected by echinostome and other cercariae were protected from S. mansoni infection at about 73 to 87%, respectively. The time of the first infection may have some impact on additional cercarial infections, and internal mechanisms repel further cercarial infections in an already infected snail. Fluke development in intermediate hosts depends on genetic factors as well as environmental factors, which might facilitate eggs and miracidia dispersion (Williams and Esch, Reference Williams and Esch1991; Fernandez and Esch, Reference Fernandez and Esch1991a, Reference Fernandez and Esch1991b). Since the trematode infection is usually harmful to the mollusk host, the impact of double or even triple infections on the snail host should be investigated in the future. In addition, a similar study in a paddy rice field that received no irrigation is needed for comparison.
The current study revealed that the virgulate species and O. viverrini tended to infect larger snails. This is consistent with the results of Namsanor et al. (Reference Namsanor, Sithithaworn, Kopolrat, Kiatsopit, Pitaksakulrat, Tesana, Andrews and Petney2015) who showed that larger snails were more susceptible to virgulate infection than were small snails. For snail–trematode interactions, gigantism is often postulated to benefit the parasite as a larger host provides a greater volume for parasite occupation and reproduction (Sandland and Minchella, Reference Sandland and Minchella2003; McCarthy et al., Reference McCarthy, Fitzpatrick and Irwin2004). In the case of O. viverrini, a possible explanation involves the irrigation water. Our previous report shows that the prevalence of O. viverrini was positively associated with the size of B. s. goniomphalos in 2010, 2011 and 2013, but the relationship was reversed in 2012 (Kopolrat et al., Reference Kopolrat, Sithithaworn, Kiatsopit, Namsanor, Laoprom, Tesana, Andrews and Petney2020). This may be partially explained by the fact that in 2012 there were more snails available as a result of more irrigated water, hence more small snails (pre-reproductive size) were infected compared with larger snails in the rainy and cool-dry seasons.
In conclusion, this study demonstrated that B. s. goniomphalos in paddy-field habitats supports a high diversity of trematode fauna led by virgulate cercariae and followed by O. viverrini. Irrigation water for rice cultivation has a strong influence on the transmission dynamics of the trematodes by supporting the abundance of snails and hence shifting the peak of all trematode prevalence in the snails from the cool-dry to the hot-dry season. The findings of very low levels of mixed trematode infections and potential interference of cercarial emergence in virgulate cercariae, but not O. viverrini, indicates the importance of trematodes developing in the snail, which may contribute to host gigantism and parasite-induced mortality. Taken together, the observed results suggest that interactions between host and parasite depend not only on a specific characteristic of the parasite but also the environmental factors including irrigation practices for rice cultivation, which affects the transmission dynamics. In addition, this 4-year study shows that variation between years can be considerable and that short-term studies are unlikely to unravel the complexity of factors influencing trematode population biology.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021001992.
Acknowledgements
We would like to acknowledge Prof David Blair for editing the manuscript via Publication Clinic KKU, Thailand. We thank the Provincial Meteorological Office of Sakon Nakhon Province, Thailand, for providing climate and environmental data. We are also grateful to the chief of Phang Khon District Agriculture Department, Sakon Nakhon Province, Thailand, for data for the rice production area and rice production. Data for the irrigation water were supplied by the Royal Irrigation Department, Sakon Nakhon Province, Thailand.
Author contribution
Conceived and designed the experiments: PS, ST and KYK. Performed the experiments: KYK, NK, JN, OP, PY and PS. Analysed and interpreted the data: KYK and PS. Contributed reagents/materials/analysis tools: PS, ST and WS. Writing-original draft: KYK. Writing-review and editing: PS, RHA and TNP
Financial support
This study described here was supported by the Cholangiocarcinoma Research Institute, Khon Kaen University, and Fluke-Free Thailand, National Research Council of Thailand.
Conflict of interest
None.