INTRODUCTION
Parasites have evolved a wide array of holdfast mechanisms that maximize the likelihood of successful attachment upon recruitment to their hosts and minimize the risk of subsequent dislodgment (Poulin, Reference Poulin2009; Randhawa and Poulin, Reference Randhawa and Poulin2010). Selective pressures on morphology are especially strong in parasites living in the lumen of the gastrointestinal tract, where physical disturbance in the form of peristalsis and food movement can exert powerful drag on attached parasites (Poulin, Reference Poulin2009). Acanthocephalans, in particular, have developed a proboscis armed with hooks that anchor to the gut of their definitive host (Taraschewski, Reference Taraschewski2000). Many species also have trunk spines that engage on the gut surface, sometimes playing a significant role in attachment (Van Cleave, Reference Van Cleave1952; Aznar et al. Reference Aznar, Bush, Fernández and Raga1999a, Reference Aznar, Bush and Raga2002a). It has been argued that investment in these primary holdfast structures is optimized for the species of host and the particular microhabitat where each species of acanthocephalan lives (Poulin, Reference Poulin2007). A possible reason is that attachment structures are costly to produce and, therefore, it would not be advantageous for a worm to produce them larger than the size necessary to ensure attachment (Poulin, Reference Poulin2007). Also, depending on the size of the animal, the size of holdfast structures should also be bounded within certain limits to ensure that attachment performance is functional (Van Cleave, Reference Van Cleave1952; see also Koehl, Reference Koehl1996).
Interestingly, both the proboscis and trunk spines of acanthocephalans are generated prior to being used for attachment, and this raises the question of how investment in such structures could optimally be allocated through ontogeny. The first larval stage, the acanthor, hatches from the egg and passes through 2 subsequent stages, the acanthella and the cystacanth, within an intermediate arthropod host; many acanthocephalans may also use a paratenic host (usually a vertebrate) in which the cystacanth gets encysted in the mesentery without further development (Schmidt, Reference Schmidt, Crompton and Nickol1985). The cystacanth is the infective stage that is consumed by the definitive vertebrate host and already has all the primary attachment structures of the adult. Van Cleave (Reference Van Cleave1952) and Petrochenko (Reference Petrochenko1956) suggested that, in most species, attachment structures are fully formed at the cystacanth stage, perhaps as an investment priority of the developing worm to secure successful establishment upon arrival to the definitive host. Indeed, to the best of our knowledge, the number and arrangement of hooks in the proboscis and the extension of spines on the trunk are never modified in the definitive host (Van Cleave, Reference Van Cleave1952). However, the extent to which the proboscis, proboscis hooks, and trunk spines grow during the adult development is an open question. Some authors reported no changes in the size of proboscis and/or proboscis hooks between cystacanths and adults of some species (Podesta and Holmes, Reference Podesta and Holmes1970; Amin et al. Reference Amin, Heckmann, Mesa and Mesa1995, Reference Amin, Heckmann and Van Ha2004). Other authors, however, noted an increase in the size of proboscis hooks or trunk spines in adults of different species compared to cystacanths (Podesta and Holmes, Reference Podesta and Holmes1970; Amin et al. Reference Amin, Heckmann, Mesa and Mesa1995), or juveniles i.e. recently recruited worms in the definitive host (Amin, Reference Amin1986, Reference Amin1987).
In any of the above studies it is difficult to separate the putative growth of the holdfast from measurement error because none used inferential statistics. However, it seems likely that the timing of growth of attachment structures may differ among species of acanthocephalan depending on their body size. Adult acanthocephalans are subject to the unsteady flow of digested food generated by peristalsis (Poulin, Reference Poulin2007). Although the physical properties of the flow of digesta are far from clear (see Schulze, Reference Schulze2006), acanthocephalans are theoretically expected to experience 3 types of dislodging forces i.e. frictional drag, pressure drag, and acceleration reaction, which are proportional to the surface area, sectional area, and volume of the body, respectively (see Koehl, Reference Koehl1984, for details). Thus, everything else being equal, dislodging forces should increase disproportionately as the body grows, and larger acanthocephalans could therefore need a finer adjustment of their holdfast structures during the adult growth, particularly if they experience a greater change of body size from the cystacanth to the adult stage (see Poulin et al. Reference Poulin, Wise and Moore2003).
In this study we compared the size of trunk spines between cystacanths and adults of 2 congeneric species of acanthocephalans from the Southern Hemisphere that clearly differ in size, namely Corynosoma cetaceum and C. australe (Fig. 1). Individuals of C. cetaceum inhabit the stomach and upper duodenum of small cetaceans, whereas C. australe is found in the intestine, mainly in the ileum and jejunum, of pinnipeds (Aznar et al. Reference Aznar, Bush, Balbuena and Raga2001, Reference Aznar, Cappozzo, Taddeo, Montero and Raga2004, Reference Aznar, Hernández-Orts, Suárez, García-Varela, Raga and Cappozzo2012; Sardella et al. Reference Sardella, Mattiucci, Timi, Bastida, Rodríguez and Nascetti2005). We focused on trunk spines because they play a key role in the attachment of species of Corynosoma (Van Cleave, Reference Van Cleave1952; Aznar et al. Reference Aznar, Bush, Fernández and Raga1999a; Reference Aznar, Bush, Balbuena and Raga2001) and can be measured in any specimen; the proboscis is rarely found fully evaginated in adult specimens, and cannot be induced to withdraw because worms are collected dead from hosts. The goals of our study were 2-fold. First, we obtained, for the first time, statistical evidence on whether spines grow during the adult development of an acanthocephalan. Second, we investigated the factors that may account for patterns of spine growth, including body size.

Fig. 1. Diagrammatic comparison of the body size and spine coverage in two species of Corynosoma. (A) Male Corynosoma cetaceum, (B) Female C. cetaceum, (C) Male C. australe, (D) Female C. australe. Dashed lines indicate the relative body size of cystacanths. Scale bar=2 mm.
MATERIALS AND METHODS
Data collection
Specimens of Corynosoma cetaceum were collected in several localities along the coast of Argentina. Cystacanths (20 females and 26 males) were obtained from the mesentery of 2 individuals of Argentine sandperch Pseudopercis semifasciata in the neighbourhood of Península Valdés (42°00′−42°45′S). Adults (43 females and 42 males) were collected from the pyloric stomach of 5 franciscana dolphins, Pontoporia blainvillei, that were found drowned in shark fishery gillnets in Necochea (38°27′S, 58°50′W) and Claromecó (38°52′S, 60°05′W). Sampling of Corynosoma australe was conducted in the north coast of Patagonia (42°45′S, 62°30′W): cystacanths (33 females and 24 males) were collected from the mesentery of 11 individuals of the flounder Paralichthys isosceles, whereas adults (35 females and 35 males) were collected from the intestine of 3 South American sea lions, Otaria flavescens stranded on Patagonian beaches. Acanthocephalan specimens were generally washed in saline and fixed and conserved in 70% ethanol. Cystacanths of C. cetaceum were fixed in 4% buffered formaldehyde and preserved in 70% ethanol. No significant morphometric differences were found between cystacanths fixed in ethanol or formaldehyde (MANOVA, P >> 0·05).
Acanthocephalans were examined under a stereomicroscope (X100) and identified following the taxonomic criteria of Aznar et al. (Reference Aznar, Bush and Raga1999b) and Sardella et al. (Reference Sardella, Mattiucci, Timi, Bastida, Rodríguez and Nascetti2005). Then, each specimen was drawn in profile with the aid of a drawing tube (Fig. 2). Trunk length (L) and disk diameter (D) were measured using homologous landmarks that were unaffected by the degree of fore-trunk invagination (Fig. 2). Four body size variables directly related to attachment performance were obtained from each specimen as follows. (1) Disk area. In species of Corynosoma, the disk covered with spines is used as a key attachment device (Van Cleave, Reference Van Cleave1952; Aznar et al. Reference Aznar, Bush, Fernández and Raga1999a, Reference Aznar, Pérez-Ponce de León and Raga2006). The disk surface is roughly circular, thus its area was estimated as the area of a circle. (2) Sectional area (Fig. 2). This variable is related to pressure drag (Koehl, Reference Koehl1984). To obtain it, the drawing in profile of each specimen was scanned and the area was calculated using Image Tool v. 3.0 (UTHSCSA). (3) Surface area. This variable is related to skin friction drag (Koehl, Reference Koehl1984). The body of species of Corynosoma can faithfully be reproduced just by bending a cone (Aznar et al. unpublished data; see Fig. 1). Therefore, surface area can be approximated using the formula for a cone surface, without considering the area of the disk (the disk is attached to the intestine so it is not exposed to drag). (4) Body volume. This variable is related to ‘virtual buoyancy’, a lifting force proportional to the mass of fluid displaced by the body (Koehl, Reference Koehl1984). Volume was calculated assuming a conical body shape.

Fig. 2. Morphometric measurements taken in specimens of Corynosoma cetaceum and C. australe. L, trunk length; D, disk diameter; SL, spine length. The shadowed area is sectional area. Regions where spines were measured are also indicated (see Materials and Methods section for details).
To measure spines, each specimen was cut with a razor blade through the sagittal plane and one half was temporarily mounted on a slide with lactic acid to clear the tegument. Using this procedure, specimens could be re-accommodated, if necessary, for spines to be drawn in profile minimizing tilt-related error. Three spines were drawn under a light microscope (X1000) from each of the 3 sites indicated in Fig. 2 i.e. the disk border, the interfold area, and the posterior hind-trunk (see Aznar et al. Reference Aznar, Bush and Raga2002a for details). For brevity, we will refer to the spines from these sites as Spines 1, 2 and 3, respectively. Spine length was measured as indicated in Fig. 2, and the values taken from 3 spines randomly selected from each site were averaged to obtain a single value per site and specimen.
Statistical analyses
A preliminary analysis indicated that the factor ‘host individual’ did not have a significant effect on average values of morphometric variables either in paratenic or definitive hosts (MANOVA, P>0·05 in all 4 tests), thus, this factor was not considered in further analyses.
The effect of developmental stage, sex, and species on body size variables was examined with MANOVA, using disk area, sectional area, surface area and volume as dependent variables. The 3 factors were considered as fixed. Concerning the ‘species’ effect, we were specifically interested in the interaction of ‘species’ with ‘developmental stage’ and ‘sex’ because this analysis allowed investigation of whether patterns of body growth differed between species, a point that was relevant for the interspecific differences observed in spine growth (see the Results section).
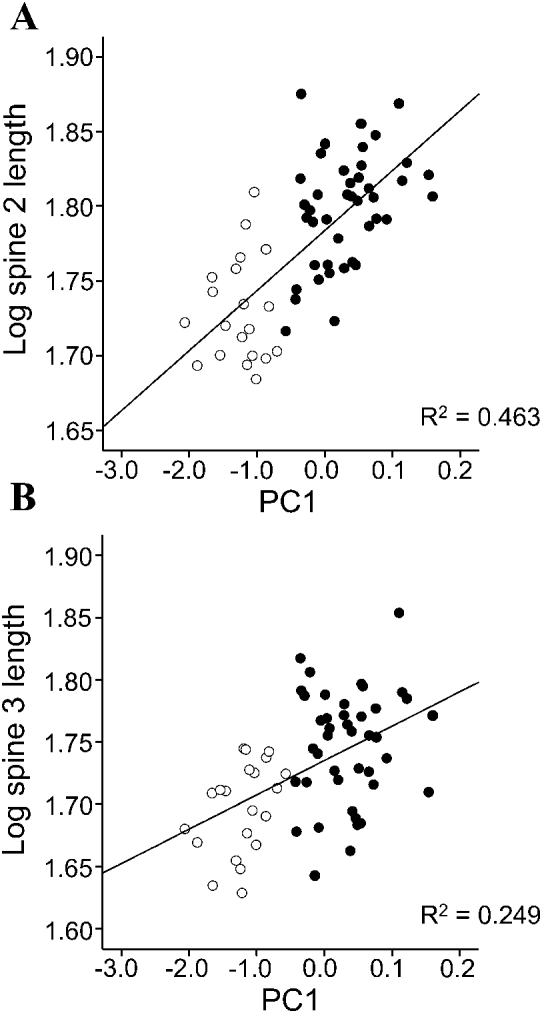
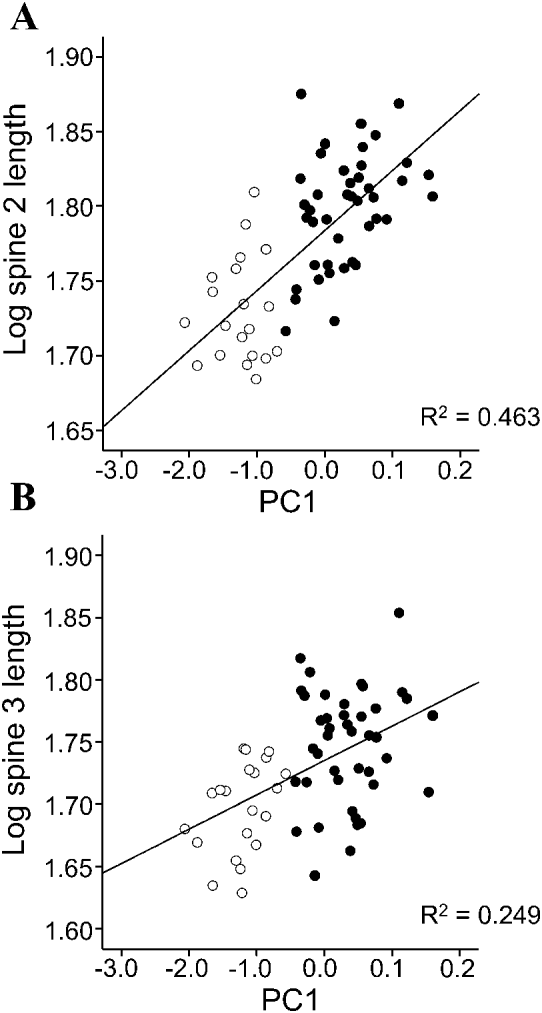
Multivariate analysis of covariance (MANCOVA) was used to examine patterns of spine growth within each species. Values of Spines 1, 2 and 3 were treated as dependent variables and ‘developmental stage’ and ‘sex’ as fixed factors. In addition, we used principal component analysis on the 4 body variables to obtain scores on the first axis (PC1) i.e. a multivariate measure of body size (Klingerberg, Reference Klingenberg, Marcus, Corti, Loy, Naylor and Slice1996). The scores in PC1 were then included in the model as a co-variate. The inclusion of PC1 is pertinent to explore the relationships between static and ontogenetic allometry in spine size growth (see Klingerberg, Reference Klingenberg, Marcus, Corti, Loy, Naylor and Slice1996). Static allometry results from co-variation between morphometric traits among individuals of the same age or developmental stage; in our case cystacanths or adults (Fig. 3A). Ontogenetic allometry deals with co-variation between morphometric traits during growth i.e. the population of cystacanths and adults considered as a whole (Fig. 3A). Both allometric patterns are usually, but not necessarily, similar (Cock, Reference Cock1966; Klingerberg, Reference Klingenberg, Marcus, Corti, Loy, Naylor and Slice1996). In our model, the way to compare allometric patterns was by examining the interaction between PC1 and developmental stage: if the interaction was significant, this would mean that static and ontogenetic allometries did not coincide. In other words, the relationship between body size and spine size would differ between cystacanths and adults, thus indicating changes in relative growth rate during the adult development in the definitive host (Fig. 3B, C). When interaction terms with the co-variate were not significant, they were removed from models to increase the sensitivity of the analysis and to correctly interpret main effects (Engqvist, Reference Engqvist2005).

Fig. 3. Theoretical relationships between static and ontogenetic allometry. (A) Levels of co-variation between spine length and body size in 2 developmental stages of an acanthocephalan i.e. cystacanth and adult (redrawn from Klingenberg, Reference Klingenberg, Marcus, Corti, Loy, Naylor and Slice1996). Static allometry (dashed rectangle) refers to co-variation among individuals of the same developmental stage (e.g. cystacanth). Ontogenetic allometry (dotted rectangle) refers to co-variation due to growth from the cystacanth to the adult stage. (B) Hypothetical relationship between static and ontogenetic allometry in which relative growth rate do not change between the cystacanth and the adult stage. (C) Hypothetical relationship between static and ontogenetic allometry in which both levels of allometry differ because the relative growth of spines changes during the adult development.
MANCOVA models were also used to explore whether variability in spine size within sites (i.e. the disk border, the interfold area, and the posterior hind-trunk) differed between sexes and developmental stages; PC1 was used as a co-variate. For each specimen, the coefficient of variation (CV) of each set of 3 spines was calculated (i.e. for Spines 1, 2 and 3). These CVs were treated as dependent variables in the MANCOVA models.
Statistical analyses were carried out with SPSS v. 17. Statistical significance was set at P<0·05.
RESULTS
Patterns of body growth
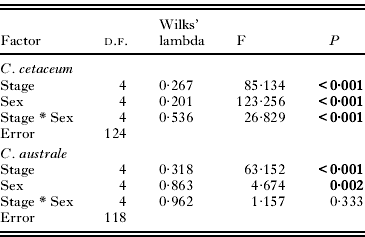
Data on morphometric variables are shown in Table 1. In C. cetaceum, highly significant differences were found in body dimensions, not only between developmental stages, but also between sexes. Also, a highly significant interaction ‘developmental stage * sex’ was observed (Table 2). Univariate ANOVAs revealed that disk area did not differ between sexes (F(1, 127)=1·864, P=0·175), but males had a significantly larger sectional area (F(1, 127)=14·427, P<0·001), surface area (F(1, 127)=81·202, P<0·001) and body volume (F(1, 127)=17·469, P<0·001) than females (Fig. 4). Significant univariate differences concerned surface area and volume (interaction ‘developmental stage * sex’: surface area, F(1, 127)=16·607, P<0·001; body volume, F(1, 127)=8·287, P<0·005). These variables grew comparatively faster in males than in females (Fig. 4). In C. australe, significant differences in body dimensions were also found between developmental stages and sexes (Table 2). However, sexual dimorphism was slight because none of the univariate ANOVAs was found to be significant (minimum nominal P=0·221) (Fig. 4). Also, the interaction ‘developmental stage * sex'was not significant (Table 2).

Fig. 4. Mean values (bars: standard error) of 4 body size variables in cystacanths (open symbols) and adults (solid symbols) of individuals from each sex of Corynosoma cetaceum and C. australe.
Table 1. Mean values (s.d.) [Coefficient of Variation] of body dimensions and length of trunk spines in cystacanth and adult specimens of the acanthocephalans Corynosoma cetaceum and C. australe

Table 2. Results from a multivariate analysis of variance that examines the effects of sex and developmental stage (cystacanth and adult) on 4 body variables i.e. disk area, sectional area, surface area and volume in the acanthocephalans Corynosoma cetaceum and C. australe
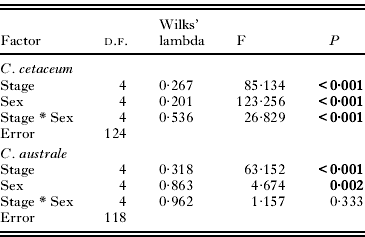
As an interspecific comparison, we tested whether the relative amount of growth from cystacanth to adult differed between C. cetaceum and C. australe. In males, the multivariate interaction ‘developmental stage * species’ was highly significant (Wilks' Lambda=0·279, F(4, 117)=75·556, P<0·001), as were interactions of these factors for each dependent variable (P<0·001). Average relative size of adult males compared to cystacanths was as follows (C. cetaceum vs C. australe): disk area: 175% vs 94%; sectional area: 172% vs 111%; surface area: 247% vs 94%; and volume: 473% vs 93% (see the Table 1). In females, a highly significant interaction ‘developmental stage * species’ was also detected (Wilks' Lambda=0·354, F(4, 125)=57·031, P<0·001), but significant univariate differences concerned sectional area and volume only (sectional area, F(1, 128)=6·500, P<0·012; volume, F(1, 128)=22·603, P<0·001). Sectional area and volume in adult females of C. cetaceum increased 164% and 255%, respectively, compared to cystacanths; however, in C. australe, these figures were just 113% and 91% (see the Table 1). In summary, during adult development individuals of C. cetaceum grew comparatively more than those of C. australe.
Patterns of spine growth
Individuals of C. cetaceum had larger spines than those of C. australe regardless of developmental stage and sex (Fig. 5; see also the Table 1).

Fig. 5. Mean length (bar: standard error) of spines measured at 3 sites (see Fig. 2) in cystacanths (open symbols) and adults (solid symbols) of both sexes in 2 species of Corynosoma. (A) Female C. cetaceum; (B) Male C. cetaceum; (C) Female C. australe; (D) male C. australe.
In C. cetaceum, spine length significantly differed between developmental stages and sexes, and the overall relationship between spine size and body size was not significant (Table 3). However, a highly significant interaction ‘developmental stage * sex’ was found and, therefore, analyses were carried out for each sex separately to tear apart the effects of developmental stage (ontogenetic allometry) and body size (static allometry). In females, the full factorial MANCOVA indicated that adults had longer spines than cystacanths (Fig. 5A) but neither the overall effect of PC1 on spine size nor the interaction ‘developmental stage *PC1’ were significant (Table 3). After removing the interaction term, a significant main effect of PC1 was found (Table 3). Univariate ANOVAs indicated that PC1 correlated significantly (P<0·05) with spine length only in Spines 2 and 3 (Fig. 6). In males of C. cetaceum, there was no significant difference in spine length between cystacanths and adults, nor was there any indication of a significant relationship between body size and spine size in cystacanths or adults (Table 3, Fig. 5B). In summary, (1) females of C. cetaceum had longer spines than males; (2) all spines were longer in adults, but only in females, and (3) there was a significant relationship between spine length and body size only in females (both cystacanths and adults), and only for Spines 2 and 3 (hind-trunk spines).
Table 3. Models of multivariate analysis of covariance that examine the effects of developmental stage (cystacanth and adult), sex, and a multivariate measure of body size (PC1, the first principal component of the 4 morphometric variables indicated in Table 1) on the length of trunk spines from 3 sites in the acanthocephalan Corynosoma cetaceum

In C. australe, spine length significantly differed between both developmental stages and sexes (Table 4). Again, a significant interaction ‘developmental stage * sex’ was found and, therefore, separate analyses were performed for each sex. In females, spine length differed between cystacanths and adults (Table 4); the univariate ANOVAs revealed that only Spine 1 was significantly larger in adults (P=0·003) (Fig. 5C). However, the effect of PC1 on spine size was not significant, even after removing the interaction ‘developmental stage *PC1’ in the model (Table 4). In males, none of the predictors of spine length was significant in any model (Table 4; Fig. 5D). In summary, (1) females of C. australe had longer spines than males; (2) disk spines (Spine 1) were longer in adults than in cystacanths, but only in females, and (3) there was no significant pattern of static allometry between spine length and body size in either sex or developmental stage.
Table 4. Models of multivariate analysis of covariance that examine the effects of developmental stage (cystacanth and adult), sex, and a multivariate measure of body size (PC1, the first principal component of the 4 morphometric variables indicated in Table 1) on the length of trunk spines from 3 sites in the acanthocephalan Corynosoma australe

None of the MANCOVA models for each species involving CVs of Spines 1, 2 and 3 revealed significant effects of sex or developmental stage on spine variability; an overall MANOVA using ‘species’ as a single factor also did not(results not shown).
DISCUSSION
Results from this study provide, for the first time, statistical evidence that trunk spines of 2 species of acanthocephalan grow during the worm development in the definitive host. Unexpectedly, spines appear to grow only in females and exhibit a different pattern of growth depending on the species. A preliminary question that must be addressed is whether there are sampling and/or measurement artifacts that could confound these results. First, cystacanths and adults of C. cetaceum could not be sampled in the same locality, but in places 600 km apart. Since there is evidence of morphological divergence between populations of C. cetaceum from South America and Australia (Aznar et al. Reference Aznar, Bush and Raga1999b), perhaps some degree of divergence might also occur at the geographical scale covered in our study, thus potentially affecting the morphometrical comparison between developmental stages. This does not appear to be the case because the morphology of all specimens of C. cetaceum thus far collected along the coast of southwestern Atlantic from Uruguay to Patagonia is very uniform (Aznar et al. Reference Aznar, Bush and Raga1999b, Reference Aznar, Berón-Vera, Crespo and Raga2002b). Second, spines of C. australe were clearly smaller than those of C. cetaceum, and small structures may exhibit greater levels of variability just because their measurement is less precise (see Aznar et al. Reference Aznar, Bush and Raga2002a). Although all spines had been measured at the same magnification regardless of species, coefficients of variation were very similar between C. australe and C. cetaceum. Therefore, the smaller size effect that was observed for spine growth in C. australe could hardly be accounted for by higher measurement error.
According to our results, both females and males of C. australe are roughly equal in size and grow at a similar rate from the cystacanth to the adult stage, whereas females of C. cetaceum are clearly smaller and grow less than males. However, females of both species have longer spines, and only in females do spines grow significantly during the adult development. Therefore, spine growth does not seem to follow simple allometric rules, nor does it conform to simple biomechanical principles i.e. females are not predicted to suffer stronger dislodgment forces than males according to their body size (Koehl, Reference Koehl1984; Poulin, Reference Poulin2007, Reference Poulin2009). So why do spines grow only in females? One hypothesis is that males require no further growth of spines beyond the cystacanth stage because they develop other attachment devices (i.e. the proboscis, the disk) more than females during late ontogeny. We could not provide an overall test for this hypothesis because most adult specimens had an invaginated proboscis. However, our results clearly indicate that the area of the attachment disk does not differ between sexes. Also, information obtained from other datasets indicate that, in both species of Corynosoma, the proboscis and hooks are significantly smaller in adult males, and the field of spines covers a roughly similar extension of the trunk in both sexes (Hernández-Orts et al. unpublished data; see also Aznar et al. Reference Aznar, Bush and Raga1999b; Sardella et al. Reference Sardella, Mattiucci, Timi, Bastida, Rodríguez and Nascetti2005). Thus, adult males appear to have a less-developed holdfast than females.
A second hypothesis would suggest that factors other than body size exert stronger overall selective pressures on females to develop more efficient attachment devices, including spines. In this context, Petrochenko (Reference Petrochenko1956) argued that adult females of acanthocephalans need to develop larger attachment structures than males because they must stay in the definitive host for longer to produce and release the eggs. Following this argument, the larger size of spines could be viewed as an adaptation of females to reduce the likelihood of being ripped loose by peristaltic movements and passing food (see Poulin, Reference Poulin2009). Females would also require a fine-tuned adjustment of the spine size to the specific microhabitat conditions they encounter during the adult development. Note that the latter strategy is not unusual: after recruitment to the definitive host, females, but not males, of the polymorphid Filicollis anatis inflate the anchored proboscis as a device that obviously improves attachment performance (Van Cleave, Reference Van Cleave1952; Petrochenko, Reference Petrochenko1958).
The hypothesis mentioned above is supported by 2 lines of evidence. First, females of C. cetaceum and C. australe appear to have indeed a longer lifespan than males, as indicated by the strongly female-biased sex ratios observed in the definitive host (Aznar et al. Reference Aznar, Bush, Balbuena and Raga2001, Reference Aznar, Cappozzo, Taddeo, Montero and Raga2004). A longer lifespan of females has also been recorded in other species of Corynosoma using controlled infections in experimental hosts (Valtonen and Helle, Reference Valtonen and Helle1982; Castro and Martínez, Reference Castro and Martínez2004). Unfortunately, we lack direct quantitative data from natural hosts, although information obtained from an allied species of comparable size, Polymorphus minutus, suggests that the lifespan of females could be at least 1·5-fold than that of males (see data from Crompton and Whitfield, 1968).
Second, it is likely that lifespan differences between sexes may have a selective impact on attachment devices because carnivorous marine mammals are hosts that impose very harsh conditions for a gut-dwelling helminth (Petrochenko, Reference Petrochenko1956). Both cetaceans and pinnipeds have higher metabolic rates than terrestrial mammals of comparable size (Williams et al. Reference Williams, Haun, Davis, Fuiman and Kohin2001), and high metabolic rates are often associated with high rates of food intake and short transit times of food along the gut (Karasov and Diamond, Reference Karasov and Diamond1985). With regard to food intake, carnivorous marine mammals need to feed often (Kastelein et al. Reference Kastelein, Hardeman, Boer, Read, Wiepkema and Nachtigall1997a), and on prey that are patchily distributed in the environment, so that large quantities of food are consumed when the occasion arises (Gaskin, Reference Gaskin1978; Williams et al. Reference Williams, Haun, Davis, Fuiman and Kohin2001). Accordingly, acanthocephalans must suffer the frequent but unpredictable passing of a great amount of digested food. On the other hand, marine mammals have comparatively long alimentary tracts associated with their elevated metabolic rates (Williams et al. Reference Williams, Haun, Davis, Fuiman and Kohin2001), but the transit time of food is generally shorter than that of terrestrial mammals of similar size (Kastelein et al. Reference Kastelein, Nieuwstraten, Verstegen, Read, Wiepkema and Nachtigall1997b; Hall-Aspland et al. Reference Hall-Aspland, Rogers, Canfield and Tripovich2011). Therefore, the flow of digesta must be, not only frequent, but fast. In summary, we believe that the need to withstand extreme flow conditions for periods of different extent might have driven a different investment and development schedule of holdfast structures in males and females of C. cetaceum and C. australe.
Another non-exclusive hypothesis is also compatible with the observed sexual differences in investment and development schedule of spines in species of Corynosoma i.e. males and females differ in sexual behaviour. The mating system of acanthocephalans appears to be polygamous; males have a more active role in copulation than females, seeking and mating with several females (Parshad and Crompton, Reference Parshad and Crompton1981). In the intestine of Saimaa ringed seals (Phoca hispida saimensis), Sinisalo et al. (Reference Sinisalo, Poulin, Högmander, Juuti and Valtonen2004) found evidence of significant competition between males of Corynosoma magdaleni for the access to females, with large-sized males firstly approaching non-mated females. Therefore, sexual selection could favour strong, permanent attachment in females of Corynosoma, but only short-term attachment in males as they need to move in search of mates.
Our study also indicates that patterns of spine growth differ between females of each species of Corynosoma. Attempting to infer adaptation in this 2-species comparison inevitably involves the confounding of independent variables (Garland and Adolph, Reference Garland and Adolph1994). In other words, each species lives within a different species of host and selects a different microhabitat and, therefore, each species is subject to different ecological regimes, including the degree of physical disturbance and food availability, which have never been quantified in the system under study. Therefore, we have no reasonable clue about the actual factors that account for the differences in morphology and growth patterns between species. Nonetheless, it is worth noting that spines on the disk border are the ones that grow in both species. Perhaps this is not surprising because the disk is a major attachment device in Corynosoma (Van Cleave, Reference Van Cleave1952), with the disk border exerting a wedge-like force against the host tissue (Aznar et al. Reference Aznar, Bush, Fernández and Raga1999a). In contrast, hind-trunk spines are apparently used only as a secondary holdfast (Aznar et al. 2002). On the other hand, it seems clear that females of C. cetaceum fine-tune the size of spines during the development in the definitive host more than C. australe. All else being equal, this might be adaptive because (i) the relative increment in volume from cystacanth to adult in C. cetaceum is almost 3-fold that of C. australe, and (ii) females of C. cetaceum also achieve a larger adult size, a trait that correlates with stronger dislodging forces (Koehl, Reference Koehl1984; Poulin, Reference Poulin2007, Reference Poulin2009) and, possibly, with a longer lifespan (see Sorci et al. Reference Sorci, Morand and Hugot1997).
Rather surprisingly, we found no significant patterns of static allometry between body size and the size of spines on the disk border in females of either C. cetaceum or C. australe. However, co-variation was significant for hind-trunk spines in females of C. cetaceum. This suggests that the final size of spines may or may not match the adult body size achieved by each individual worm depending on the body region where the spine grows. Again, it seems premature to speculate on the reasons for these differences as we lack information about the factors that control spine morphogenesis (Aznar et al. 2002), and the specific attachment performance of disk or hind trunk spines (see Koehl, Reference Koehl1996). It should be pointed out, however, that narrow co-variation between spine size and final body size must not functionally be required if slight increases in spine size suffice for secure attachment within a range of body sizes (see Poulin, Reference Poulin2009).
In conclusion, this study sheds light on the question regarding whether or not the holdfast of acanthocephalans is fully developed prior to entering the definitive host. In particular, it suggests that temporal allocation of investment in attachment structures may differ, not only between congeneric species, but also between sexes of the same species, possibly due to the different selective pressures that each population subset faces. Future studies should address whether life span and body size are also relevant factors affecting development of other attachment structures (e.g. the proboscis) in a multi-species context.
ACKNOWLEDGEMENTS
The authors thank N. García and M. Aversa for technical assistance with the sample collection. We also thank Prefectura Naval Argentina and ALPESCA S.A. for allowing us to collect our material in hake trawlers. Institutional support was given by Centro Nacional Patagónico (CONICET, Argentina). Permits were provided by Secretaría de Áreas Protegidas y Turismo, and Dirección de Fauna y Flora Silvestre, of the Chubut Province (Argentina).
FINANCIAL SUPPORT
This study was supported by the Ministry of Science and Innovation of Spain (project number CGL2007-6321), Fundación BBVA (BIOCON 04) and the Valencian Government (PROMETEO 2011-040). J.S.H.-O. benefited from a Ph.D. student grant from the National Council on Science and Technology (CONACYT) of Mexico.