Introduction
Paramphistomosis is a serious endemic infection of ruminant livestock in tropical and sub-tropical regions (Rojo-Vázquez et al. Reference Rojo-Vázquez, Meana, Valcárcel and Martínez-Valladares2012), and in recent years it has been identified as an emerging infection in Western Europe (Huson et al. Reference Huson, Oliver and Robinson2017). Calicophoron daubneyi has been confirmed in a number of studies as the primary rumen fluke species infecting ruminant livestock across Western Europe (Ferreras et al. Reference Ferreras, González-Lanza, Pérez, Fuertes, Benavides, Mezo, González-Warleta, Giráldez, Martínez-Ibeas, Delgado, Fernández and Manga-González2014; Malrait et al. Reference Malrait, Verschave, Skuce, Van Loo, Vercruysse and Charlier2015) including the UK and Ireland (Gordon et al. Reference Gordon, Roberts, Lean, Zadoks, Sargison and Skuce2013; Martinez-Ibeas et al. Reference Martinez-Ibeas, Munita, Lawlor, Sekiya, Mulcahy and Sayers2016; Jones et al. Reference Jones, Brophy, Mitchell and Williams2017). Morbidity and mortality attributed to paramphistome infections is invariably associated with acute disease, where ingested paramphistome metacercariae excyst in the small intestine, and the resulting newly excysted juvenile (NEJs) cause significant damage to the intestinal tissues as they move from the small intestine lumen to the sub-mucosa (Millar et al. Reference Millar, Colloff and Scholes2012; Pavan Kumar et al. Reference Pavan Kumar, Syaama Sundar and Devi Prasad2016). Immature paramphistomes are thought to remain in the small intestine for up to 3 months, feeding on host tissue, before they complete their migration to the rumen where they mature and infections become patent (Sanabria and Romero, Reference Sanabria and Romero2008).
Currently, where an active case of paramphistomosis is suspected, there is no diagnostic test available, which can confirm pre-patent acute disease in an animal, therefore clinical paramphistomosis can only be confirmed during post-mortem examination. Mature infections may only be diagnosed by fecal egg count tests unless a post-mortem examination is performed by a veterinarian or in the abattoir. The therapeutic treatment and control of paramphistomosis at present relies on a single anthelmintic compound; oxyclozanide (Arias et al. Reference Arias, Sanchís, Francisco, Francisco, Piñeiro, Cazapal-Monteiro, Cortiñas, Suárez, Sánchez-Andrade, Paz-Silva, Sanchis, Francisco, Francisco, Pineiro, Cazapal-Monteiro, Cortinas, Suarez, Sanchez-Andrade and Paz-Silva2013) but this is often used off-licence as it is only approved for use against fasciolid infection (asides from a single formulation of oxyclozanide to treat paramphistomosis, licensed only in France: Douvistome). Clearly, the lack of an appropriate diagnostic test and approved treatment options are not desirable in the face of this emerging parasitic infection, which has the potential to cause significant clinical disease where large numbers of metacercariae are encountered and ingested by their ruminant hosts.
In order to develop both the diagnostic tools and anthelmintic treatments for paramphistomosis, the identification of suitable diagnostic and anthelmintic targets is required. To facilitate this, researchers require access to the infective (and most pathogenic) stages, namely the C. daubneyi NEJs and immature small intestine-dwelling flukes. These specimens are impractical to obtain from naturally infected animals in the abattoir, as is common for the collection of mature rumen fluke. Therefore, a reliable protocol is required to excyst C. daubneyi metacercariae in vitro. When successfully excysted and maintained in vitro, the resulting C. daubneyi NEJs will facilitate the study of infective stage-specific parasite molecules to support diagnostic development through proteomic or transcriptomic experiments (Robinson et al. Reference Robinson, Menon, Donnelly, Dalton and Ranganathan2009), as well as providing a source of infective stage parasites for in vitro studies such as the screening of existing/novel anthelmintics (Panic et al. Reference Panic, Ingram and Keiser2013). However, anecdotal evidence from the research community suggested that C. daubneyi metacercariae were difficult to excyst using protocols largely developed for the liver fluke, Fasciola hepatica. Here, six previously published methods, which had been developed for the in vitro excystment of other trematode species, were modified and tested against C. daubneyi metacercariae. An optimal protocol consistently giving >80% parasite excystment under in vitro conditions is described.
Materials and methods
Parasites
Calicophoron daubneyi metacercariae (Miskin isolate) were obtained from Ridgeway Research (Gloucestershire, UK). Metacercariae were harvested from Galba truncatula snails which had been previously infected with C. daubneyi miracidia. Metacercariae were washed briefly in water before use.
In vitro excystment of C. daubneyi metacercariae
Six methods which have been previously described for the in vitro excystment of various trematode parasite species; F. hepatica (McGonigle et al. Reference McGonigle, Mousley, Marks, Brennan, Dalton, Spithill, Day and Maule2008), Fasciola gigantica (Nagar et al. Reference Nagar, Raina, Varghese, Kumar, Samanta, Prasad, Gupta, Banerjee, Singh, Rao, Tewari, Paul, Jayraw, Chandra and Garg2010), Zygocotyle lunata (Fried et al. Reference Fried, Robbins and Nelson1978), Paramphistomum spp (Huesca-guillén et al. Reference Huesca-guillén, Ibarra-Velarde and Sánchez-González2007), Acanthoparyphium spinulosum (Bass and LeFlore, Reference Bass and LeFlore1984) and Neascus pyriformis (Schroeder et al. Reference Schroeder, Johnson and Mohammad1981) were selected to test with C. daubneyi metacercariae. Whilst other methods were available, the selected methods were chosen to avoid testing highly similar protocols. Some modifications, based on preliminary observations and the availability of reagents, were made to the published methods. These are detailed in Table 1. All excystment experiments performed here included incubations at 39 °C (the approximate body temperature of the major definitive hosts of C. daubneyi, namely cattle, sheep and goats) with gentle agitation at 60 rpm in a shaking incubator.
Table 1. Details of any modifications made to the original excystment methods tested against Calicophoron daubneyi metacercariae

a Trematode species the excystment protocol was originally designed for.
Initially all six protocols were tested in parallel, with 20 metacercariae per treatment. With the exception of method 1 (incubation in 0·5% sodium hypochlorite) all groups of metacercariae were incubated at 39 °C in dH2O for 10 mins then washed twice in dH2O with a 2 min, 500 × g centrifugation applied between washes. Protocols were then followed as detailed in Table 2, with two washes in dH2O performed between all media changes, but with no centrifugation of the metacercariae after incubation in the activation media. Excystment of NEJs was monitored after 2, 4 and 6 h incubation in the excystment media and after an overnight incubation (Fig. 2). Where the excystment protocol called for a salt solution to be used, Locke's solution (LS) (0·9% NaCl, 0·042% KCl, 0·02% NaHCO3, 0·024% CaCl2) at pH 7·4 was used in all cases.
Table 2. Details of the six in vitro excystment methods tested against Calicophoron daubneyi metacercariae

LS, Locke's solution.
Post-excystment maintenance of NEJs
Excysted NEJs were collected from the respective excystment media by pipette under a dissecting microscope, and transferred to a 2 mL microcentrifuge tube containing 1 mL of warm (39 °C) RPMI 1640 culture media, supplemented with 100 IU mL−1 penicillin and 100 mg mL−1 streptomycin. One change of the RPMI 1640 media was performed after collection of the last NEJs into each tube and parasites were then maintained for a 24 h period at 39 °C in an incubator. NEJs were observed for activity at 4, 8 and 24 h of incubation. All chemicals were purchased from Sigma-Aldrich unless otherwise stated.
Results
Three of the six methods tested (methods 4, 5 and 6) showed no or minimal parasite excystment and were not carried forward for further trials. The remaining three methods were tested in triplicate with 20 metacercariae/treatment, with an average excystment rate of 11/20 (53%) for method 1, 14/20 (70%) for method 2 and 18/20 (90%) for method 3, respectively. These excystment levels were compared using a one-way ANOVA with Tukey's pairwise comparison using PAST (Hammer et al. Reference Hammer, Harper and Ryan2001) and the level of excystment seen for method 3 was shown to be significantly higher than the excystment achieved with both method 1 (P < 0·01) and method 2 (P < 0·05), as detailed in Fig. 1. To obtain optimal levels of excystment of metacercariae it was necessary to incubate the activated metacercariae overnight and collect NEJ parasites the following day after approximately 20 h incubation in excystment media (Fig. 2).

Fig. 1. Excystment rates obtained from the three methods which showed promising initial results (>50% excystment) against Calicophoron daubneyi metacercariae. ** = P < 0·01, * = P < 0·05.

Fig. 2. Percentage excystment of Calicophoron daubneyi metacercariae after incubation in excystment media over a 20 h period. Mean values are shown for those methods (1–3) which typically gave >50% excystment.
It was observed that excystment of C. daubneyi NEJs occurred after much activity of the parasite within the cyst, with an aperture appearing at a single point in the cyst wall through which the NEJ could escape, as shown in Fig. 3. Following excystment, NEJs were successfully maintained for 24 h in the RPMI1640 medium supplemented with 100 IU mL−1 penicillin and 100 mg mL−1 streptomycin, and showed constant movement when observed.
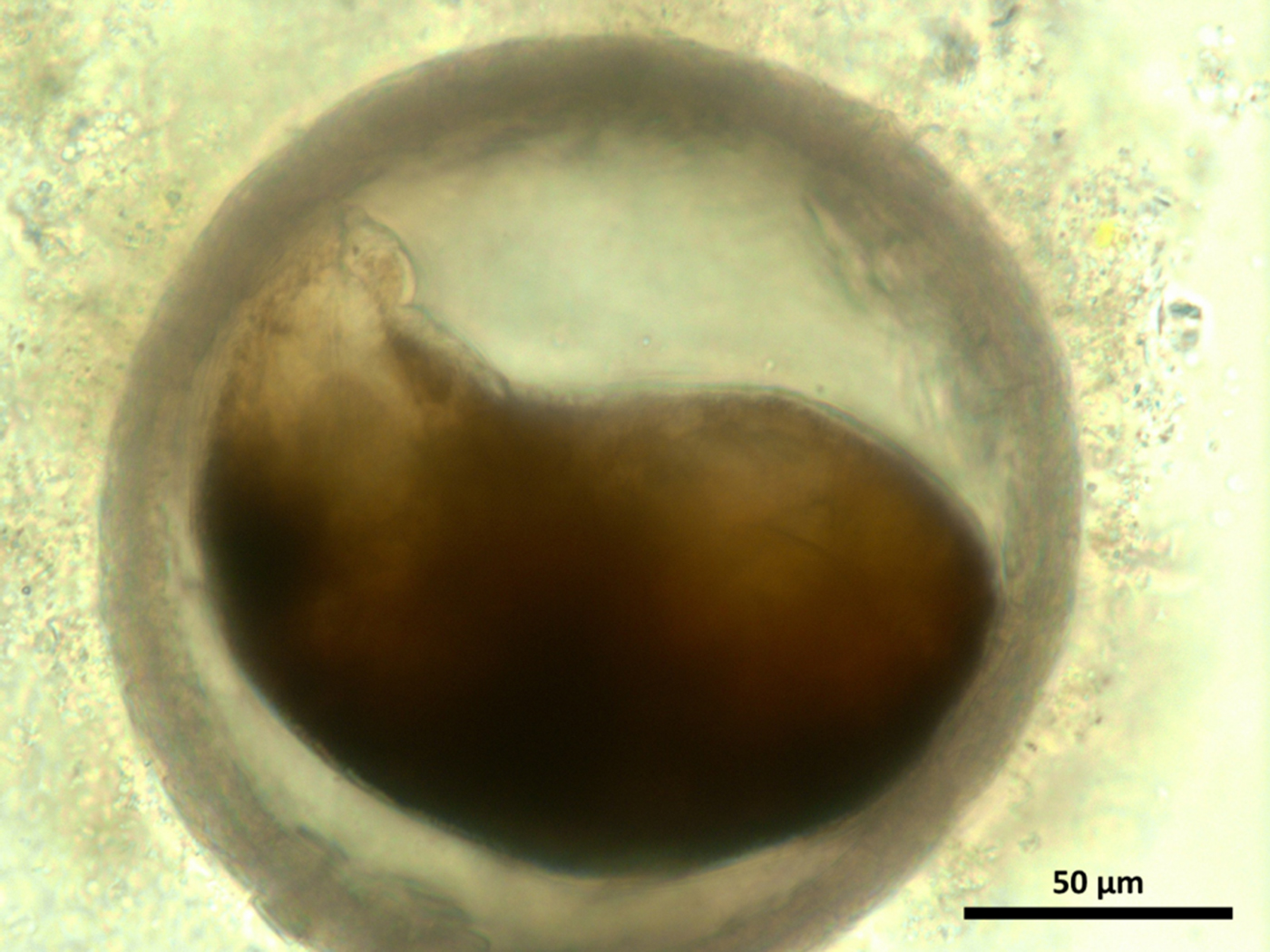
Fig. 3. Activated Calicophoron daubneyi metacercaria (pre-excystment).
Method 3, modified from the protocol described by Fried et al. (Reference Fried, Robbins and Nelson1978) for excystment of Z. lunata, was the most successful. This protocol was further tested with groups of 100, 500 and 1000 metacercariae. Here the alkaline excystment medium was filter sterilized (0·22 µm, Millipore Ltd, Hertfordshire, UK) before the addition of 100 IU mL−1 penicillin, 100 µg mL−1 streptomycin, and 2 µg mL−1 amphotericin B to remove any undissolved bile salts and possible microbial contaminants. These further excystment trials demonstrated that the protocol is still highly successful when applied to larger numbers of metacercariae. These tests yielded 84, 86 and 80% excystment rates, respectively. By the time the final 1000 metacercariae excystment test was performed, metacercariae had been stored post harvesting for up to 10 weeks at 4 °C, and >80% excystment of active, viable NEJs was still observed.
Discussion
Paramphistomosis, caused by C. daubneyi, is on the increase throughout Europe and is thought to be more prevalent than the liver fluke, F. hepatica, in some parts of the UK and Ireland (Toolan et al. Reference Toolan, Mitchell, Searle, Sheehan, Skuce and Zadoks2015; Jones et al. Reference Jones, Brophy, Mitchell and Williams2017). Whilst the impact of chronic rumen fluke infection on animal health and production remains largely unknown, clinical disease and mortality linked to significant immature parasite burdens in the small intestine, although rare, have been reported in both sheep and cattle (Foster et al. Reference Foster, Otter, O'Sullivan, Cranwell, Twomey, Millar and Taylor2008; Mason et al. Reference Mason, Stevenson, Cox and Dick2012; Millar et al. Reference Millar, Colloff and Scholes2012). To begin to understand how NEJ and immature C. daubneyi parasites contribute to the pathology of infected animals, and to aid the development of diagnostic tools and treatment options, we must first be able to study these life cycle stages in vitro. Towards this goal, we describe for the first time a successful method for the in vitro excystment of C. daubneyi metacercariae.
Previously, treatments including exposure to CO2 (Dixon, Reference Dixon1966), reducing conditions (Bass and LeFlore, Reference Bass and LeFlore1984), acid-pepsin treatment and the presence of both bile salts and trypsin (Fried et al. Reference Fried, Robbins and Nelson1978) have all been suggested to be necessary for the in vitro excystment of trematode parasites. Here, the two protocols which included a 15 min acid-pepsin treatment (methods 2 and 3) produced the highest levels of excystment, although this step does not appear to be an absolute requirement for the emergence of NEJs given the 54% average excystment seen in method 1 where no acid-pepsin treatment was included. Furthermore, no excystment was seen with method 5 which included an hour long acid-pepsin treatment, perhaps indicating that prolonged exposure to such conditions may be detrimental to the excystment process. Greater levels of excystment were also seen with the two protocols that included a sodium dithionite treatment (methods 2 and 3), whereas a lower average excystment was seen in method 1 where L-cysteine was used to create reducing conditions. The removal of the 1% trypsin from the alkaline/bile salt medium in method 4 was necessary as during initial trials it was seen that, although up to 90% excystment was achieved, the NEJs that emerged were rapidly digested by the trypsin. Hence, it is possible that the trypsin used in the previously described protocol from which method 4 was adapted, and other protocols where trypsin has been included at a similar concentration (LeFlore and Bass, Reference LeFlore and Bass1983), was only minimally active when included at 1% (w/v). Li et al. (Reference Li, Chung, Chung, Choi, Yu and Hong2004) also included trypsin in their excystment protocol for F. gigantica, but at a much lower final concentration of 0·01%. The percentage trypsin used by Li et al. (Reference Li, Chung, Chung, Choi, Yu and Hong2004) is likely much closer to the in vivo concentration of trypsin in the host intestine, with an average of 143 µg mL−1 trypsin (=0·0143%) reported in human intestinal fluid (Metheny et al. Reference Metheny, Stewart, Smith, Yan, Diebold and Clouse1997). Our results, however, indicate that the presence of trypsin is not required for the in vitro excystment of C. daubneyi metacercariae given the success of methods 1–3, which all lacked this supplement.
For all treatment groups in which NEJs successfully emerged, whilst a considerable number of NEJs appeared after 6 h incubation in excystment media, maximal excystment was achieved following prolonged incubation, typically overnight (up to 20 h). This is similar to the excystment time required by Nagar et al. (Reference Nagar, Raina, Varghese, Kumar, Samanta, Prasad, Gupta, Banerjee, Singh, Rao, Tewari, Paul, Jayraw, Chandra and Garg2010) to obtain the maximum number of F. gigantica NEJs. Although shorter incubation times have been reported to achieve excystment in other trematode species, the success of the overnight incubation period, with no impact on the motility of the NEJs recovered after this time, makes this an efficient and convenient protocol for excystment of C. daubneyi metacercariae in vitro. It has recently been shown that F. hepatica NEJs can be excysted and maintained in vitro for long-term studies of their growth and development (McCusker et al. Reference McCusker, McVeigh, Rathinasamy, Toet, McCammick, O'Connor, Marks, Mousley, Brennan, Halton, Spithill and Maule2016). Our development of a successful method for in vitro excystment of C. daubneyi metacercariae now allows similar refinement of culture conditions that permit long-term studies of rumen fluke.
The development of the current method for the excystment of C. daubneyi metacercariae opens the door for a wide range of in vitro experiments using the infective stage of this emerging parasite. One research priority is the study of transcriptome and proteome profiles relating specifically to this infective stage (Huson et al. Reference Huson, Oliver and Robinson2017). This would not only inform our knowledge of how these parasites establish and maintain infections in the definitive ruminant host but would also facilitate the discovery of potential diagnostic antigens and vaccine candidates. In addition, the successful development of a method to produce C. daubneyi NEJs paves the way for further in vitro studies to improve our understanding of the developmental and molecular biology of these parasites, along with the development of in vitro culture tools for drug susceptibility studies.
Acknowledgements
This work was supported by the Biotechnology and Biological Sciences Research Council (M.W.R. grant number BB/N017757/1), Agrisearch and AHDB Beef & Lamb.