INTRODUCTION
Domestic dogs represent a preferential food source for many arthropod vectors of pathogens, some of which of zoonotic concern (Otranto and Eberhard, Reference Otranto and Eberhard2011). Among filarioids (superfamily Filarioidea), Dirofilaria immitis and Dirofilaria repens (Spirurida, Onchocercidae) are recognized as major causes of infestations in animals and humans and are often diagnosed in the veterinary practice. Differently, Acanthocheilonema reconditum is less pathogenic, probably due to the fact that adults are beneath the subcutaneous tissues of the limbs and back (Nelson, Reference Nelson1962), seldom of the trunk, hind legs and the fat near the kidney (Grassi and Calandruccio, Reference Grassi and Calandruccio1890; Korkejian and Edeson, Reference Korkejian and Edeson1978; Sonin, Reference Sonin1985). Nonetheless, A. reconditum has a global distribution and, in many geographical areas of the Mediterranean Basin, Middle East, South Africa, South America and Oceania, it is the sole (Mazzotti and Chabaud, Reference Mazzotti and Chabaud1962; Korkejian and Edeson, 1974; Minnaar and Krecek, Reference Minnaar and Krecek2001) or the most prevalent (Pennington and Phelps, Reference Pennington and Phelps1969; Boreham and Atwell, Reference Boreham and Atwell1985; Pampiglione et al. Reference Pampiglione, Poglayen and Capelli1986; Saleh et al. Reference Saleh, Kirkpatrick, De Haseth and Lok1988; Ortega-Mora et al. Reference Ortega-Mora, Gomez-Bautista, Rojo-Vazquez, Rodenas and Guerrero1991; Aranda et al. Reference Aranda, Panyella, Eritja and Castella1998; Alves et al. Reference Alves, de Almeida Silva, Faustino, McCall, Supakonderj, Labarthe, Sanchez and Caires1999; Cringoli et al. Reference Cringoli, Rinaldi, Veneziano and Capelli2001; Reifur et al. Reference Reifur, Thomaz-Soccol and Montiani-Ferreira2004; Giannetto et al. Reference Giannetto, Poglayen, Gaglio and Brianti2007) filarioid species infesting dogs. Differently from other filarioids transmitted by mosquitoes (e.g., D. immitis and D. repens) or ticks (e.g., Cercopithifilaria spp.) to dogs, A. reconditum completes its life cycle in and is vectored by fleas (i.e., Ctenocephalides canis, Ctenocephalides felis, Pulex irritans, P. simulans, Echidnophaga gallinae) or lice (i.e., Heterodoxus spiniger, Linognathus setosus) (Newton and Wright, Reference Newton and Wright1956; Nelson, Reference Nelson1962; Pennington and Phelps, Reference Pennington and Phelps1969; Bain and Beaucournu, Reference Bain and Beaucournu1974). Nonetheless, a few studies assessed the potential role of Rhipicephalus sanguineus, the brown dog tick, as a vector of this filarioid species (Pennington and Phelps, Reference Pennington and Phelps1969; Korkejian and Edeson, Reference Korkejian and Edeson1978; Cringoli et al. Reference Cringoli, Rinaldi, Veneziano and Capelli2001).
In spite of its wide distribution and its finding in the eye of a human (Huynh et al. Reference Huynh, Thean and Maini2001), scant information is available on the biology of A. reconditum. There is evidence indicating that A. reconditum blood microfilariae (mfs) are found in peripheral blood after a pre-patent period of 67–101 days, in experimentally infected dogs (Lindsay, Reference Lindsay1962; Sawyer et al. Reference Sawyer, Rubin and Jackson1965; Farnell and Faulkner, Reference Farnell and Faulkner1978; Lindemann and McCall, Reference Lindemann and McCall1984). Nonetheless, no further information is available on the patent period of the infestation in naturally infested animals as well as on the prevalence and incidence of the infestation in any confined dog population. Moreover, information on the rate of infestation in its intermediate hosts is limited to old reports (Pennington and Phelps, Reference Pennington and Phelps1969; Korkejian and Edeson, Reference Korkejian and Edeson1978). In this perspective, the aim of the present study was to investigate the prevalence and incidence of A. reconditum in a confined population of dogs living in an endemic area of Sicily (southern Italy). The presence of A. reconditum was also searched for in fleas and ticks collected from the same dog population. Furthermore, the periodicity of microfilaraemia was monitored in highly microfilaraemic dogs to investigate the circadian rhythm of this nematode in its definitive host.
MATERIALS AND METHODS
Study population
The study was carried out in a confined dog population in a municipal shelter of Messina, northern Sicily (38.2416° N, 15.5218° E), an area of A. reconditum endemicity (Giannetto et al. Reference Giannetto, Poglayen, Gaglio and Brianti2007). In this shelter, a total of 371 dogs were housed in single or collective mesh fences, being grouped according to character and gender compatibility. Animals included in the study had a stray origin and had been hosted in the shelter for at least 2 years. Dogs sampled were older than 6 months and in general good health conditions. All dogs were untreated against ectoparasites individually but pyrethroids (flumethrine 6%, 1 ml per litre of water) were sprayed in the environment on late spring (e.g., May 2010) to reduce the level of tick and flea infestation. Dogs were treated against gastrointestinal helminths using commercial compounds (i.e., pyrantel embonate, praziquantel and febantel) once brought to the shelter and, subsequently, twice a year.
Sampling design
In May 2010, 152 clinically healthy dogs (i.e., 85 males and 67 females) aged between 2 and 13 years were bled and the locations of each animal within the fences were recorded. One year later (May 2011) a second blood collection was performed on the same individual dogs to estimate both the duration/persistence of microfilaraemia in animals positive at the first sampling and 1 year cumulative incidence of the infestation in those that were negative. At the second sampling time, dogs were checked for the presence of ticks and fleas by visual inspection and combing, respectively. Ectoparasites collected were stored in labelled vials and transported, within 4 h, to the laboratory for dissection (see below).
Two dogs, namely dog 1 and dog 2, presenting a high level of blood mfs (i.e., about 1000 mfs per ml of blood) at the first sampling were systematically bled since May 2010, twice a day (i.e., 8 a.m. and 8 p.m.) for 10 days and, later on, every 2 weeks for 1 year (May 2010–May 2011). These 2 dogs were treated monthly against ectoparasites (FrontlineCombo®, Merial SAS) to prevent new A. reconditum infestation. In addition, to investigate the existence of a circadian rhythm of microfilaraemia, a high microfilaraemic dog, namely dog 3, was bled every 3 h for 4 days (i.e., a total of 32 blood samples in 96 h). From these dogs only a small amount of blood (i.e., 2 ml) was withdraw. Animals were kept (i.e., housing, food) under their usual housing conditions before, during, and after the study. Clinical signs suggestive of A. reconditum infestation (e.g., dermatitis and/or alopecia) were recorded at every blood collection.
The study design and the experimental procedures were approved and authorized by the Animal Ethics Committee of the University of Messina (Italy).
Laboratory procedures
All blood samples were stored in 3 ml tubes with pre-added anticoagulant (EDTA K3) and examined for the presence of mfs within 5 h post-collection. In particular, 1 ml of blood was processed according to the modified Knott's technique (Balbo and Panichi, Reference Balbo and Panichi1968) and mfs found in the samples were identified at species level using morphometric criteria, counted and the burden expressed as mfs per ml of blood (mfs/ml). The morphological diagnostic characters were compared with measurements and features of the most common filarioids retrieved in dogs in Italy, namely, D. immitis, D. repens and A. reconditum (Fülleborn, Reference Fülleborn1912; Bernard and Bausche, Reference Bernard and Bausche1913; Lent and Freitas, Reference Lent and Freitas1937; Olmeda-Garcia and Rodriguez-Rodriguez, Reference Olmeda-Garcia and Rodriguez-Rodriguez1994).
The collected fleas and ticks were individually identified at species level, dissected in a drop of saline solution under a stereomicroscope and examined for the presence of A. reconditum developmental stages. Once dissected, a drop of methylene blue (1%) was added and a coverslip placed on the preparation in order to better visualize larval nematodes (Brianti et al. Reference Brianti, Otranto, Dantas-Torres, Weigl, Latrofa, Gaglio, Napoli, Brucato, Cauquil, Giannetto and Bain2011). Larvae found were identified and staged according to the protocols of Pennington and Phelps (Reference Pennington and Phelps1969) and Bain and Beaucournu (Reference Bain and Beaucournu1974). A system for image analysis, AxioVision rel. 4.8 (Carl Zeiss AG, Germany) connected with digital camera mounted directly on the microscope was used to take pictures and to measure the larvae.
Data analysis
Cumulative incidence was calculated as the proportion of non-microfilaraemic dogs at the first sampling (May 2010) that had become microfilaraemic after 1 year, subtracting those dogs who died or that were adopted (Thrusfield, 1996). Differences in the frequencies of infestation between sexes were assessed by chi-square test (Yates corrected) whereas the differences in the number of blood mfs between samples (i.e., morning/evening or May 2010/May2011) were analysed by paired Student's t-test. Variations in the mean number of blood mfs in dog 3 among the 4 days of sampling were analysed using one-way ANOVA. The normality distribution of data was tested by Kolmogorov-Smirnov method (Hawkins, Reference Hawkins2005). The critical significance level (α) was set at 5% (0·05) and all tests were performed two-sided. Statistical analyses were done using the statistical package SPSS v. 13.0.
RESULTS
Prevalence, incidence and duration of microfilaraemia
At the first blood collection (May 2010), 17 (11·2%) out of 152 sampled dogs were microfilaraemic. Microfilariae were identified as A. reconditum based on their measurements and morphology (i.e., 273·1±9·4 in length and of 5·4±0·2 in width). Positive animals had a mean number of blood mfs of 184·5 mfs/ml (± 392·4) with a range from 3 to 1427. At the 1-year follow-up (May 2011), 16 (13·3%) out of the remaining 120 dogs were positive for A. reconditum mfs with a mean number of 199·1 mfs/ml (± 639·5), ranging from 2 to 2576. In particular, the presence of mfs was persistently detected in 10 (58·8%) out of the 17 dogs positive in May 2010 (Table 1). Three (17·7%) of the initially positive dogs were negative at the second screening. New cases of infestation were recorded in 6 dogs that were negative at the first sampling, thus resulting in a cumulative incidence of 5·9%. All the newly infested dogs were co-housed with A. reconditum microfilaraemic dogs. No significant differences were observed in the rate of infestation between males and females either at the first or second blood collections and no clinical signs suggestive of A. reconditum infestation were observed in positive dogs.
Periodicity of microfilaraemia
In the 2 dogs bled twice a day for 10 days, in 8 out of 10 days of observation, a significantly greater mean number of blood mfs was recorded in the morning samples than in the evening (paired t-test=2·970, P=0·008) (Fig. 1). In these animals, the number of circulating mfs ranged from 610 and 732 mfs/ml up to 1329 and 1269 mfs/ml in dog 1 and dog 2, respectively. Later on, during the 1-year follow-up with blood collection every 2 weeks, both dogs showed a persistence of microfilaraemia with different patterns. In particular, from May 2010 to May 2011, dog 1 maintained a constant high level of circulating mfs, even if with monthly variation ranging from 916 to 5040 mfs/ml; dog 2 showed a progressive decrease in the mean number of mfs from 889 to 2 mfs/ml (Fig. 2). In dog 3, bled every 3 h for a total of 4 days, the number of circulating mfs varied from 2640 to 5524 mfs/ml (median 3684±774) showing different microfilaraemia patterns for each of the fourth days of monitoring (Fig. 3). Briefly, in the first day the microfilaraemia peaked over the median value only in the afternoon (i.e., 5524 mfs/ml at 5 p.m.); in the second day a total of 3 peaks (i.e., 4576 mfs/ml at 2 a.m., 5204 mfs/ml at 5 p.m. and 5060 mfs/ml at 11 p.m.) were recorded, whereas in the third day 2 peaks, one in the afternoon (i.e., 4562 mfs/ml at 2 p.m.) and one in the evening (i.e., 4816 mf/sml, at 8 p.m.) were recorded. Finally, a total of 3 peaks (i.e., 5192 mfs/ml at 5 a.m., 5152 mfs/ml at 11 a.m. and 4284 mfs/ml at 5 p.m.) were recorded in the fourth day. No statistical difference was recorded between and among each sampling time points in the same day.

Fig. 1. Mean and range of circulating microfilariae of Acanthocheilonema reconditum recorded in dog 1 and dog 2 in the morning (i.e., 8:00 am) and in the evening (i.e., 8:00 pm) for 10 days (paired t-test=2·970, P=0·008).

Fig. 2. One-year variation of the number of circulating microfilariae of Acanthocheilonema reconditum in dog 1 and dog 2. Dogs were bled every 2 weeks from May 2010 to May 2011.

Fig. 3. Periodicity of circulating microfilariae of Acanthocheilonema reconditum in a dog. The dog was bled every 3 h for 96 h (error bars=s.e.; Red bold line indicates the median value recorded over the 4 days).
Infestation in fleas and ticks
On May 2011, 78 fleas and 272 ticks were collected from a total of 21 and 31 dogs, respectively. With regard to fleas, the predominant species was C. felis (41 females and 20 males) followed by C. canis (10 females and 7 males), whereas ticks (164 males, 97 females, 11 nymphs) were identified as R. sanguineus.
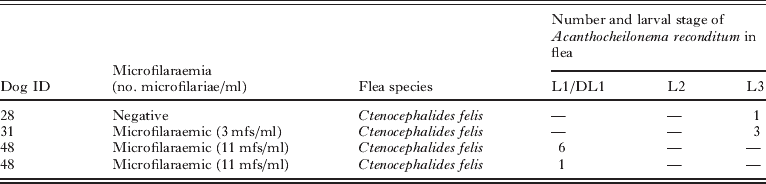
Eleven developing A. reconditum larvae were detected in 4 (i.e., 5·1%) C. felis females (Table 2). Larvae were identified as 7 first-stage (L1) and 4 infective-stage (L3) larvae (Fig. 4). L1 had a mean length of 268·7 μm and a width of 9·2 μm; L3 were 1148 μm×24 μm. As much as 3 L3 or 6 L1 were observed in a single specimen of flea. Positive fleas were collected from 3 microfilaraemic and 1 non-microfilaraemic dog. In particular, all fleas bearing L1 were collected from microfilaraemic animals whereas those with L3 were from a non-microfilaraemic one. At the dissection, no tick was found positive for A. reconditum. No correlation was found between the numbers of developing A. reconditum larvae in the fleas and the number of circulating mfs in flea-infested dogs.

Fig. 4. Developing larvae of Acanthocheilonema reconditum found in dissected fleas. (A) First-stage larva (L1), note the presence of a prominent hook at the cephalic end (head of arrow); (B) Cephalic region of third-stage infective larva (lateral view); (C) Caudal region of third-stage infective larva (lateral view); (D) Caudal region of third-stage infective larva (dorsal view), note the presence of 3 (1 medial and 2 lateral) conical lapplets arising from the caudal end (head of arrow). Scale bars=50 μm.
Table 1. Number of circulating microfilariae in dogs that have been tested positive for Acanthocheilonema reconditum in May 2010 and/or in May 2011 samplings
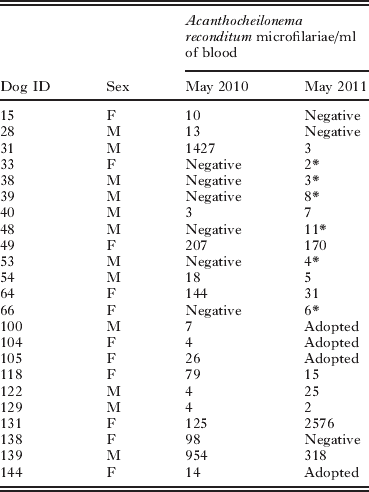
* Newly infected dogs.
Table 2. Number and stage of developing Acanthocheilonema reconditum larvae found in dissected fleas according to flea species and microfilaraemia status of the infested dogs
DISCUSSION
The study here presented reports the prevalence of A. reconditum infestation in a confined population of dogs and provides new insights into the biology and ecology of this filarioid in the definitive and intermediate host. Acanthocheilonema reconditum is endemic in the study area with prevalence of microfilaraemic animals as high as 11·2% and 13·3% in the first and second year, respectively. Information on this filarioid infestation is scant, with D. immitis and D. repens being considered the most common filarial species parasitizing dogs worldwide. In Italy, there are data indicating that A. reconditum is the most prevalent filarioid species infesting dogs in southern regions (Cringoli et al. Reference Cringoli, Rinaldi, Veneziano and Capelli2001; Otranto et al. Reference Otranto, Capelli and Genchi2009), as has also been demonstrated by an extensive survey carried out on 2512 dogs in Sicily where this nematode was the most common causative agent of filarial infection with an overall prevalence of 4·5% (95% CI 3·7%−5·4%) (Giannetto et al., Reference Giannetto, Poglayen, Gaglio and Brianti2007). Interestingly, A. reconditum was the only species found in dogs in the study site, as recorded in other geographical areas where it was the only filarioid affecting dogs (Mazzotti and Chabaud, Reference Mazzotti and Chabaud1962; Korkejian and Edeson, Reference Korkejian and Edeson1978; Minnaar and Krecek, Reference Minnaar and Krecek2001).
In contrast to previous studies (Pennington and Phelps, Reference Pennington and Phelps1969; Cringoli et al. Reference Cringoli, Rinaldi, Veneziano and Capelli2001; Giannetto et al. Reference Giannetto, Poglayen, Gaglio and Brianti2007), no differences in terms of infestation rate between animal genders were found as already recorded by Korkejian and Edeson (Reference Korkejian and Edeson1978). This finding could be due to the fact that in the studied shelter dogs were housed in the same environment, thus being exposed and infested by the same population of arthropods. To the best of our knowledge, the cumulative incidence of 5·9% recorded in our study represents the first data on the incidence of A. reconditum infestation in a dog population and could only be compared with the force of infection calculated across European countries for D. immitis and D. repens that was 0–8·4% and 0–3·3%, respectively (Troz-Williams and Trees, Reference Trotz-Williams and Trees2003). However, the cumulative incidence recorded in this study might be biased by the animal density of our study population in a shelter environment and might not represent what would be observed in other dog population.
Interestingly, new infestations were recorded in dogs sharing the same kennels with at least 1 dog microfilaraemic for A. reconditum in the previous year. This feature suggests the pivotal role of A. reconditum-infested dogs as reservoirs of this filarioid in a confined environment, being the proximity to an infested animal crucial for the occurrence of new infestations. This is probably due to the fact that adult fleas, the only stage capable in transmitting this filarial species (Newton and Wright, Reference Newton and Wright1956; Nelson, Reference Nelson1962; Bain and Beaucournu, Reference Bain and Beaucournu1974), do not drop off from their host, so that, fleas passage between and among individuals is more likely when animals are co-housed (Rust, Reference Rust1994). This ecological aspect makes the epidemiology of A. reconditum very different from that of other filarioids affecting dogs such as D. immitis and D. repens, since mosquitoes can spread the infestation for long distances (McCall et al. Reference McCall, Genchi, Kramer, Guerrero and Venco2008; Otranto et al. Reference Otranto, Capelli and Genchi2009) or as Cercopithifilaria sp. where the transstadial passage of larvae in R. sanguineus ticks can allow, at the same time, the maintenance in the environment and the transmission to new susceptible hosts (Brianti et al. Reference Brianti, Otranto, Dantas-Torres, Weigl, Latrofa, Gaglio, Napoli, Brucato, Cauquil, Giannetto and Bain2011).
The mean number of A. reconditum mfs recorded in our study (i.e., 184·5 mf/ml in 2010 and 199·1 mf/ml in 2011) is one of the highest reported either in natural (Pennington and Phelps, Reference Pennington and Phelps1969; Herd, Reference Herd1978; Cringoli et al. Reference Cringoli, Rinaldi, Veneziano and Capelli2001) or experimental infestations (Lindemann et al. Reference Lindemann, Evans and McCall1983; Lindemann and McCall, Reference Lindemann and McCall1984). However, the number of circulating microfilariae is not always correlated to the number of adult worms (Pennington and Phelps, Reference Pennington and Phelps1969) but it could be affected, as for other filarial species, by the fertility of females that seems to have a progressive rise followed by a decrease later on (Kihara, Reference Kihara1987). This may explain the reduction of circulating mfs in 7 out of the 10 dogs showing 1-year persistent microfilaraemia. Analogously, a progressive decrease of microfilaraemia (from 889 mf/ml in May 2010 to 2 mf/ml in May 2011) was recorded throughout 1 year in dog 2.
The periodicity of A. reconditum mfs was earlier investigated with contrasting results as singular microfilaraemie peaks have been reported diurnal (Newton and Wright, Reference Newton and Wright1956), nocturnal (Korkejian and Edeson, Reference Korkejian and Edeson1978; Bobade et al. Reference Bobade, Ojebuoboh and Akinboade1981), or both (Gubler, 1966; Pennington and Phelps, Reference Pennington and Phelps1969). In our work the periodicity of circulating mfs was evaluated in 3 different experiments. Although in the first experiment a higher number of mfs was recorded during the morning in 8 out of 10 days of study, the absence of any circadian rhythm was confirmed by data of the third experiment conducted by bleeding a high microfilaraemic dog every 3 h for 4 days. Indeed, our results indicate that there is no defined periodicity of A. reconditum microfilaraemia in dogs. This is in line with the short periods of flea blood feedings (about 10 min) and the absence of a defined circadian rhythm (Koehler et al. Reference Koehler, Leppla and Patterson1989).
The rate of A. reconditum infestation in fleas (5·1%) is much lower than that reported in a similar study where developing larvae of A. reconditum were found in 70·5% and 40·3% of C. canis and H. spiniger, respectively (Pennington and Phelps, Reference Pennington and Phelps1969). However, these differences are more likely due to the different methodologies employed for the collection of fleas since in the present study fleas were collected from infested dogs regardless their microfilaraemic status, whereas in previous studies fleas were collected exclusively from microfilaraemic dogs. Nevertheless, only 1 of the 4 positive fleas found in our study was collected from a non-microfilaraemic dog that, however, shared the same kennel with a microfilaraemic, flea-infested dog. None of the 272 ticks dissected carried developing forms of A. reconditum (data not shown) providing further evidence that R. sanguineus does not act as a vector of A. reconditum, as already suggested by other researchers (Nelson, Reference Nelson1962; Pennington and Phelps, Reference Pennington and Phelps1969; Bain and Beaucournu, Reference Bain and Beaucournu1974).
No microfilaraemic dog displayed clinical signs suggestive of infestation by A. reconditum, which is considered one of the less pathogenic filarioids of dogs (Bobade et al. Reference Bobade, Ojebuoboh and Akinboade1981; Hubert, Reference Hubert1985; Chauve, Reference Chauve1990). Accordingly, a study of dogs experimentally infested by A. reconditum demonstrated that this nematode is not a parasite of clinical significance (Lindemann et al. Reference Lindemann, Evans and McCall1983) even though some of the experimentally infested dogs presented significantly greater counts of leukocytes and eosinophils than control dogs. However, further studies should be addressed to better understand the real pathogenic role of this filarioid in dogs and its potential risk for humans in areas where it is endemic.
ACKNOWLEDGEMENTS
The veterinary clinic ‘Peloro’ and Dr Rosamaria Mirabito are thanked for the support provided in dog hospitalization. The authors wish to thank the veterinarian Dr Marilena Di Pietro and all the personnel of Messina dog shelter for the professional assistance provided during the study.








