Introduction
Neurocysticercosis (NCC) is a human disease which arises when larvae of the cestode Taenia solium infect the central nervous system (CNS) (Mahanty and Garcia, Reference Mahanty and Garcia2010). The most common symptom of this infection is the development of epileptic seizures, which occurs in 70–90% of symptomatic NCC cases (Carpio and Romo, Reference Carpio and Romo2014). As such, NCC is thought to be the leading cause of acquired epilepsy. Epilepsy affects 50 million people worldwide with about 80% of cases in the developing world, constituting a critical global health concern. NCC is typically prevalent in developing countries, but with increasing migration from – and travel to – endemic countries, it is steadily becoming a global phenomenon (Burneo and Cavazos, Reference Burneo and Cavazos2014; Carpio and Romo, Reference Carpio and Romo2014). This is concerning as NCC not only impacts heavily on the quality of life of those infected, but also presents a significant drain on medical and economic resources (Roman et al., Reference Roman, Sotelo, Del Brutto, Flisser, Dumas, Wadia, Botero, Cruz, Garcia, de Bittencourt, Trelles, Arriagada, Lorenzana, Nash and Spina-Franca2000; Bhattarai et al., Reference Bhattarai, Budke, Carabin, Proaño, Flores-Rivera, Corona, Cowan, Ivanek, Snowden and Flisser2011).
Despite the global impact of NCC, there is still much that is uncertain about the disorder. Precisely how, for example, cerebral infection with T. solium relates to the development of seizures remains unclear. Furthermore, there exists a need for additional therapeutic options for patients with epilepsy secondary to NCC, as many of these patients suffer from seizures that are refractory to currently available treatment (Burneo and Cavazos, Reference Burneo and Cavazos2014; Carpio and Romo, Reference Carpio and Romo2014; Mahanty et al., Reference Mahanty, Orrego, Mayta, Marzal, Cangalaya, Paredes, Gonzales-Gustavson, Arroyo, Gonzalez, Guerra-Giraldez, Garcia and Nash2015). The study of NCC also represents a unique opportunity for understanding how neuroinflammatory processes contribute to the development of seizures more generally (Nash et al., Reference Nash, Mahanty, Loeb, Theodore, Friedman, Sander, Singh, Cavalheiro, Del Brutto, Takayanagui, Fleury, Verastegui, Preux, Montano, Pretell, White, Gonzales, Gilman and Garcia2015). As such, it is essential that we continue to develop new ways in which to study this disease. In this review, we explore the various model systems used to study NCC. We critically evaluate their relative strengths and weaknesses and summarize how they have contributed to our current understanding of disease processes. Finally, we discuss the potential for novel research strategies, which could enable progress in understanding pathogenic mechanisms in NCC.
A brief background on NCC
Taenia solium life cycle: how does NCC come about?
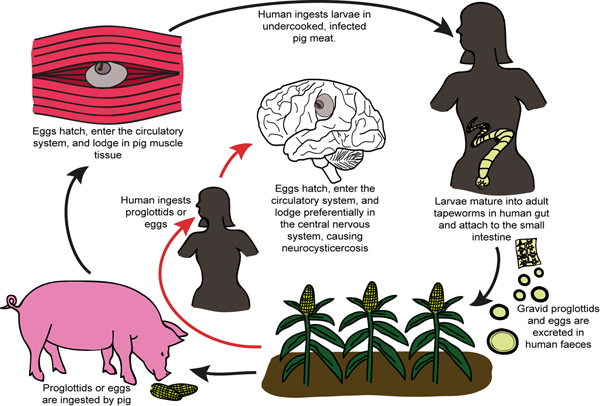
The adult worm of T. solium is found in the small intestine of Homo sapiens, the only known definitive host of T. solium (White, Reference White2000) (Fig. 1). These worms can produce up to 2 00 000 and 4 00 000 infectious oncospheres (eggs) per day, which are excreted in the human feces (White, Reference White2000). If infected feces are ingested by a pig, the oncospheres become activated/mature in the presence of bile salts and intestinal enzymes, and force their way through the gut wall and into the bloodstream (White, Reference White2000). At blood vessel terminations, the activated oncospheres lodge in muscle, nervous, subcutaneous and ocular tissue (White, Reference White2000). There, each mature oncosphere evolves into a vesicular larva with an invaginated scolex (also known as a cysticercus) over a period of weeks or months (White, Reference White2000). These cysticerci have a lifespan of a few years (White, Reference White2000). If pork meat containing a viable cysticercus is ingested by a human, the scolex of the cysticercus evaginates in the small intestine (due to a change in osmotic pressure), and attaches to the intestinal wall. Here the larva develops once more into an adult worm (White, Reference White2000) (see Fig. 1).
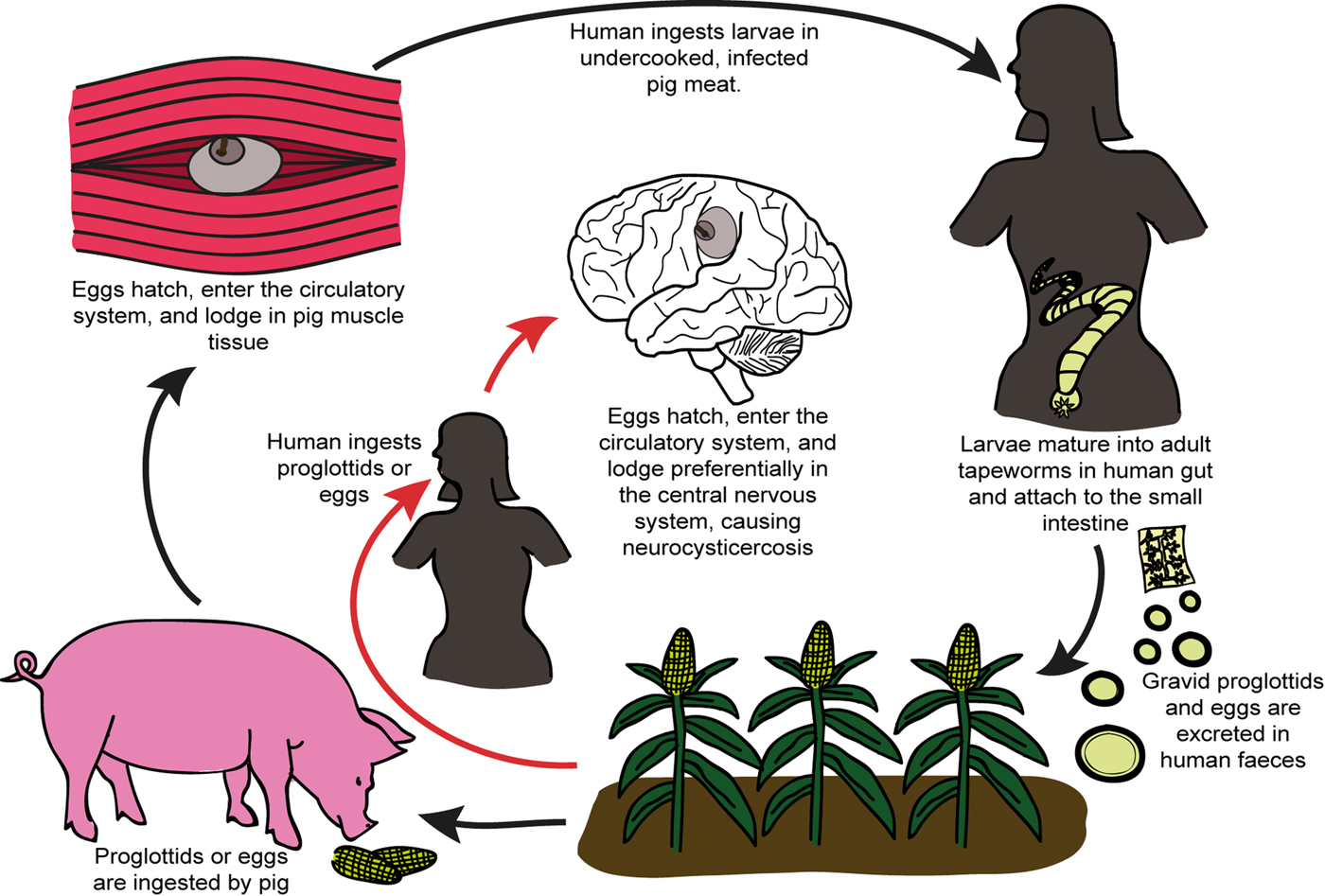
Fig. 1. Schematic representation illustrating the life cycle of Taenia solium and the process which results in human infection and neurocysticercosis.
NCC occurs when humans accidentally ingest the oncospheres of T. solium (Carpio, Reference Carpio2002). This may occur via food or water in areas where water sources are contaminated by human feces, or via accidental ingestion of tiny amounts of the feces of an adult tapeworm carrier in the household (Flisser, Reference Flisser1994). Oncospheres are also activated in the human gut as they would be in the pig gut, and are able to penetrate the gut wall and pass into the bloodstream (Carpio, Reference Carpio2002). The activated oncospheres may then lodge in muscle, ocular, subcutaneous or nervous tissue, with nervous system infection being of the greatest clinical concern (Carpio, Reference Carpio2002).
NCC disease progression and manifestation in humans
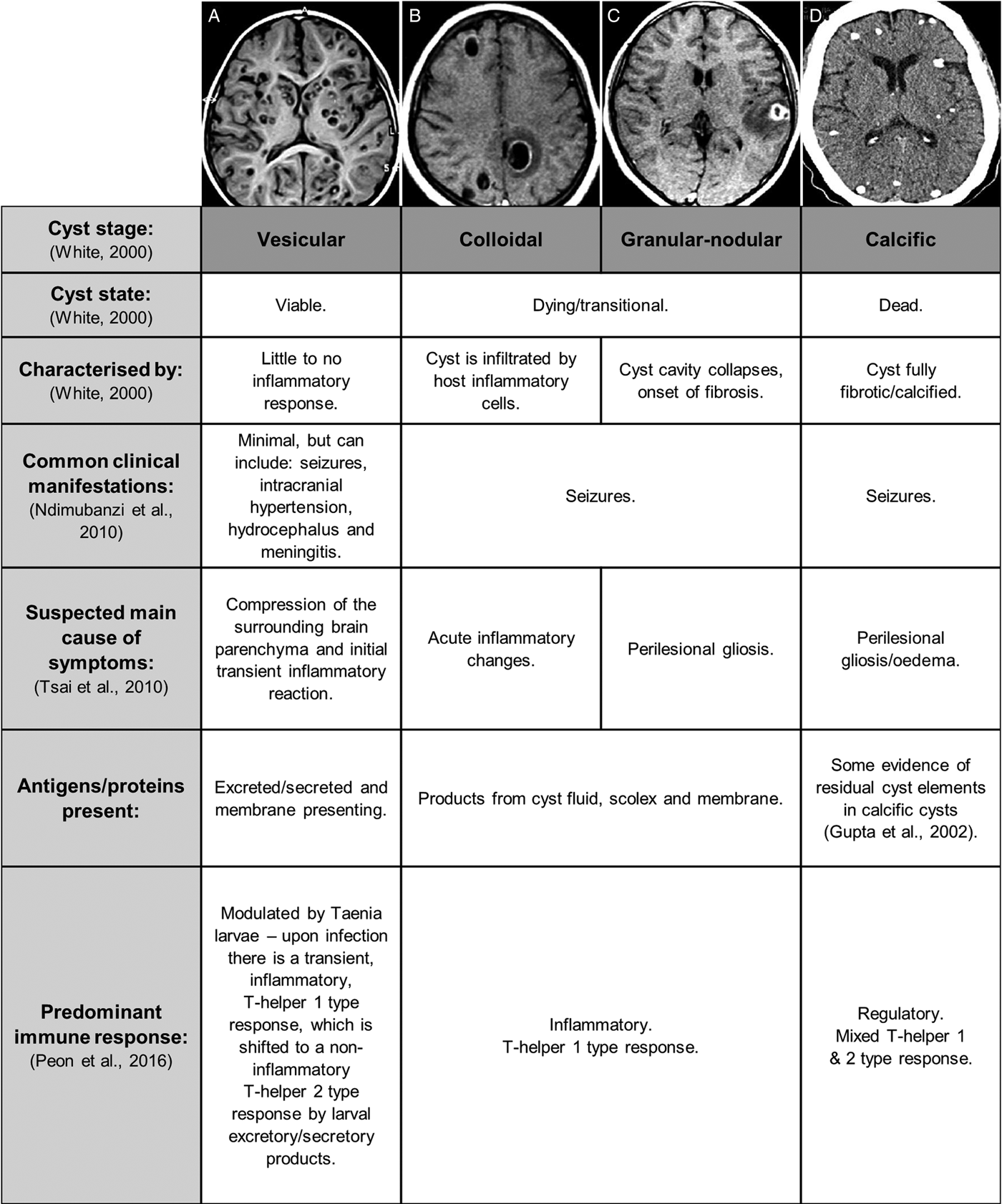
Following initial infection and the establishment of cysticerci in the brain parenchyma, sub-arachnoid space or ventricles, there is usually a lengthy period (months to years) in which the host shows little to no immune or inflammatory response to the infection and experiences no clinical symptoms (White, Reference White2000). This may be because the viable cysticerci employ various immune modulatory mechanisms to remain largely unaffected by the host immune system (White, Reference White2000). Viable cysticerci are also referred to as being in the vesicular stage (White, Reference White2000). At some point, however, the cysts appear to lose their ability to control the host immune response and the cyst wall and fluid become infiltrated by host inflammatory cells. This is termed the colloidal phase. Thereafter the cyst cavity collapses and the host response progresses to surrounding the cyst with fibrosis, resiulting in the granular–nodular phase. Together, cysts in the colloidal or granular–nodular phase are often termed as transitional phase cysts. Eventually the entire cyst is replaced with fibrosis and calcifies and is then said to be in the calcific stage (White, Reference White2000).
Manifestations of NCC are often studied and described in reference to the stage of the cyst (White, Reference White2000; Fleury et al., Reference Fleury, Cardenas, Adalid-Peralta, Fragoso and Sciutto2016; Gonzales et al., Reference Gonzales, Rivera and Garcia2016) (see Fig. 2). Cyst stages are largely determined using imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) scans, as well as post-mortem brain dissections (Carpio and Romo, Reference Carpio and Romo2014). A systematic review of the prevalence of different clinical manifestation of NCC reports seizures or epilepsy as the most common symptom, followed by headaches, intracranial hypertension, hydrocephalus and meningitis (Carabin et al., Reference Carabin, Ndimubanzi, Budke, Nguyen, Qian, Cowan, Stoner, Rainwater and Dickey2011). Seizures occur more commonly in patients with calcified cysts, although this symptom of NCC is still prevalent in those with transitional/active cysts. Intracranial hypertension, hydrocephalus and meningitis seem to be much more common in patients with transitional/active cysts (see Fig. 2). There are numerous other, extremely varied, symptoms secondary to NCC that have been reported (Patel et al., Reference Patel, Jha and Yadav2006; Mahanty and Garcia, Reference Mahanty and Garcia2010; Peon et al., Reference Peon, Ledesma-Soto and Terrazas2016). This diversity in symptoms has been attributed to the diversity in cyst antigens, numbers, stages, positions in the nervous system and the level of inflammation induced (Yakoleff-Greenhouse et al., Reference Yakoleff-Greenhouse, Flisser, Sierra and Larralde1982; Fleury et al., Reference Fleury, Dessein, Marie, Dumas, Tapia, Larralde and Sciutto2004).
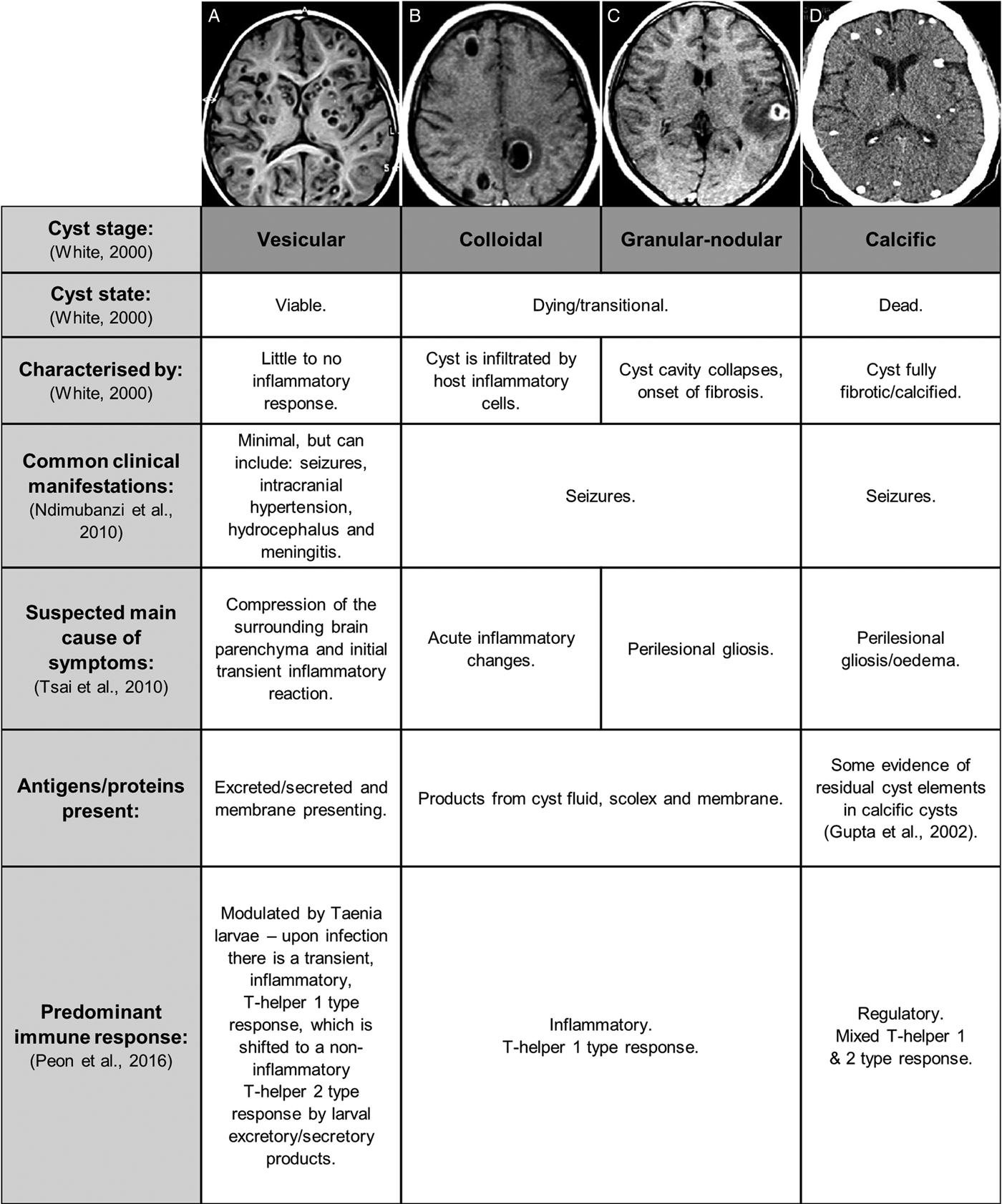
Fig. 2. Key differences in clinical, molecular and immune characteristics associated with Taenia solium cysts in neurocysticercosis [photographs sourced, with permission, from Carpio and Romo (Reference Carpio and Romo2014)].
Studying NCC disease processes in humans
NCC is most commonly studied in patients with the disease, that is, in vivo in humans naturally infected with T. solium larvae. These studies frequently make use of imaging techniques, such as CT and MRI. Such images have then been used in conjunction with clinical presentation of the disease in order to start disentangling what it is that underlies symptom development in NCC. Such studies have provided many valuable insights including that the presence of vesicular cysts may contribute to a patient remaining asymptomatic, even when other stages of cyst are present (Prasad et al., Reference Prasad, Gupta, Pradhan, Tripathi, Pandey and Prasad2008); that patients with calcified parenchymal cysts appear to be more susceptible to depressive symptoms (Leon et al., Reference Leon, Saito, Mehta and McMurtray2015); that some cysts which appear to be inactive on an MRI scan are actually surrounded by gliosis, which may contribute to seizure recurrence (Pradhan et al., Reference Pradhan, Kathuria and Gupta2000); and that the best drug regimens vary for different NCC presentations (Garcia et al., Reference Garcia, Gonzales, Lescano, Bustos, Pretell, Saavedra and Nash2014; Zhao et al., Reference Zhao, Jiang, Ma, Jin, Li, Lu, Nakajima, Huang, Sun, Chen and Chen2016; Del Brutto et al., Reference Del Brutto, Roos, Coffey and Garcia2006).
Another common way of studying disease processes in humans is through the analysis of blood serum or cerebrospinal fluid samples from NCC patients. Such studies have revealed proteins that appear to be specific to active, symptomatic NCC (Chung et al., Reference Chung, Bahk, Huh, Kang, Kong and Cho1999; Ferrer et al., Reference Ferrer, Gonzalez, Foster-Cuevas, Cortez, Davila, Rodrıguez, Scuitto, Harrison, Parkhouse and Garate2005); that T. solium larvae appear to induce a regulatory T-cell response in the host, which creates an environment favourable to their survival (Arce-Sillas et al., Reference Arce-Sillas, Álvarez-Luquín, Cárdenas, Casanova-Hernández, Fragoso, Hernández, Proaño Narváez, García-Vázquez, Fleury, Sciutto and Adalid-Peralta2016); and that there appear to be certain genetic polymorphisms associated with individuals who have symptomatic vs asymptomatic NCC (Verma et al., Reference Verma, Prasad, Gupta, Singh, Nyati, Rizwan, Pandey and Paliwal2010).
More rarely, researchers may obtain brain tissue samples from NCC patients that undergo neurosurgical procedures as a part of their standard clinical care (Restrepo et al., Reference Restrepo, Llaguno, Sandoval, Enciso and Teale1998; Robinson et al., Reference Robinson, Garza, Weinstock, Serpa, Goodman, Eckols, Firozgary and Tweardy2012). One such study has reported that substance P (a neuropeptide involved in neuropathic inflammation and possibly seizure induction) is prevalent in cells adjacent to NCC granulomas, but not in areas distant from granulomas, nor in brain tissue from individuals without NCC (Robinson et al., Reference Robinson, Garza, Weinstock, Serpa, Goodman, Eckols, Firozgary and Tweardy2012). Another examined the cellular and molecular immune response surrounding cysts and reported that there are at least four distinct types of immune responses in NCC (Restrepo et al., Reference Restrepo, Llaguno, Sandoval, Enciso and Teale1998).
Human NCC is also studied in vitro utilizing normal, healthy cell culture lines of nervous system immune cells, or extracted and cultured immune cells from NCC infected patients. Such cell cultures have been utilized in conjunction with T. solium extracts to study immune responses in NCC (Uddin et al., Reference Uddin, Garcia, Gilman, Gonzalez and Friedland2005, Reference Uddin, Gonzalez, Gilman, Thomas, Rodriguez, Evans, Remick, Garcia and Friedland2010; Amit et al., Reference Amit, Nath, Rakesh, Shweta, Sanjeev, Vimal and Mukesh2011). Notable discoveries include that cysts treated with anti-parasitic agents elicit greater chemokine secretion in monocytes than those left untreated (Uddin et al., Reference Uddin, Gonzalez, Gilman, Thomas, Rodriguez, Evans, Remick, Garcia and Friedland2010); that astrocytes play a key role in the inflammatory response to certain T. solium larval elements (Uddin et al., Reference Uddin, Garcia, Gilman, Gonzalez and Friedland2005); that healthy human monocytes respond differently to T. solium brain cysts than to T. solium muscle cysts from pigs (Uddin et al., Reference Uddin, Gonzalez, Gilman, Thomas, Rodriguez, Evans, Remick, Garcia and Friedland2010); and that different cyst elements (scolex, membrane or fluid) elicit different responses in both monocytes and lymphocytes (Uddin et al., Reference Uddin, Gonzalez, Gilman, Thomas, Rodriguez, Evans, Remick, Garcia and Friedland2010; Amit et al., Reference Amit, Nath, Rakesh, Shweta, Sanjeev, Vimal and Mukesh2011).
Challenges to NCC research in humans and the necessity for animal models
A major challenge for the study of NCC in humans is that the disease can only be studied as it occurs naturally. This means that there are large numbers of variables which cannot be controlled for in human studies, making it hard to isolate conditions which lead to symptom onset. Additionally, when patients present with symptomatic NCC, there exists an ethical obligation to start treatment as soon as possible, which also obstructs the understanding of the disease processes underlying symptom development (Cardona et al., Reference Cardona, Restrepo, Jaramillo and Teale1999). Study of the disease in humans is costly, as the only definitive diagnostic techniques are MRI or CT scans, which are typically not available in endemic areas with the highest prevalence rates. In addition, the large variability in human pathology and symptom presentation means that large sample sizes are required to obtain statistical significance. Longitudinal studies in humans are also challenging due to the lengthy time course of the disease, both in terms of symptom onset and progression of the cysts (Cardona et al., Reference Cardona, Restrepo, Jaramillo and Teale1999).
Studies using human tissue are also limited, as most rely on specimens that are collected using only minimally invasive procedures such as the collection of blood samples. Beyond that, samples of brain tissue or cerebrospinal fluid can only be sourced in cases where these are sampled out of clinical or diagnostic need. This is fairly rare, making it difficult to obtain large sample sizes (Cardona et al., Reference Cardona, Restrepo, Jaramillo and Teale1999). Taken together, these challenges greatly limit the exploration of the cellular and molecular processes underlying disease progression in NCC.
Animal models of NCC offer the potential to overcome many of these limitations: they allow for the experimental infection of animals in a controlled environment and for the study of disease progression both with and without treatment interventions. They are often much more cost-effective, as definitive infection can be confirmed post-mortem without neuroimaging, and smaller sample sizes can be used due to the controlled experimental environment. They facilitate longitudinal studies, as the time course of disease, especially in smaller animals, is much shorter and can be accelerated experimentally. Most importantly, animal models allow for unrestricted access to brain tissue and cerebrospinal fluid, thereby enabling more extensive cellular and molecular exploration. Although animal models have great utility in the study of disease processes in NCC, one should always bear in mind that findings from animal studies may not necessarily extrapolate to the human condition, as no animal model can fully recapitulate the human disease state.
In the section that follows, we review existing models of NCC, whilst describing their specific strengths and weaknesses.
Parasites utilized in animal model systems of neurocysticerosis
Model systems for studying NCC typically consist of two components: a cestode species and a host organism. In this review, we will discuss model systems involving three different cestode species.
Taenia solium
Taenia solium (T. solium) is the ‘gold standard’ organism in the study of NCC, as it is the cestode responsible for pathology in humans. Further, the genome of T. solium has recently been sequenced, allowing for the use of powerful genetic tools (Tsai et al., Reference Tsai, Zarowiecki, Holroyd, Garciarrubio, Sanchez-Flores, Brooks, Tracey, Bobes, Fragoso, Sciutto, Aslett, Beasley, Bennett, Cai, Camicia, Clark, Cucher, De Silva, Day, Deplazes, Estrada, Fernandez, Holland, Hou, Hu, Huckvale, Hung, Kamenetzky, Keane, Kiss, Koziol, Lambert, Liu, Luo, Luo, Macchiaroli, Nichol, Paps, Parkinson, Pouchkina-Stantcheva, Riddiford, Rosenvit, Salinas, Wasmuth, Zamanian, Zheng, Consortium, Cai, Soberon, Olson, Laclette, Brehm and Berriman2013). However, T. solium has numerous practical limitations for use within the laboratory setting. It is highly infectious to humans and its experimental use requires strict biosafety measures. It is also challenging to obtain T. solium larvae, and extremely hard to maintain a steady experimental supply of the larvae. Larvae (or cysticerci) can be obtained in three ways: they can be harvested directly from a naturally or experimentally infected pig; they can be produced experimentally by feeding oncospheres [obtained from gravid proglottids in the stool of infected human patients, or from experimentally infected, immunosuppressed, chinchillas or hamsters (Arora et al., Reference Arora, Tripathi, Kumar, Mondal, Mishra and Prasad2017)] to a host in which they will be activated and develop naturally into cysticerci (Nguekam et al., Reference Nguekam, Zoli, Vondou, Pouedet, Assana, Dorny, Brandt, Losson and Geerts2003); or they can be produced by activating oncospheres in vitro and then injecting the activated oncospheres into the brain to develop into cysts (Liu et al., Reference Liu, Li and Hao2002; Verastegui et al., Reference Verastegui, Mejia, Clark, Gavidia, Mamani, Ccopa, Angulo, Chile, Carmen, Medina, Garcia, Rodriguez, Ortega and Gilman2015). Taenia solium may also not be infectious to animals utilized in animal models, thus requiring direct intracranial application, but the larvae of T. solium are large, and may displace most of the brain tissue in small animals.
Taenia crassiceps
Taenia crassiceps (T. crassiceps) is the most commonly utilized model organism for T. solium. The two cestodes are closely related, belonging to the same genus, and have been shown to have significant antigenic similarity (Larralde et al., Reference Larralde, Montoya, Sciutto, Diaz, Govezensky and Coltorti1989; Sciutto et al., Reference Sciutto, Fragoso, Trueba, Lemus, Montoya, Diaz, Govezensky, Lomeli, Tapia and Larralde1990). Taenia crassiceps very rarely infect humans, making it a reasonably safe laboratory model, not requiring extensive biosafety measures (Willms and Zurabian, Reference Willms and Zurabian2010). This is particularly the case for the ORF strain of T. crassiceps, which has entirely lost the ability to infect a definitive host and mature into adult worms, meaning that it does not present an infection risk to animals (Willms and Zurabian, Reference Willms and Zurabian2010). A major advantage of T. crassiceps as a model organism for T. solium is that T. crassiceps larvae are able to rapidly asexually divide by budding in the intermediate host (usually mice), providing a simple way to maintain a steady experimental supply of the organism (Stringer et al., Reference Stringer, Marks, White and Robinson2003; Willms and Zurabian, Reference Willms and Zurabian2010). The larvae are also able to survive for several weeks in in vitro culture.
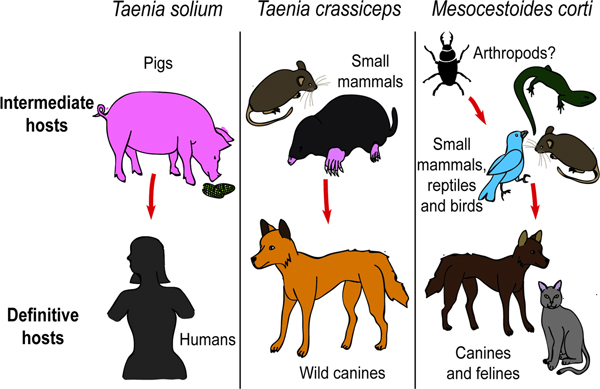
The use of T. crassiceps as a model organism in the study of NCC also has its limitations. The definitive hosts for T. crassiceps are carnivores, most often wild canines (Willms and Zurabian, Reference Willms and Zurabian2010), whilst that of T. solium is humans (see Fig. 3). Taenia crassiceps larvae are usually hosted by rodents and small moles (Willms and Zurabian, Reference Willms and Zurabian2010), whilst pigs host larval T. solium (see Fig. 3). As a result, there must exist differences in the antigens and species-specific immune responses induced by the two organisms (Sciutto et al., Reference Sciutto, Fragoso and Larralde2011). Another concern when utilizing T. crassiceps is that it has been found that many of the strains undergo morphological and genetic changes when they are maintained via serial intraperitoneal inoculation in mice, which may affect their immunogenicity and increase their dissimilarity to T. solium larvae (Zurabian et al., Reference Zurabian, Aguilar, Jiménez, Robert and Willms2008; Willms and Zurabian, Reference Willms and Zurabian2010). Lastly, T. crassiceps are difficult to use where intracranial inoculation of small model animals is desired (e.g. mice), as the cysticerci displace most of the brain tissue (Alvarez et al., Reference Alvarez, Mishra, Gundra, Mishra and Teale2010).

Fig. 3. The intermediate and definitive hosts of Taenia solium, Taenia crassiceps and Mesocestoides corti.
Mesocestoides corti
Mesocestoides corti (M. corti) is thought to infect anthropods as the initial host, small mammals, birds, reptiles and amphibians in the larval form, and carnivores, such as dogs and cats, as an adult worm (Crosbie et al., Reference Crosbie, Padgett and Boyce2000) (see Fig. 3). Mesocestoides corti is not known to infect humans, making it a safe laboratory model not requiring extensive biosafety measures. Mesocestoides corti asexually divides both as cysticerci in the intermediate host (Alvarez et al., Reference Alvarez, Mishra, Gundra, Mishra and Teale2010) and as adult worms in the definitive host (Schmidt and Todd, Reference Schmidt and Todd1978). The larvae have also been shown to be able to survive and divide under the right in vitro culturing conditions (Voge and Coulombe, Reference Voge and Coulombe1966; Vendelova et al., Reference Vendelova, Hrckova, Lutz, Brehm and Komguep2016). A colony of M. corti can therefore be experimentally produced and maintained with relative ease (Schmidt and Todd, Reference Schmidt and Todd1978). Mesocestoides corti is closely related to T. solium, but is not of the same genus, which means that it may have greater antigenic difference to T. solium than does T. crassiceps (Alvarez et al., Reference Alvarez, Mishra, Gundra, Mishra and Teale2010). A major advantage of M. corti is that the cysticerci are significantly smaller than those of T. solium and T. crassiceps, which makes it easier to use for intracranial inoculation of small model animals such as rodents (Alvarez et al., Reference Alvarez, Mishra, Gundra, Mishra and Teale2010). However, unlike T. solium and T. crassiceps, M. corti has not been known to infect the CNS of any of its hosts during its natural cycle, which means that intracranial injection by the experimenter is the only way in which M. corti can enter the CNS (Alvarez et al., Reference Alvarez, Mishra, Gundra, Mishra and Teale2010).
Animal model systems utilized in the study of NCC
Taenia crassiceps in mice
Mice are attractive model organisms for studying NCC due to their popularity across the life sciences. They are relatively cheap to maintain and have a rapid breeding cycle. Most importantly, the relative ease of modifying the mouse genome means that transgenic strains allowing molecular dissection of immunological and neurological pathways are now widely available.
NCC has been modelled in mice by intracranially injecting T. crassiceps larval extracts or intact early-stage larvae (Matos-silva et al., Reference Matos-silva, Reciputti, De Paula, Oliveira, Moura, Vinaud, Oliveira and Lino-Júnior2012; Robinson et al., Reference Robinson, Garza, Weinstock, Serpa, Goodman, Eckols, Firozgary and Tweardy2012; Leandro et al., Reference Leandro, Fraga, de Souza Lino and Vinaud2014). Different strains of mice show differing susceptibility to T. crassiceps intracranial infection (Matos-silva et al., Reference Matos-silva, Reciputti, De Paula, Oliveira, Moura, Vinaud, Oliveira and Lino-Júnior2012). There has been one report of T. crassiceps in the brain of a wild mouse (Kroeze and Freeman, Reference Kroeze and Freeman1982), which is suggestive that further experimentation on the oral administration of T. crassiceps oncospheres in mice (perhaps using immunocompromised individuals) may have potential for the development of a model somewhat more congruent to the condition in humans. One strength of this model is that intracranial injection of peritoneal granulomas can produce seizures in the host (Robinson et al., Reference Robinson, Garza, Weinstock, Serpa, Goodman, Eckols, Firozgary and Tweardy2012), although it is not known whether these seizures could persist chronically.
Intracranial administration of T. crassiceps in mice has provided some valuable insights: one study has shown that early-stage granuloma extracts containing substance P may be responsible for seizure activity; another has shown this model results in encephalitis closely resembling that in human NCC; and a third has shown that Taenia larvae in the brain are highly adaptable when faced with adverse conditions (Matos-silva et al., Reference Matos-silva, Reciputti, De Paula, Oliveira, Moura, Vinaud, Oliveira and Lino-Júnior2012; Robinson et al., Reference Robinson, Garza, Weinstock, Serpa, Goodman, Eckols, Firozgary and Tweardy2012; Leandro et al., Reference Leandro, Fraga, de Souza Lino and Vinaud2014).
Mesocestoides corti in mice
Mesocestoides corti (M. corti) can be utilized in conjunction with cultured mouse primary microglia to explore helminth-associated immunomodulation. One study utilizing this model system elucidated an immunosuppressive mechanism that may help to explain the delay in the onset of neuroinflammation seen in human NCC (Sun et al., Reference Sun, Chauhan, Sukumaran, Sharma, Singh and Mishra2014). More commonly, however, M. corti larvae are administered intracranially in mice (as they do not migrate to the CNS if administered orally) to model human NCC (Cardona et al., Reference Cardona, Restrepo, Jaramillo and Teale1999, Reference Cardona, Gonzalez and Teale2003; Cardona and Teale, Reference Cardona and Teale2002; Alvarez and Teale, Reference Alvarez and Teale2007; Alvarez et al., Reference Alvarez, Mishra, Gundra, Mishra and Teale2010). This model presents with an initial relative lack of immune responsiveness, which is thought may be useful as a comparative model for the study of asymptomatic NCC (Cardona et al., Reference Cardona, Restrepo, Jaramillo and Teale1999). Thus far no seizures have been reported in this model system of NCC, but it has provided much insight into potential mechanisms that dictate the severity of inflammation, blood–brain barrier breakdown, parasite burden and neuronal pathology (Cardona et al., Reference Cardona, Restrepo, Jaramillo and Teale1999, Reference Cardona, Gonzalez and Teale2003; Cardona and Teale, Reference Cardona and Teale2002; Alvarez and Teale, Reference Alvarez and Teale2006, Reference Alvarez and Teale2007).
Taenia crassiceps in rats
Extracts of T. crassiceps larvae have been administered intracranially in rats, but to date no studies have been performed where intact oncospheres/cysts are injected. Studies using this model have shown that the intracranial administration of early-stage granulomal extracts can induce seizures in the host (Stringer et al., Reference Stringer, Marks, White and Robinson2003; Robinson et al., Reference Robinson, Garza, Weinstock, Serpa, Goodman, Eckols, Firozgary and Tweardy2012). This is a very promising finding in terms of its potential use in the study of seizures secondary to NCC. It should be noted, however, that these studies are limited by the fact that the granulomas were produced peripherally in mice, and immune responses differ in different hosts, as well as peripherally as compared with the CNS, so the content of these granulomas may not be reflective of brain cysts. Brain activity was monitored acutely in these studies, so it is not yet known whether recurrent seizures would present in these rats.
Taenia solium in rats
Taenia solium does not naturally infect rodents, and as such very little research has been done using the T. solium–rat combination. In one study, however, activated oncospheres were intracranially injected into rats, and these were found to form cysts in roughly half of the rats after about 4 months (Verastegui et al., Reference Verastegui, Mejia, Clark, Gavidia, Mamani, Ccopa, Angulo, Chile, Carmen, Medina, Garcia, Rodriguez, Ortega and Gilman2015). Importantly, infection is much more successful in younger rats. The authors report that this model presents with many NCC characteristics typical of human infection and that 9% of infected rats present with chronic seizures (Verastegui et al., Reference Verastegui, Mejia, Clark, Gavidia, Mamani, Ccopa, Angulo, Chile, Carmen, Medina, Garcia, Rodriguez, Ortega and Gilman2015). Although this is not a very efficient model of epilepsy secondary to NCC, the induction of chronic seizures is intriguing, and it may be worth exploring whether this model could be optimized for seizure occurrence. The study further revealed diverse cyst distribution and immunopathology, similar to what is observed in humans. A major advantage of this model over other rodent models is the use of the parasite responsible for human disease.
Taenia solium in pigs
The study of T. solium NCC in pigs often involves the utilization of pigs reared for agricultural purposes that have naturally acquired the infection. Pigs can also be experimentally infected by oral administration of T. solium eggs to induce NCC, with between 20 and 100% of pigs dosed with high numbers of eggs developing NCC (de Aluja et al., Reference de Aluja, Villalobos, Plancarte, Rodarte, Hernandez and Sciutto1996; Santamaria et al., Reference Santamaria, Plancarte and de Aluja2002; Nguekam et al., Reference Nguekam, Zoli, Vondou, Pouedet, Assana, Dorny, Brandt, Losson and Geerts2003). Older pigs appear to be more resistant to infection than younger pigs (Santamaria et al., Reference Santamaria, Plancarte and de Aluja2002). Recently, a new model of pig NCC was developed whereby activated T. solium oncospheres are surgically implanted into the subarachnoid space (Fleury et al., Reference Fleury, Trejo, Cisneros, García-Navarrete, Villalobos, Hernández, Hernández, Hernández, Rosas, Bobes, de Aluja, Sciutto and Fragoso2015). All infected pigs developed brain cysts, although at very low infection efficiencies (Fleury et al., Reference Fleury, Trejo, Cisneros, García-Navarrete, Villalobos, Hernández, Hernández, Hernández, Rosas, Bobes, de Aluja, Sciutto and Fragoso2015). This model surprisingly did not result in any neurological signs (Fleury et al., Reference Fleury, Trejo, Cisneros, García-Navarrete, Villalobos, Hernández, Hernández, Hernández, Rosas, Bobes, de Aluja, Sciutto and Fragoso2015).
The pig model system of NCC has been extremely useful in characterizing immunopathological and proteomic changes in response to T. solium in the brain, both in the normal course of disease and after a vaccination or treatment protocol. Significant overlap between human and pig reactions has been found (Molinari et al., Reference Molinari, Meza and Tato1983; Sikasunge et al., Reference Sikasunge, Johansen, Phiri, Willingham and Leifsson2009; Guerra-Giraldez et al., Reference Guerra-Giraldez, Marzal, Cangalaya, Balboa, Orrego, Paredes, Gonzales-Gustavson, Arroyo, García, González, Mahanty and Nash2013; Singh et al., Reference Singh, Prasad, Prasad, Tripathi, Gupta and Husain2013; Mahanty et al., Reference Mahanty, Orrego, Mayta, Marzal, Cangalaya, Paredes, Gonzales-Gustavson, Arroyo, Gonzalez, Guerra-Giraldez, Garcia and Nash2015; Christensen et al., Reference Christensen, Trevisan, Leifsson and Johansen2016; Navarrete-Perea et al., Reference Navarrete-Perea, Isasa, Paulo, Corral-Corral, Flores-Bautista, Hernández-Téllez, Bobes, Fragoso, Sciutto, Soberón, Gygi and Laclette2017). A recent study reports severe seizures in naturally infected pigs, which could be extremely valuable in aiding progress towards understanding the most common symptomatic presentation in NCC (Trevisan et al., Reference Trevisan, Mkupasi, Ngowi, Forkman and Johansen2016). The pigs that presented with seizures in this study were much older than the others in the sample group, suggesting that a longer infection/experimental period may be necessary for neurological symptoms to present (Trevisan et al., Reference Trevisan, Mkupasi, Ngowi, Forkman and Johansen2016). NCC in pigs also presents with great variation in the infection characteristics and antibody response, which suggests that porcine models may be able, to some extent, to recapitulate the great variation in pathology and disease progression that is observed in infected humans (Prasad et al., Reference Prasad, Chawla, Prasad, Tripathi, Husain and Gupta2006; Saenz et al., Reference Saenz, Ramirez, Aluja, Escobar, Fragoso, Morales, Perez-Tamayo, Rosetti, Larralde, Sciutto and Fleury2008).
Limitations of the pig–T. solium model system includes that this model can prove very time and resource intensive, with T. solium cysts taking as long as 350 days to form in pigs (de Aluja et al., Reference de Aluja, Villalobos, Plancarte, Rodarte, Hernandez and Sciutto1996), pig handlers requiring training and larger animals requiring more resources to feed and keep (de Aluja et al., Reference de Aluja, Villalobos, Plancarte, Rodarte, Hernandez and Sciutto1996; Arora et al., Reference Arora, Tripathi, Kumar, Mondal, Mishra and Prasad2017).
Taenia solium in rhesus monkeys
Primate studies of neurological conditions are rare due to the significant ethical (Greene et al., Reference Greene, Schill, Takahashi, Bateman-House, Beauchamp, Bok, Cheney, Coyle, Deacon, Dennett, Donovan, Flanagan, Goldman, Greely, Martin, Miller, Mueller, Siegel, Solter, Gearhart, McKhann and Faden2005) and legal (Fox, Reference Fox2009) implications of using primates for research purposes. Many countries have laws in place either preventing primate research or restricting their use to cases where all other options have been exhausted or found unsuitable (Greene et al., Reference Greene, Schill, Takahashi, Bateman-House, Beauchamp, Bok, Cheney, Coyle, Deacon, Dennett, Donovan, Flanagan, Goldman, Greely, Martin, Miller, Mueller, Siegel, Solter, Gearhart, McKhann and Faden2005; Fox, Reference Fox2009).
Taenia solium has, however, been reported to infect several non-human primates in its larval form, although this is considered an ‘accidental infection’ since T. solium does not require infection of non-human primates to complete its life cycle (Kuntz, Reference Kuntz and Bourne1973; Johnston et al., Reference Johnston, Dyer, Madison-Antenucci, Mergen, Veeder and Brice2016). NCC can be reliably induced in rhesus monkeys by feeding them large doses of activated T. solium oncospheres. The infected monkeys present with seizures and clinical symptoms very similar to those in humans within a matter of days, and if not treated may eventually die from the infection (Saleque et al., Reference Saleque, Chowdhury, Iyer and Baruah1988; Chowdhury et al., Reference Chowdhury, Saleque, Sood and Singla2014). Symptom presentation is delayed and attenuated in monkeys receiving smaller numbers of oncospheres, which may more closely resemble the human condition (Chowdhury et al., Reference Chowdhury, Saleque, Sood and Singla2014). It is interesting to note that symptom onset could be induced within a matter of days, in contrast to a study reporting a case of naturally acquired NCC in an 8-year-old rhesus monkey, which presented with no symptoms (Johnston et al., Reference Johnston, Dyer, Madison-Antenucci, Mergen, Veeder and Brice2016). This could be explained by the high dosage of oncospheres used in the experimental studies and serves as a reminder of the importance of dose in eliciting disease phenotypes in models of NCC.
Future roles for animal model systems in the study of NCC
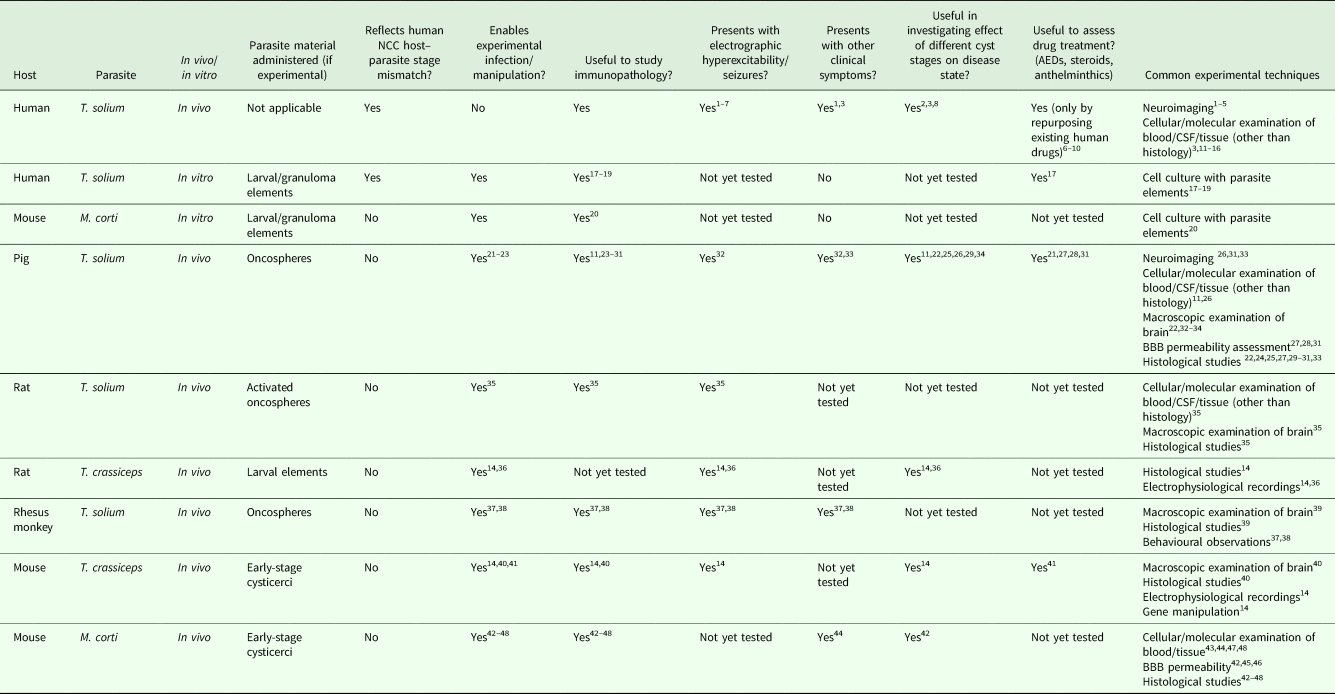
Due to the remaining uncertainty surrounding disease mechanisms in NCC and the limitations of studying the disease in humans, there is a need for continued exploration and improvement of animal models that recapitulate the human disease process. Table 1 summarizes the respective utility of currently available model systems used in NCC research and highlights the fact that there still exist many areas that remain unexplored.
Table 1. A summary of the characteristics and utilities of existing model systems for the study of neurocysticercosis
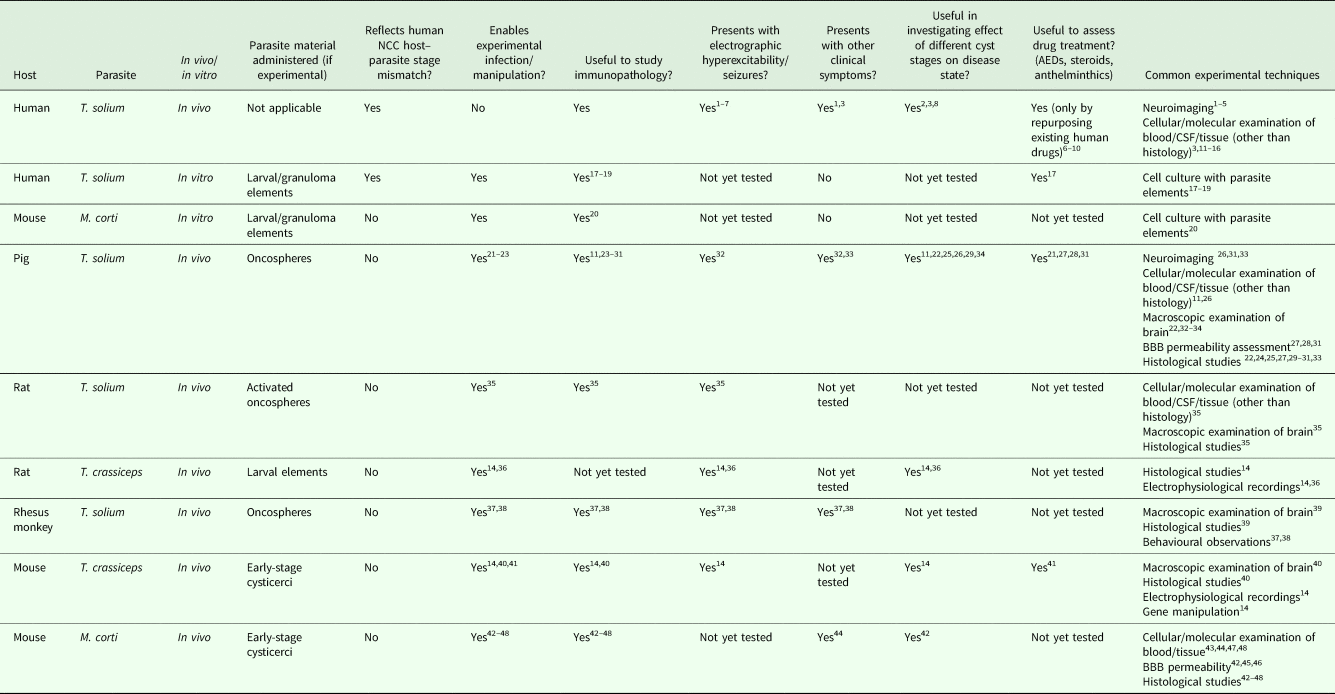
Table reference guide: 1 – Leon et al. (Reference Leon, Saito, Mehta and McMurtray2015); 2 – Prasad et al. (Reference Prasad, Gupta, Pradhan, Tripathi, Pandey and Prasad2008); 3 – Fleury et al. (Reference Fleury, Dessein, Marie, Dumas, Tapia, Larralde and Sciutto2004); 4 – Pradhan et al. (Reference Pradhan, Kumar and Gupta2003); 5 – Pradhan et al. (Reference Pradhan, Kathuria and Gupta2000); 6 – Das et al. (Reference Das, Mondal, Banerjee, Mukherjee and Singh2007); 7 – Garcia et al. (Reference Garcia, Gonzales, Lescano, Bustos, Pretell, Saavedra and Nash2014); 8 – Del Brutto et al. (Reference Del Brutto, Roos, Coffey and Garcia2006); 9 – Zhao et al. (Reference Zhao, Jiang, Ma, Jin, Li, Lu, Nakajima, Huang, Sun, Chen and Chen2016); 10 – Nash et al. (Reference Nash, Singh, White, Rajshekhar, Loeb, Praono, Takayanagui, Gonzalez, Butman, DeGiorgio, Del Brutto, Delgado-Escueta, Evans, Gilman, Martinez, Medina, Pretell, Teale and Garcia2006); 11 – Ferrer et al. (Reference Ferrer, Gonzalez, Foster-Cuevas, Cortez, Davila, Rodrıguez, Scuitto, Harrison, Parkhouse and Garate2005); 12 – Chung et al. (Reference Chung, Bahk, Huh, Kang, Kong and Cho1999); 13 – Arce-Sillas et al. (Reference Arce-Sillas, Álvarez-Luquín, Cárdenas, Casanova-Hernández, Fragoso, Hernández, Proaño Narváez, García-Vázquez, Fleury, Sciutto and Adalid-Peralta2016); 14 – Robinson et al. (Reference Robinson, Garza, Weinstock, Serpa, Goodman, Eckols, Firozgary and Tweardy2012); 15 – Restrepo et al. (Reference Restrepo, Llaguno, Sandoval, Enciso and Teale1998); 16 – Verma et al. (Reference Verma, Prasad, Gupta, Singh, Nyati, Rizwan, Pandey and Paliwal2010); 17 – Uddin et al. (Reference Uddin, Gonzalez, Gilman, Thomas, Rodriguez, Evans, Remick, Garcia and Friedland2010); 18 – Uddin et al. (Reference Uddin, Garcia, Gilman, Gonzalez and Friedland2005); 19 – Amit et al. (Reference Amit, Nath, Rakesh, Shweta, Sanjeev, Vimal and Mukesh2011); 20 – Sun et al. (Reference Sun, Chauhan, Sukumaran, Sharma, Singh and Mishra2014); 21 – de Aluja et al. (Reference de Aluja, Villalobos, Plancarte, Rodarte, Hernandez and Sciutto1996); 22 – Fleury et al. (Reference Fleury, Trejo, Cisneros, García-Navarrete, Villalobos, Hernández, Hernández, Hernández, Rosas, Bobes, de Aluja, Sciutto and Fragoso2015); 23 – Nguekam et al. (Reference Nguekam, Zoli, Vondou, Pouedet, Assana, Dorny, Brandt, Losson and Geerts2003); 24 – Molinari et al. (Reference Molinari, Meza and Tato1983); 25 – Sikasunge et al. (Reference Sikasunge, Johansen, Phiri, Willingham and Leifsson2009); 26 – Singh et al. (Reference Singh, Prasad, Prasad, Tripathi, Gupta and Husain2013); 27 – Marzal et al. (Reference Marzal, Guerra-Giraldez, Paredes, Cangalaya, Rivera, Gonzalez, Mahanty, Garcia, Nash, Gilman, Arroyo, Gonzales-Gustavson, Tsang, Verastegui, Zimic, Mayta, Orrego, Saenz, Gonzales and Saavedra2014); 28 – Guerra-Giraldez et al. (Reference Guerra-Giraldez, Marzal, Cangalaya, Balboa, Orrego, Paredes, Gonzales-Gustavson, Arroyo, García, González, Mahanty and Nash2013); 29 – Alvarez et al. (Reference Alvarez, Londoo, Alvarez, Trujillo, Jaramillo and Restrepo2002); 30 – Christensen et al. (Reference Christensen, Trevisan, Leifsson and Johansen2016); 31 – Cangalaya et al. (Reference Cangalaya, Bustos, Calcina, Vargas-Calla, Suarez, Gonzalez, Chacaltana, Guerra-Giraldez, Mahanty, Nash and García2016); 32 – Trevisan et al. (Reference Trevisan, Mkupasi, Ngowi, Forkman and Johansen2016); 33 – Prasad et al. (Reference Prasad, Chawla, Prasad, Tripathi, Husain and Gupta2006); 34 – Saenz et al. (Reference Saenz, Ramirez, Aluja, Escobar, Fragoso, Morales, Perez-Tamayo, Rosetti, Larralde, Sciutto and Fleury2008); 35 – Verastegui et al. (Reference Verastegui, Mejia, Clark, Gavidia, Mamani, Ccopa, Angulo, Chile, Carmen, Medina, Garcia, Rodriguez, Ortega and Gilman2015); 36 – Stringer et al. (Reference Stringer, Marks, White and Robinson2003); 37 – Chowdhury et al. (Reference Chowdhury, Saleque, Sood and Singla2014); 38 – Saleque et al. (Reference Saleque, Chowdhury, Iyer and Baruah1988); 39 – Johnston et al. (Reference Johnston, Dyer, Madison-Antenucci, Mergen, Veeder and Brice2016); 40 – Matos-silva et al. (Reference Matos-silva, Reciputti, De Paula, Oliveira, Moura, Vinaud, Oliveira and Lino-Júnior2012); 41 – Leandro et al. (Reference Leandro, Fraga, de Souza Lino and Vinaud2014); 42 – Cardona et al. (Reference Cardona, Restrepo, Jaramillo and Teale1999); 43 – Cardona et al. (Reference Cardona, Gonzalez and Teale2003); 44 – Cardona and Teale (Reference Cardona and Teale2002); 45 – Alvarez and Teale, (Reference Alvarez and Teale2007); 46 – Alvarez and Teale, (Reference Alvarez and Teale2006); 47 – Gundra et al. (Reference Gundra, Mishra, Wong and Teale2011); 48 – Mishra et al. (Reference Mishra, Gundra and Teale2011).
One aspect of the disease that may be useful consider when designing model systems is that the disease state in humans involves a mismatch between the host and the parasite stage. Humans are not natural hosts for the larval life stage of T. solium (Fig. 1). Therefore, it may be worth considering using or creating model systems where this mismatch is replicated. Inducing NCC using T. crassiceps or M. corti in animals that usually act as the definitive host for these parasites (such as cats or dogs – see Fig. 3), for example. Reports exist of CNS infections by T. crassiceps and T. solium occurring naturally in cats or dogs, and although seizures are not amongst the neurological symptoms reported in these cases, they provide an encouraging precedent for experimental models in these animals (Rogers et al., Reference Rogers, Pandey and Bleakley1989; Crosbie et al., Reference Crosbie, Padgett and Boyce2000; Wünschmann et al., Reference Wünschmann, Garlie, Averbeck, Kurtz and Hoberg2003; Jull et al., Reference Jull, Browne, Boufana, Schöniger and Davies2012). By recreating the host–parasite stage mismatch, models in canines offer a potential new avenue for NCC research, although this would need to be weighed against the ethical and cultural concerns of using these animals for research purposes.
Another potential avenue of exploration towards the expansion of animal model systems is the use of novel model parasites. Taenia taeniaeformis, for example, has been used to experimentally induce cysticercosis in rodents and been shown to have significant antigenic similarity to T. solium (Shukla et al., Reference Shukla, Husain, Jyotsna, Gupta and Husain2008; Preet and Prakash, Reference Preet and Prakash2011). Further, another species of Taenia, Taenia serialis, has been reported to cause cerebral cysticercosis in cats, and as such may also be an interesting experimental parasite to explore (Jull et al., Reference Jull, Browne, Boufana, Schöniger and Davies2012; Orioles et al., Reference Orioles, Beltran, Stewart, Boufana and Holloway2014).
There currently exists a paucity of model systems that result in the development of seizures, and even fewer that result in recurrent seizures (refer to Table 1). One way in which research into this aspect of NCC could be relatively easily expanded is through the use of in vitro/ex vivo models using neural tissue, such as acute or organotypic brain slices (De Simoni and Yu, Reference De Simoni and Yu2006). Whilst these model systems lack many key components necessary for fully recapitulating the disorder (e.g. adaptive immunity), brain slice models do allow for unprecedented experimental and molecular access to the tissue in ways that are difficult or impossible to accomplish in vivo. The expansion and optimization of in vivo animal models, that result in seizures would also be extremely valuable. Such a rodent model would be particularly well received, as experimental tools for observing and manipulating neural circuits and seizure activity in rodents have progressed remarkably over the past two decades. For example, wireless telemetry now enables chronic (>3 months) EEG recordings in freely moving mice and rats, which can document the development of seizures over time (Wykes et al., Reference Wykes, Heeroma, Mantoan, Zheng, MacDonald, Deisseroth, Hashemi, Walker, Schorge and Kullmann2012); and transgenic mouse lines can selectively knockout immunological pathways (Ndlovu and Brombacher, Reference Ndlovu and Brombacher2014) or allow in vivo calcium imaging to better understand circuit-level changes which result in seizures (Madisen et al., Reference Madisen, Zwingman, Sunkin, Oh, Zariwala, Gu, Ng, Palmiter, Hawrylycz, Jones, Lein and Zeng2010). Any animal model of NCC which results in recurrent seizures would have additional worth as a general inflammatory model of epilepsy, for which reliable animal models are still lacking (Nash et al., Reference Nash, Mahanty, Loeb, Theodore, Friedman, Sander, Singh, Cavalheiro, Del Brutto, Takayanagui, Fleury, Verastegui, Preux, Montano, Pretell, White, Gonzales, Gilman and Garcia2015).
Whilst genetically altered mice are an underutilized resource in the study of NCC, there is also much potential for the development of molecular tools for modifying the genomes of model parasites. There is one research group who, for example, are developing a ‘reporter’ strain of T. crassiceps where larvae express fluorescent proteins (Moguel et al., Reference Moguel, Moreno-Mendoza, Bobes, Carrero, Chimal-Monroy, Díaz-Hernández, Herrera-Estrella and Laclette2015). This could prove valuable for tracking the parasites in vivo. Further, genetic knock-out cestode strains could help isolate parasite functions that are crucial for disease progression, and thereby help elucidate the mechanisms of disease.
Conclusion
NCC is an important global health challenge that is still poorly understood. Whilst the study of NCC in patients has provided important insights into the disease, there exist innate limitations that can only be overcome through the use and continued development of animal models. Model systems utilizing T. solium, T. crassiceps and M. corti in mice, rats, pigs and even rhesus monkeys have generated invaluable knowledge on disease mechanisms in NCC. However, there is a great need for animal models of NCC which result in seizures and epilepsy. New rodent model systems of NCC would allow researchers to take advantage of the latest technological advances to explore the disease at unprecedented molecular and cellular detail. We believe that there is still huge potential that could be realized in animal model systems, and that this represents the key to ultimately unlocking a definitive understanding – and treatment of NCC.
Author ORCIDs
Anja de Lange 0000-0002-7117-8979
Acknowledgements
We would like to acknowledge Hayley Tomes and Laura de Lange for valuable discussions, which informed the contents of this manuscript.
Financial support
This work was supported by the Royal Society (J.V.R., Newton Advanced Fellowship), the South African National Research Foundation (J.V.R, Competitive Funding for Rated Researchers; A.D., Scarce Skills Doctoral Scholarship), the Oppenheimer Memorial Trust (A.D., Doctoral Scholarship) and the University of Cape Town (A.D., Doctoral Research Scholarship).
Conflict of interest
None.
Ethical standards
Not applicable.