THE PARASITE
There is perhaps no other metazoan parasite that evokes such awe and revulsion within the fishing fraternity world-wide than the plerocercoid stage of the pseudophyllidean cestode, Ligula intestinalis. This is primarily due to the impressive size which this parasite can obtain in the body cavity of its fish intermediate host, and yet it is the size of the parasite and its relationship with its fish host which has made this parasite such a valuable model to study parasite/host interactions at the molecular, cellular, organismal and population levels. As a highlight of the size which the infection can attain, Barus and Prokes (Reference Barus and Prokes1994) noted that the weight of parasite tissue can be greater than that of the fish tissue (Fig. 1). This relationship has been studied utilising the parasitisation index (parasite weight/fish weight×100) which normally lies in the range of 1–20% (Claridge et al. Reference Claridge, Hardisty, Potter and Williams1985) or up to 40% as recorded by Morrison (Reference Morrison1977). It is perhaps not surprising therefore that the infection has been associated with a distension of the body wall which leads to separation of the scales which may allow entry of pathogens (e.g. Sweeting, Reference Sweeting1977), and effects on the body wall and musculature (Richards and Arme, Reference Richards and Arme1981; Loot et al. Reference Loot, Lek, Brown and Guegan2001c). In some rare instances, perforation of the body wall and intrusion of the parasite into the aquatic environment has been noted (e.g. Barus et al. Reference Barus, Sebela and Prokes1997). The authors proposed that this may represent a possible means for a free-living phase of the plerocercoid or, at least, prolong the window for transmission into a definitive host for several days after death of the host.

Fig. 1. Ligulosed roach, Rutilus rutilus, fixed in Bouins fixative and cleared in xylene. Note plerocercoid burden (P) within the body cavity of the fish host. (Picture reproduced by kind permission of Professor C. Arme, Keele University, UK.)
The plerocercoid of L. intestinalis occurs in a wide range of fish hosts. In Europe it has been found in several species of cyprinids, e.g. roach (Rutilus rutilus), rudd (Scardinius erythrophthalmus), dace (Leuciscus leuciscus), gudgeon (Gobio gobio), bream (Abramis brama), bleak (Alburnus alburnus), minnow (Phoxinus phoxinus), chub (Leuciscus cephalus), tench (Tinca tinca) and silver bream (Blicca bjoerkna) (Orr, Reference Orr1968; Arme and Owen, Reference Arme and Owen1968; Harris and Wheeler, Reference Harris and Wheeler1974; Adamek et al. Reference Adamek, Barus and Prokes1996; Museth, Reference Museth2001; Loot et al. Reference Loot, Brosse, Lek and Guegan2001a, Reference Loot, Fancisco, Santoul, Lek and Gueganb, Reference Loot, Lek, Brown and Gueganc, Reference Loot, Lek, Dejean and Guegand, Reference Loot, Aulagnier, Lek, Thomas and Guegan2002a, Reference Loot, Poulin, Lek and Gueganb, Reference Loot, Park, Lek and Brosse2006; Hecker et al. Reference Hecker, Sanderson and Karbe2007). Dubinina (Reference Dubinina1966) noted 49 species of fish in the former USSR were hosts of the parasite, whilst there are several authors who have indicated the presence of the worm in the North American continent. For example, in Canada, Szalai et al. (Reference Szalai, Yang and Dick1989) noted the parasite in white suckers (Catostomus commersoni), yellow perch (Perca flavescens), quillback (Carpoides cyprinus) and spottail shiners (Notropis hudsonius), and Groves and Shields (Reference Groves and Shields2001) and Shields et al. (Reference Shields, Groves, Rombaugh and Bellmore2002) found the parasite in the Crooked River system and Haystack Reservoir in central Oregon. Ligula has also been found in the Middle East (Ergonul and Altindag, Reference Ergonul and Altindag2005a, Reference Ergonul and Altindagb; Sasi, Reference Sasi2005; Kir and Tekin-Ozan, Reference Kir and Tekin-Ozan2005; Hatice et al. Reference Hatice, Erdogan and Coz-rakovac2006; Shargh et al. Reference Shargh, Shamsaii and Karimi2008; Hajirostamloo, Reference Hajirostamloo2008; Aydogdu et al. Reference Aydogdu, Selver and Cirak2008; Tekin-Ozan and Kir, Reference Tekin-Ozan and Kir2008), Africa (Dejen et al. Reference Dejen, Vijverberg and Sibbing2006; Cowx et al. Reference Cowx, Rollins and Tumwebaze2008; Britton et al. Reference Britton, Jackson and Harper2009) and Australasia (Pollard, Reference Pollard1974; Weekes and Penlington, Reference Weekes and Penlington1986; Morgan, Reference Morgan2003). In China, the parasite can cause problems in culture systems (Xianghua and Zhixin, Reference Xianghua and Zhixin1987; Li and Liao, Reference Li and Liao2003). The large range of fish species that the parasite has been recorded from is also extended to the association between the definitive host and the adult parasite, for example, Dubinina (Reference Dubinina1966) claimed 72 species of bird can serve as final hosts for the Ligulidae. Dubinina has also given an account of the development cycle of Ligula in which parasite eggs, which first appear in the uterus 45–50 hours after infection, pass out in the birds' faeces and hatch after 5–8 days to release the free-swimming coracidia. These are ingested by the copepod first intermediate host in which the procercoid develops, and the fish becomes infected by consuming the infected copepod. The ubiquitous nature of Ligula in terms of host fish and geographical range has meant that this parasite has been a good model to study speciation and diversity in fish parasites.
LIGULA AS AN ECOLOGICAL MODEL
Although there have been numerous studies on the ecological interactions between fish parasites and their hosts, the majority have concentrated on limited time spans with, on some occasions, speculation on the long-term implications of infection. None of the studies undertaken, except some of those carried out on Ligula, have considered what happens to the epizootic and the implications for the aquatic community over an extended time period. Although some studies (Bauer and Stolyarov, Reference Bauer, Stolyarov, Dogiel, Petrushevski and Polyanski1961; Black and Fraser, Reference Black and Fraser1984; Izyumova, Reference Izyumova1987) have revealed that Ligula may persist for several years within a single water body, the majority of studies, for example those carried out in the UK (Wilson, Reference Wilson1971; Sweeting, Reference Sweeting1976; Morrison, Reference Morrison1977; Tobin, Reference Tobin1986; Bean and Winfield, Reference Bean and Winfield1992), have noted that a decline of fish host populations leads to reduction in transmission rates of the parasite. This reduction is thought to be due primarily to an increase in fish mortality in infected fish caused by either a reduction in their ability to survive over winter (Wyatt and Kennedy, Reference Wyatt and Kennedy1988) and/or by making the infected fish more susceptible to predation both by birds and fish (Van Dobben, Reference Van Dobben1952; Holmes and Bethel, Reference Holmes, Bethel, Canning and Wright1972; Sweeting, Reference Sweeting1976; Hoole, Reference Hoole, Pike and Lewis1994). Studies on metazoan parasite/host systems in natural environments are invariably, due to funding restrictions or available opportunities, restricted to short time periods. The effects of Ligula on host populations noted above make this parasite a unique model to study long-term effects of parasitisation on host dynamics. This has been achieved by several eloquent studies carried out by Kennedy and co-workers over a period of 31 years utilising Ligula infections in Chew Lakes, Slapton Ley, Devon, UK (Kennedy et al. Reference Kennedy, Shears and Shears2001). The events that pre-disposed the lake for introduction of Ligula began in the 1960s when increasing eutrophic conditions and an expansion of the roach population led to intra-specific competition and the presence of numerous small, stunted roach and a decline in the dominant rudd population. This change in the fish population dynamics and availability of an ample small sized food source attracted Great Crested Grebes, Podiceps cristatus, one of the definitive hosts for L. intestinalis and it was not surprising to note the appearance of ligulosed roach in 1973. The prevalence peaked at 28% in 1975 and resulted in a decline in the roach population and a subsequent recovery in the rudd numbers. This was the first cycle of Ligula infection whose details were described by Burrough and Kennedy (Reference Burrough and Kennedy1979), Kennedy and Burrough (Reference Kennedy and Burrough1981) and Wyatt and Kennedy (Reference Wyatt and Kennedy1988, Reference Wyatt and Kennedy1989). The decrease in roach numbers led to increased growth rates, but adverse winter conditions during 1984–1985 caused substantial fish deaths and the parasite re-appeared in 1991 in the 1989, 1990 and 1991 year classes. In this second wave of infection, prevalence levels were very high i.e. 75% peaking in 1992. A decline in infection occurred between 1993–1994. It would appear that a third cycle also occurred from 1999 and although infection occurred in 0, 1 and 2 year old fish, prevalence never exceeded 14%. The studies on Slapton Ley are therefore unique, not only in their duration, but also in the fact that 3 distinct infection cycles were clearly observed although this does not imply any regulation or stability within the system as infection cycles varied. In previous short-term studies (Arme and Owen, Reference Arme and Owen1968; Harris and Wheeler, Reference Harris and Wheeler1974; Sweeting, Reference Sweeting1976, Reference Sweeting1977; Bean and Winfield, Reference Bean and Winfield1992), biotic factors such as fish feeding behaviour and copepod levels were suggested to affect Ligula predominance in smaller fish. In contrast, in the second cycle at Slapton Ley, fish of all ages may have been infected. Kennedy et al. (Reference Kennedy, Shears and Shears2001) proposed that whilst the long-term study may suggest controlled population regulation, the first and second cycles were in fact independent events and the cycles reflect changes in habitat. Recently Ligula intestinalis has proved an invaluable model to elucidate how parasites may modify their host phenotypic appearance, and the impact that this has on the ecological interactions between parasites and their hosts, and transmission success. In 1999, Lafferty defined a phenomenon which resulted in an increase in parasite fitness mediated by evolutionary processes, the so-called ‘Parasite Increased Trophic Transmission – PITT’. Extensive studies carried out since 2000 by Loot and co-workers on ligulosed roach populations in France have made a significant contribution to testing the PITT hypothesis in natural systems. Detailed morphological investigations on roach collected from the Lavernose-Lacasse gravel pit complex in Toulouse revealed that extensive morphological changes occurred in ligulosed roach which were dependent on total parasite biomass (Loot et al. Reference Loot, Lek, Brown and Guegan2001c). Further studies by Loot et al. (Reference Loot, Lek, Dejean and Guegan2001d) showed that in the Lavernose, Muret and Pareloup lake systems the distribution of the plerocercoid of L. intestinalis was spatially and temporally clumped within their fish hosts although this aggregation was far more pronounced in the Lavernose Lake suggesting the presence of site-related affects. Loot and co-workers (Reference Loot, Brosse, Lek and Guegan2001a) also noted that ligulosed roach were more highly parasitised closer to the bank and that parasite occurrence and abundance were both highly significant parameters in accounting for this spatial distribution. They also showed that these two parameters decreased in fish older than 3 years of age, suggesting that Ligula infection resulted in host death. Three hypotheses were considered to explain why parasite infection was greatest near the bank of the lake. Whilst the possibilities that this infection distribution was due to an accidental side-effect of parasitisation or perhaps was correlated to the localisation of infected first intermediate copepod hosts were considered, the authors proposed that Ligula increased feeding motivation into the highly productive littoral areas of the lake and the reduced swimming efficiency increased predation by the bird definitive host. These results thus support the PITT hypothesis. Detailed studies on the interaction between L. intestinalis and its fish host have primarily concentrated on the Euro-Mediterranean clades and it is only recently that the PITT hypothesis has been investigated in other geographical localities. Britton et al. (Reference Britton, Jackson and Harper2009) investigated Ligula host specificity in the fish population of Lakes Baringo and Naivasha in Kenya's Rift Valley. It was noted that the parasite had a restricted second intermediate host range occurring in two cyprinid fish species, Barbus lineomaculatus and B. paludinosus, and, in comparison to the Euro-Mediterranean clade, multiple infections were not observed frequently. The authors suggested that since parasite prevalence was correlated to habitat the results obtained could be interpreted as supportive of the PITT hypothesis. The complex life cycle of L. intestinalis has also recently been utilised to establish the contributing role that host ecological dynamics and physiological compatibility has on parasite transmission and evolution. Loot et al. (Reference Loot, Park, Lek and Brosse2006), using measurements of host abundances over time, noted that in the Lavernose-Lacasse gravel pit system used in earlier studies, the favoured Ligula hosts were the copepod, Eudiaptomus gracilis, the roach Rutilus rutilus and the great crested grebe (Podiceps cristatus). It was suggested that the similar temporal dynamics and frequent associations between hosts and parasite created a stable system that promoted the successful completion of the Ligula life cycle. Such an association, it was proposed, affected the evolution of L. intestinalis specificity. It was hypothesised that the selection of hosts was driven by the probability of successive hosts encountering each other which aided parasite transmission and whilst, biochemical compatibility between host and parasite was important, this may be secondary to spatial and temporal dynamics.
There have been extensive studies on the pathological and biochemical interactions between Ligula and its fish hosts (for example see Arme et al. Reference Arme, Griffiths and Sumpter1982; Hoole, Reference Hoole, Pike and Lewis1994) which have primarily been stimulated by the large parasite burdens that can occur in some hosts and the subsequent extensive size that infected hosts can attain. The possibility that host gigantism occurs in Ligula infection has thus been recently investigated by Loot et al. (Reference Loot, Poulin, Lek and Guegan2002b) who revealed that the parasite induced an enhanced growth rate during the first 2 years of the life of the roach host. Whilst the possibility that the parasite produced growth enhancers or may divert energy from gonadal development to somatic growth were considered, the authors proposed that since cestode-associated growth in the heaviest infected fish was only found in one out of the three localities studied, that the effect may result from a change in foraging behaviour of the fish. What these and other studies have highlighted however is the intricate association between Ligula and the reproductive potential of its fish host.
LIGULA: THE MODEL OF ENDOCRINE DISRUPTION
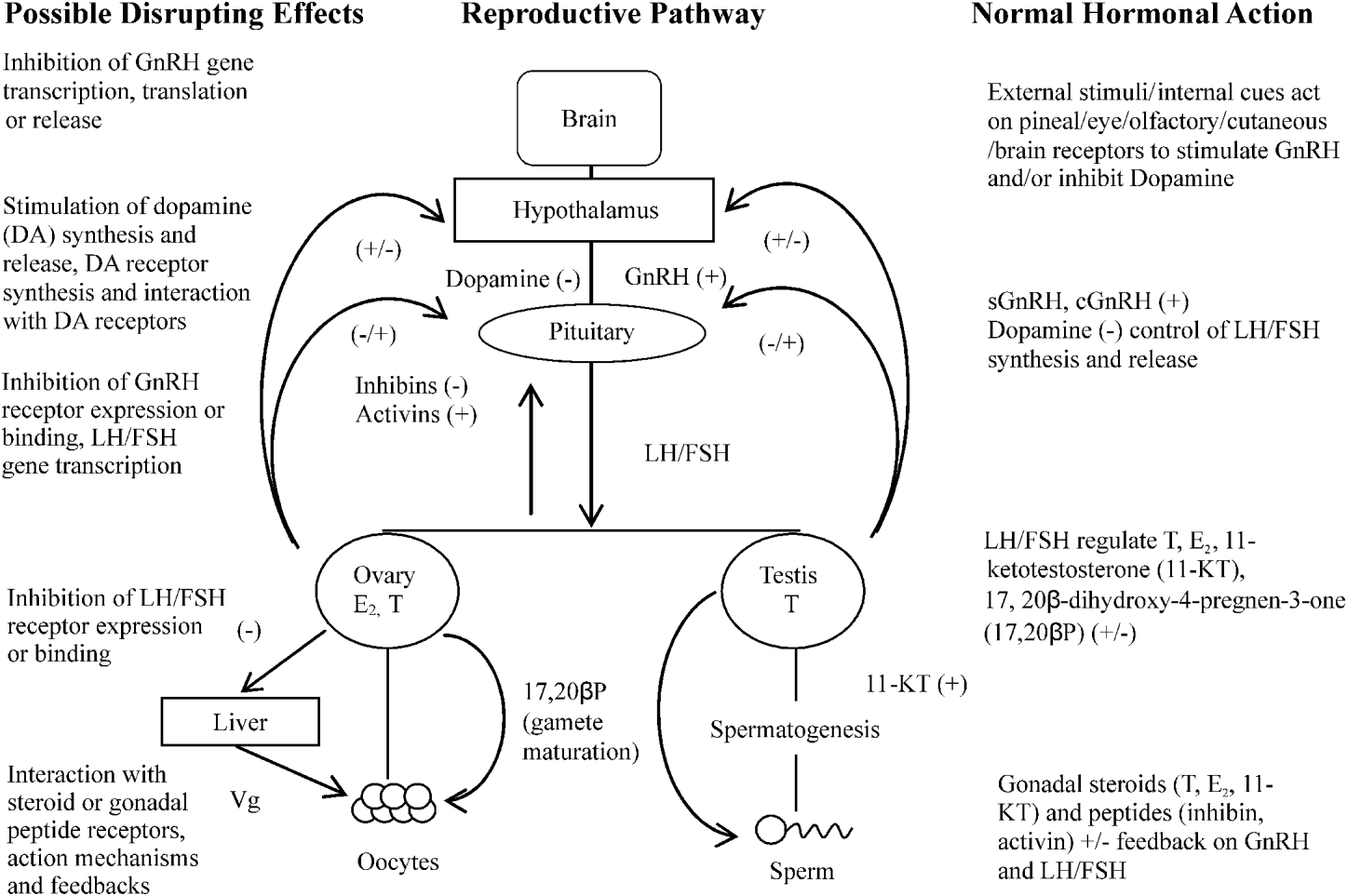
Perhaps the most significant impact of Ligula on the fish host population is its ability to inhibit reproductive function of its host. Whilst it has been established previously that parasite effects on host reproduction occur in several host/parasite interactions, for example the snail, Lymnea stagnalis infected with Trichobilharzia ocellata (Joose and van Elk, Reference Joose and Van Elk1986); Schistosoma mansoni infections of Biomphalaria glabrata (Crews and Yoshino, Reference Crews and Yoshino1989) and in vertebrate hosts – the tapeworms Taenia taeniaeformis affect the testis in rats and T. crassiceps induces feminisation in mice (Lin et al. Reference Lin, Rikihisa, Kono and Gu1990; Larralde et al. Reference Larralde, Morales, Terrazas, Govezensky and Romano1995, respectively) – it is the intricate relationship between Ligula and the reproductive endocrine status of the fish that has fascinated and perplexed biologists. Studies by Kerr (Reference Kerr1948) and Arme (Reference Arme1968) noted that in ligulosed roach, putative gonadotrophs were reduced in number and had a reduced granular content than their uninfected counterparts. This effect on reproductive potential has been reported subsequently by Arme and Owen (Reference Arme and Owen1968), Mahon (Reference Mahon1976), Sweeting (Reference Sweeting1977), Bean and Winfield (Reference Bean and Winfield1989) and Cowx et al. (Reference Cowx, Rollins and Tumwebaze2008) and has driven the idea that the effect of Ligula on reproductive inhibition is mediated through the host's pituitary gland. The effects of the parasite on the pituitary gland were observed 6 weeks post-implantation of a small worm into a mature female roach and also occurred in non-host species such as Xenopus laevis (Arme, Reference Arme1968, Reference Arme, Jennings and Lee1975). These observations not only indicate that the effects on the pituitary gland may not be dependent on worm burden, but suggest a general endocrine effect rather than an inhibition which is species specific. The mechanism by which this effect is mediated is unknown, but is thought to be via the hypothalamus/pituitary gland/gonadal axis. This axis is a complex array of hormones and feedback mechanisms, any number of which could be affected by Ligula (Fig. 2). To understand the possible intricate effects of Ligula on fish reproduction thus requires a brief consideration of the complexity that is the reproductive endocrine control mechanism in fish. Gonadotrophin Releasing Hormone (GnRH) is regarded as the first key hormone in the cascade controlling reproduction. GnRH occurs in a variety of forms which are thought to have arisen from gene duplication and mutations (O'Neill et al. Reference O'Neill, Powell, Standen, Youson, Warby and Sherwood1998; Okubu and Aida, Reference Okubu and Aida2001) and interacts with receptors present on the pituitary gonadotrophs. The expression of these receptors dictates the sensitivity of the pituitary to the GnRH, as increased receptor numbers at spawning enhance the preovulatory gonadotrophin surge (Yu et al. Reference Yu, He, Chik, Lin, Chang and Peter1998). During the reproductive cycle, the GnRH content in the hypothalamus and pituitary gland of goldfish increases to a maximum on the day of aggregation for spawning and is lowest in preovulatory and immature fish.
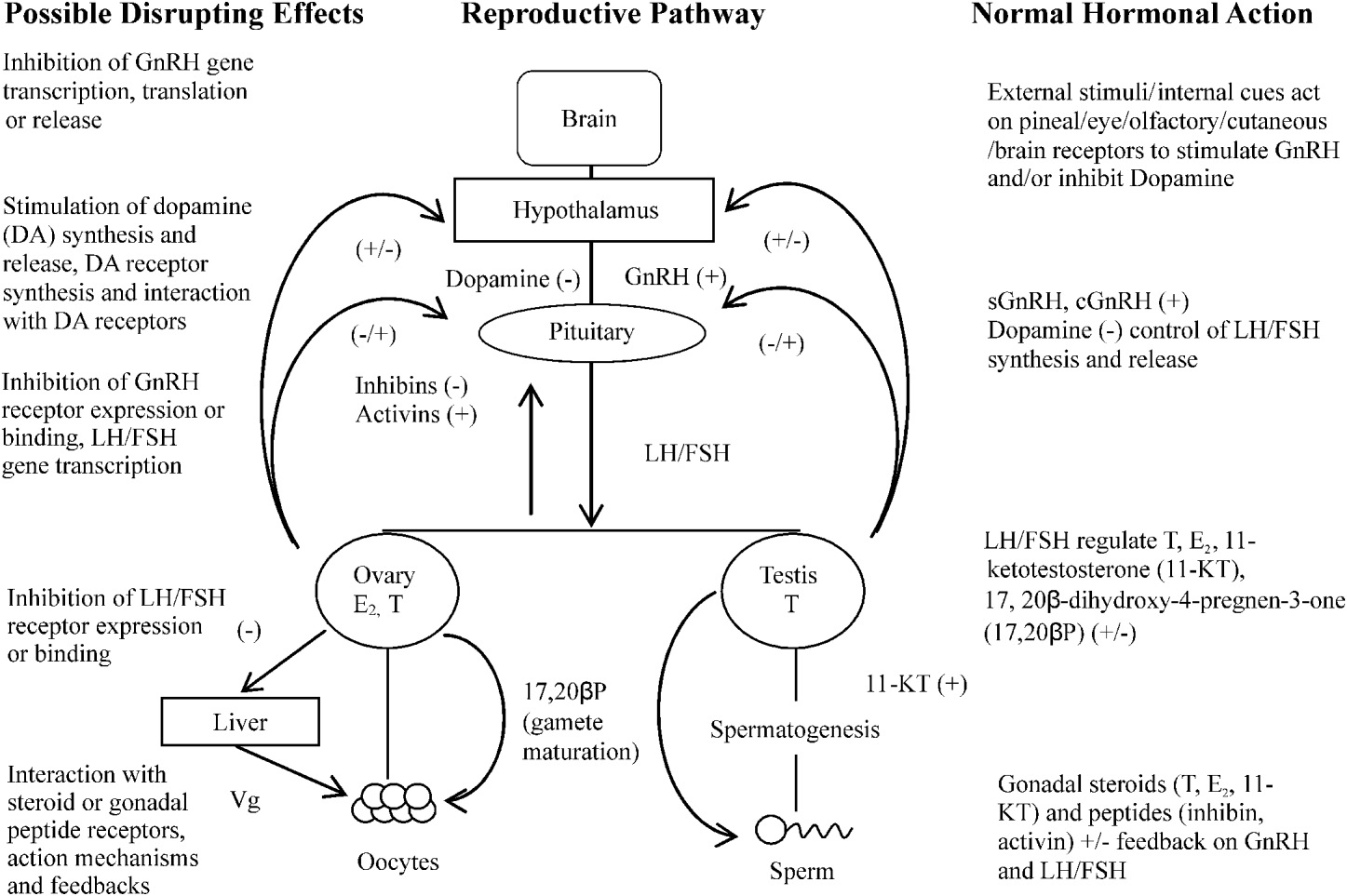
Fig. 2. Possible actions of Ligula on the roach reproductive pathway, +=stimulation, −=inhibition.
As revealed by the pioneer studies of R.E. Peter and coworkers in the goldfish, pituitary gonadotropic cells of teleosts may be subjected to regulation, not only by a stimulatory control of GnRH as in mammals, but also as direct inhibition by dopamine (for review see Peter et al. Reference Peter, Chang, Nahorniak, Omeljaniuk, Sokolowska, Shih and Billard1986). GnRH and dopamine are, respectively, the principal stimulatory and inhibitory neurohormones controlling gonadotrophin release (e.g. Hernandez-Rauda et al. Reference Hernandez-Rauda, Otero, Rey, Rozas and Aldegunde1996; Trudeau, Reference Trudeau1997). In the goldfish, dopamine was shown to be involved in the inhibition of the final steps of gametogenesis in mature fish (final oocyte maturation and ovulation in females, spermiation in males), a role that was also found in the other cyprinids studied, as well in other, but not all, teleost species (for review see Dufour et al. Reference Dufour, Weltzien, Sebert, Le Belle, Vidal, Vernier and Pasqualini2005). The possible inhibitory role of dopamine in the inhibition of earlier steps of gametogenesis was investigated in the European eel, in which a strong dopaminergic inhibition was shown to be involved in the prepubertal blockade of sexual maturation, before the oceanic reproductive migration (Vidal et al. Reference Vidal, Pasqualini, Le Belle, Holland, Sbaihi, Vernier, Zohar and Dufour2004; Dufour et al. Reference Dufour, Weltzien, Sebert, Le Belle, Vidal, Vernier and Pasqualini2005). A dopaminergic role in the inhibition of puberty was also recently found in the mullet (Nocillado and Elizur, Reference Nocillado and Elizur2008) but not in some other teleost species such as the striped bass or seabream (for review: Dufour et al. Reference Dufour, Weltzien, Sebert, Le Belle, Vidal, Vernier and Pasqualini2005). This complexity thus gives Ligula a range of potential strategies to affect the endocrine status and reproduction of its fish host.
The gonadotrophic hormones, synthesised and released from the anterior pituitary gland have a controlling role on reproduction. GtHI (FSH) mediates gonadal growth (Tyler et al. Reference Tyler, Santos and Prat1999), whilst GtHII (LH) regulates the final stages of maturation and ovulation/spermiation. These gonadotrophins act primarily on the ovary and testes to promote gametogenesis. Receptors for GtH are located in both cell layers surrounding the oocytes (thecal and granulosa cells) and in the testis (Kanamori et al. Reference Kanamori, Kagawa and Nagahama1987; Yan et al. Reference Yan, Swanson and Dickhoff1992). Fish gonads have the capacity to synthesise steroid hormones such as 17β-estradiol, testosterone, 11-KT and 17-20βP (Nagahama, Reference Nagahama1999), as well as gonadal peptides (inhibin, activin) which exert feedback control onto the hypothalamus and pituitary gland in a classical feedback loop, and their regulation is dependent on the maturational status of the fish (Feist and Schreck, Reference Feist and Schreck1996). Ligula could therefore be affecting the secretion of GtH hormones, their receptors and/or the feedback system between the gonads and the brain. Indeed, the fact that fish usually become infected when young may suggest that the parasite is preventing puberty in its host. Ligula could therefore be considered as a useful tool to gain an understanding of the endocrine control of the development of the reproductive system as it could mediate its effect at various levels in the hypothalamus/pituitary gland/gonadal axis (Fig. 2).
Implantation of Ligula plerocercoids into uninfected roach results in atresia of developed follicles, or if implanted after ovulation, inhibited recrudescence in fish examined 3 months after implantation (Arme, Reference Arme1968). The effects are reproducible with a small plerocercoid implanted into a large mature fish, which precludes pressure effects on fish organs or general debilitation from parasite metabolic demands (Arme, Reference Arme, Jennings and Lee1975). Arme postulated that Ligula produces a substance with an inhibitory effect on the pituitary gland, and noted that the nature of the substance was similar in action to testosterone treatment and may possibly be a steroid with anti-gonadal effects on the pituitary gland. Arme et al. (Reference Arme, Griffiths and Sumpter1982) later published evidence against this hypothesis as three different assay attempts failed to identify a sex steroid in extracts of worm and in media in which the parasites had been cultured, although cholesterol (sex steroid precursors) was identified in abundance (Arme, Reference Arme1997). It is plausible that Ligula targets GnRH as two forms of gonadotrophin releasing hormone (sGnRH and cGnRH-II) have been localised in brains of ligulosed and uninfected roach by Penlington et al. (Reference Penlington, Williams, Sumpter, Rand-Weaver, Hoole and Arme1997). Although no differences in cell distribution, cell number or staining intensity were detected that could be attributed to Ligula, both GnRH forms were present in uninfected and infected fish, although cGnRH positive neurones were more numerous than sGnRH (Williams et al. Reference Williams, Penlington, King, Hoole and Arme1998).
Endocrine studies by Carter et al. (Reference Carter, Pierce, Dufour, Arme and Hoole2005) have revealed a more intricate effect of Ligula on the endocrine status of the pituitary gland in roach. The effect of parasitisation on the LH content of this gland was studied using heterologous radio-immunoassay for the LHβ subunit of Cyprinus carpio which revealed that the pituitary gland of infected roach contained 50% less LH than non-ligulosed fish. In addition, partial cloning of roach LHβ subunit allowed Carter and co-workers to show that there was a 50% reduction in LHβ mRNA in the pituitary gland of ligulosed roach. These results support the hypothesis that the pituitary gland plays a significant role in the interaction between the parasite and the reproductive status of the fish host. There is evidence however that the interaction between Ligula and the brain of fish may be multifaceted. Testosterone can have a two-fold effect on the regulation of LH transcript levels either in its own right (Huang et al. Reference Huang, Schmitz, Le Belle, Chang, Querat and Dufour1997) or after aromatisation in the pituitary to E2 in order to stimulate LH (Antonopoulou et al. Reference Antonopoulou, Mayer, Borg, Swanson, Murza and Christoforov1999). Aromatase activity, which is seasonally present in the pituitary of the goldfish (Melamed et al. Reference Melamed, Rosenfeld, Elizur and Yaron1998), as in all vertebrates, is a member of an enzyme complex including P450 aromatase and reductase which carries out the conversion of androgens to estrogens (Gonzalez and Piferrer, Reference Gonzalez and Piferrer1999; Carreau et al. Reference Carreau, Bourguiba, Lambard, Galeraud-Denis, Genissel and Levallet2002). This essential enzyme complex for estrogen biosynthesis has been demonstrated in the brain and ovary of teleosts (Cruz and Canario, Reference Cruz and Canario1999). Stimulatory effects of small quantities of testosterone are consistent with a positive feedback mechanism, which stimulates accumulation and secretion of LH. Large testosterone doses presumably exert negative effects via inhibition of LH (Berglund et al. Reference Berglund, Antonopoulou, Mayer and Borg1995). Recent studies by Hecker et al. (Reference Hecker and Karbe2005, Reference Hecker, Sanderson and Karbe2007) have further investigated the role of aromatase activity in the control of endocrine status in Abramis brama from the river Elbe in Germany. Brain aromatase activity was significantly positively correlated with plasma estradiol in females and 11-ketotestosterone in males which the authors suggested led to the disruption of reproductive parameters such as the maturation of germ cells and secondary sex characteristics. The interesting observation was that the prevalence of Ligula in the fish was correlated with a suppression of the aromatase activity.
Further studies are also required to investigate the possible impact of the parasite on the expression and production of roach FSH. Indeed, according to data in other teleosts, FSH is supposed to be mostly involved in the control of the first steps of gametogenesis (vitellogenesis in the female, spermatogenesis in the male) while LH would control the last ones (oocyte final maturation and ovulation in the female, spermiation in the male). Recently, Trubiroha et al. (Reference Trubiroha, Wuertz, Frank, Sures and Kloas2009) developed quantitative real time PCR to evaluate the impact of Ligula on the expression of LHβ and FSHβ subunits in infected roach. In agreement with the authors' previous studies (Carter et al. Reference Carter, Pierce, Dufour, Arme and Hoole2005), field studies revealed a significant decrease in both LHβ and FSHβ pituitary expression in infected roach as compared to non-infected ones. However, under controlled laboratory condition of infection, only FSHβ mRNA levels were lowered. This suggested that FSH may be a prime target of Ligula inhibitory effect on roach sexual maturation, in agreement with the early role of FSH in the induction of gametogenesis as discussed above. Considering the early blockade of sexual maturation by Ligula in the roach, development of new tools such as the use of reproductive cell lines to investigate the regulation of FSH expression and release would be highly relevant.
The possibility that Ligula is producing a substance that directly affects pituitary gland activity has been investigated in unpublished studies carried out by the authors using a pituitary primary cell culture system (PPCC) according to the method previously developed (Montero et al. Reference Montero, Le Belle, Vidal and Dufour1996; Huang et al. Reference Huang, Schmitz, Le Belle, Chang, Querat and Dufour1997). Pituitary glands cells obtained from female silver (prepubertal) eels (Anguilla anguilla) were exposed to secretions (WCM) from Ligula intestinalis and LH content measured by radioimmunoassay (Dufour et al. Reference Dufour, Delerue-Le Belle and Fontaine1983). Ligula WCM added to eel pituitary cells induced a significant increase in cellular LH content which occurred in a dose-dependent manner (0·658 μg–1·8 μg of Ligula protein). Whilst the presence of protease inhibitors did not affect the amount of LH produced when pituitary cells were exposed to whole parasite WCM, it did increase the LH content of the pituitary cells exposed to parasite fractions (e.g. 30+kDa fraction with protease inhibitors=412·8±29 ng LH/μl cell extract, without protease inhibitors=20·25±1 ng LH/μl cell extract, T =13·6, P<0·001), which suggests that this endocrine active substance is susceptible to proteolytic digestion. Indeed, there are previous reports which indicate that Ligula plerocercoids not only produce proteolytic enzymes, but also protease inhibitors (Matskasi and Juhasz, 1977; Juhasz, Reference Juhasz1979; Matskasi and Nemeth, Reference Matskasi and Nemeth1979). The protective effect of protease inhibitors was also observed on the effects of other fractions of parasite secretions (e.g. 10–30 kDa, 3–10 kDa or <3 kDa) on the LH content of pituitary cells, which suggests that multiple factors secreted by Ligula may contribute to the endocrine disruption of the host reproduction.
Although the eel pituitary cell culture model adopted indicated a possible direct effect of Ligula on pituitary gland cells, a method of maintaining pituitary cells from the natural host of Ligula, the roach, failed to give any conclusive results. The increase in LH content measured on addition of parasite products to the eel pituitary cell model may be due to several possible mechanisms, such as an increase in production of LH and a decrease in the release of LH from the pituitary gland, or no change in LH production, but a decrease in LH release. The effect of Ligula on eel pituitary cells also supports the previous suggestion by Arme (Reference Arme1968) that effects of this parasite on host reproduction may not be limited to its natural host. Further studies should aim at investigating the possible direct effects of Ligula products on the expression of roach LHβ and FSHβ subunits.
Whilst there appears to be much debate on the mechanism by which Ligula affects the reproductive status of its fish host there appears to be a consensus, at least in the speculation, as to why this should occur and how the parasite might benefit from this effect. Besides the possible effects on the energy balance in the infected fish several authors e.g. Barber and Huntingford (Reference Barber and Huntingford1996), Loot et al. (Reference Loot, Brosse, Lek and Guegan2001a, Reference Loot, Aulagnier, Lek, Thomas and Guegan2002a, Reference Loot, Poulin, Lek and Gueganb), Morgan (Reference Morgan2003), Dejen et al. (Reference Dejen, Vijverberg and Sibbing2006) have proposed that infection in fish leads to altered behaviours such as movement to shallow waters, occurrence of fish on the surface of the water, swimming impediments, absence of shoaling, and delayed response to a stimulus, all of which lead to the infected fish being more prone to predation by the avian definitive host or another fish (Museth, Reference Museth2001). This host/parasite interaction thus has an important role to play in the studies on the parasite manipulation and predator foraging behaviour (Brown et al. Reference Brown, Loot, Grenfell and Geugan2001, Reference Brown, Loot, Teriokhin, Brunel, Brunel and Guegan2002; Fenton and Rands, Reference Fenton and Rands2006).
Parasitism, and in particular with Ligula, is not the only biological factor which can affect the endocrine system and over the last 25 years there have been many studies (e.g. McMasters et al. Reference McMasters, Van der Kraak and Munkittrick1996; Harries et al. Reference Harries, Sheahan, Jobling, Matthiessen, Neall, Sumpter, Taylor and Zaman1997, Reference Harries, Janbakshs, Jobling, Matthiessen, Sumpter and Tyler1999) which have shown that chemical pollutants can affect the endocrine status of fish. Surprisingly, there are very few studies that have considered water quality in association with the Ligula/fish interaction. Recent investigations by Hecker and co-workers (Hecker and Karbe, Reference Hecker and Karbe2005; Hecker et al. Reference Hecker, Sanderson and Karbe2007) on Abramis brama infected with L. intestinalis collected from the river Elbe have attempted to relate endocrine status of the fish to parasitisation and the presence of a range of chemicals from industrial, agricultural and domestic sources. Regional differences in infection of the fish were noted along the length of the Elbe studied with highest prevalence occurring in heavily polluted areas. When the authors used a linear model to compare these regional differences in prevalence of infection with biological parameters, not all the differences observed could be attributed to Ligula. They proposed that pollution may have contributed to the observed altered reproductive and endocrine status observed in the fish. Whilst these studies in no way refute the observed effects on Ligula on the hypothalamus/pituitary gland/gonadal axis they do highlight the possible importance of other biological parameters such as water quality.
LIGULA: A MODEL FOR POLLUTION MONITORING
The association of water quality with the interaction between parasites and their fish hosts is not surprising as there have been recent studies which have highlighted the role of pollutants in host-parasite interactions (Hoole, Reference Hoole1997; Morley et al. Reference Morley, Lewis and Hoole2006, Sures, Reference Sures2008a, Reference Suresb). Several studies have investigated the effects, not only of pollutants on the immune response of the fish hosts to the parasitic fauna (Hoole, Reference Hoole1997), but also on helminth life cycles. These latter studies have primarily concentrated on trematode stages such as eggs, miracidia, cercariae and metacercariae (Abd Allah et al. Reference Abd Allah, Wanas and Thompson1997; Morley et al. Reference Morley, Crane and Lewis2001a, Reference Morley, Crane and Lewisb, Reference Morley, Crane and Lewisc; Reference Morley, Crane and Lewis2002, Reference Morley, Crane and Lewis2003; Pietrock et al. Reference Pietrock, Marcoliese and McLaughlin2002) and the bioaccumulation of heavy metals in adult acanthocephalans and cestodes in fish (Sures et al. Reference Sures, Taraschewski and Siddall1997; Sures and Siddall, Reference Sures and Siddall1999; Sures, Reference Sures2003). The relatively few studies which have been carried out on the interaction between fish tapeworms and heavy metals have indicated that these metazoan parasites possess, on some occasions, the ability to bio-accumulate pollutants at greater levels than their fish hosts. For example, the monozoic cestode, Monobothrium wageneri, had higher concentrations of both cadmium (Cd) and lead (Pb) than its host tench, Tinca tinca, whilst there was no difference between Pb burdens detected in the adult cestode, Bothriocephalus scorpii, and those present in the intestinal wall of its host turbot, Scophthalamus maximus (Sures et al. Reference Sures, Taraschewski and Siddall1997). In addition, the concentration of heavy metals is dependent on the different body parts of tapeworms analysed. For example, posterior gravid proglottids of the cestode, Bothriocephalus scorpii accumulate higher contents of Pb and Cd than the anterior immature ones (Sures et al. Reference Sures, Taraschewski and Siddall1997). This may be due to the ability of tapeworm egg shells to bio-accumulate heavy metals (Khalil et al. Reference Khalil, Furness, Polwart and Hoole2009).
The size and ubiquitous nature of Ligula has led to the large plerocercoid stage being considered as a possible model in the study of heavy metal contamination in water bodies. Tenora et al. (Reference Tenora, Barus, Kracmer and Dvoracek2000) utilised atomic absorption spectrometry to monitor lead, chromium and cadmium levels in L. intestinalis and Philometra ovata in the body cavity of three cyprind fish species (A. brama, R. rutilus, B. bjoerkna). All heavy metals studied bio-accumulated within the plerocercoid to a greater level than in the fish muscle. Further studies carried out by Tenora et al. (Reference Tenora, Barus and Prokes2002) also revealed that this ability to bio-accummulate heavy metals (Pb, Cd) was not restricted to the plerocercoid, as the adult L. intestinalis contained higher levels of these two metals than the definitive avian hosts. It would appear however, that the accumulation of heavy metals by the plerocercoid stage may be dependent on the age of the parasite. Barus et al. (Reference Barus, Tenora, Kracmar and Prokes2001) noted that whilst nickel levels were higher in young plerocercoids, the levels of the majority of heavy metals monitored, i.e. Pb, Cr and Cd, were greater in the older parasites. They hypothesised that this indicated that accumulation of heavy metals in the parasite was a gradual and long-term process which probably occurred during the growing phase of the parasite in the fish host. More recent studies by Tekin-Ozan and co-workers (Tekin-Ozan and Kir, Reference Tekin-Ozan and Kir2005, Reference Tekin-Ozan and Kir2008; Tekin-Ozan and Barlas, Reference Tekin-Ozan and Barlas2008), utilising Ligula and tench (Tinca tinca), have extended the greater bio-accummulation by the plerocercoid to a more extensive range of heavy metals and speculate that if the parasite reflects the amount of heavy metal contaminants in the water and sediments, it may provide reliable data about pollution in water bodies. Whilst this is perhaps debatable, the value of the association of the plerocercoid with heavy metals probably lies in the size of the parasite which may assist in the elucidation of how parasites can bio-accumulate pollutants with no apparent ill-effects on their biology.
LIGULA: A MODEL OF GENETIC BIODIVERSITY
One important area that underpins all of the studies mentioned above relates to the taxonomic status of the parasites used, which are from a range of fish species and geographical locations. The classification of ligulid tapeworms into the genus Ligula Bloch, 1782 and genus Digramma Cholodkovsky, 1914 has proved controversial for many years, and yet L. intestinalis with its global distribution and complex life cycle may be a valuable model to study speciation and the evolution of parasite genetic diversity. Since the turn of the century there have been several investigations which have applied molecular techniques to studies on speciation within ligulid cestodes. Li and Liao (Reference Li and Liao2003), using sequences for the 5′ end of 4 genes, i.e. 28S ribosomal ribonucleic acid (28rRNA), mitrochondrial cytochrome c oxidase subunit 1 (CO1), nicotinamide adenine dinucleotide dehydrogenase subunit 1 (ND1) and the internal transcribed spacer of the nuclear ribosomal deoxyribonucleic acid (ITS1), noted that there was low genetic divergence in these in Ligula and Digramma and suggested that the two parasites should be considered as different species within the genus Ligula. These authors had previously utilised 28rRNA and CO1 isolated from formalin-fixed specimens of Ligula obtained from Qinghai-Tibet Plateau, Russia and England and proposed that the Chinese Ligula was within the same species as that occurring in Europe. Importantly, they proposed that geographic location, host affinity and host habitat were not reliable criteria to use in the classification of Ligula (Li et al. Reference Li, Liao and Yang2000). Olson et al. (Reference Olson, Littlewood, Griffiths, Kennedy and Arme2002) provided molecular evidence that Ligula occurring in gudgeon, and roach in Lough Neagh, Northern Ireland, were separate strains which may be reflected in their effect on host gonadal development. In addition, Ligula from minnow (Phoxinus phoxinus) from Wales resembled those from the Lough Neagh roach. The authors suggested that the existence of separate strains in Lough Neagh probably resulted from the introduction of roach and an increase in the number of the definitive host, the great crested grebe. Logan et al. (Reference Logan, Horák, Štefka, Aydogdu and Scholz2004) highlighted that L. intestinalis from Turkey were genetically isolated from their European and Chinese counterparts. In a recent extensive study carried out by Bouzid et al. (Reference Bouzid, Stefka, Hypsa, Lek, Scholz, Legal, Ben Hassine and Loot2008a), the genetic variation within L. intestinalis obtained from 13 fish species originating from different localities i.e. Algeria, Australia, Canada, China, Czech Repubic, Estonia, Ethiopa, France, Germany, UK, Mexico, Poland, Russia, Tunisa and the Ukraine, was analysed using two mitochondrial genes, cytochrome oxidase 1 and cytochrome B, and the nuclear sequence of intergenic transcribed 2 (ITS2). The authors proposed that the evolutionary patterns observed were determined at the local and global levels. At the local aspect, the migrating avian definitive host was thought to be important in preventing the establishment of genetic barriers, whilst on the global scale, genetically distinct clusters were observed. In addition, the authors noted that Ligula was split into two clades, termed A and B. Clade A contained samples from Tunisia and Europe and were obtained from ‘derived cyprinids’ (Abramis, Alburnus, Phoxinus, Rutilus and Scardinius), whilst Clade B was restricted to European, Algerian, Chinese and Australasian samples from ‘basal cyprinid fish’ (Barbus, Gobio and Rhodeus). Bouzid et al. (Reference Bouzid, Lek, Mace, Ben Hassine, Etienne, Legal and Loot2008b), utilising L. intestinalis, also proposed that inter-simple sequence repeat markers was a rapid and inexpensive technique to define markers that could be used to assess genetic diversity. Recently, isolation and characterization of microsatellite loci have been used to understand the genetic complexity and diversity that occurs within the ligulids. After this technology was established as a useful tool in the case of L. intestinalis (Stefka et al. Reference Stefka, Gilleard, Grillo and Hypsa2007), Stefka and co-workers in 2009 used 15 microsatellite loci to monitor the genetic differences in populations of L. intestinalis from a range of distant geographical locations in North America, Europe, Asia, Africa and Australasia. They noted a very high level of polymorphism and strong genetic structure in Ligula from these localities and proposed some very interesting reasons for this. For example, the existence of parasite subdivisions between Europe and Tunisia was due to the Mediterranean Sea effect which, although did not prevent migration of the avian definitive host, the fact that the adult tapeworm persists in the bird for only one week (Dubinina, Reference Dubinina1966) would probably mean that the tapeworm was not transported between the two localities. In addition, they also suggested that the fish immune response may be a factor in determining host-specificity of the Ligula genotypic lineages. Unfortunately, most of the studies on the immune response to the plerocercoid stage have been carried out in roach. In a series of publications by Hoole and co-workers it was established that there is an intense cellular response to the parasite (Hoole and Arme, Reference Hoole and Arme1982, Reference Hoole and Arme1983a, Reference Hoole and Armeb; Taylor and Hoole, Reference Taylor and Hoole1989a, Reference Taylor and Hooleb, Reference Taylor and Hoole1993, Reference Taylor and Hoole1994, Reference Taylor and Hoole1995) which involved several specific and non-specific humoral components (Hoole and Arme, Reference Hoole and Arme1986, Reference Hoole and Arme1988; Williams and Hoole, Reference Williams and Hoole1992, Reference Williams and Hoole1995). However, even with the host response, substantial differences to Ligula infection occur in roach and gudgeon (Arme, Reference Arme1997), again indicating possible genetic diversity of the parasite.
In conclusion, since the classical experiments carried out by J. D. Smyth in 1947, which revealed L. intestinalis as a model to study tapeworm development in vitro, this large metazoan parasite has proved an invaluable model not only for parasitologists in general, but endocrinologists, ecologists, geneticists, immunologists and in pollution studies.