Introduction
Invasive species pose an increasing threat to global biodiversity (Floerl et al. Reference Floerl, Inglis, Dey and Smith2009). While invasives have numerous direct effects – through predation, competition or hybridization with native species (Menge, Reference Menge1972; O'Dowd et al. Reference O'Dowd, Green and Lake2003; Wanless et al. Reference Wanless, Angel, Cuthbert, Hilton and Ryan2007) – they may also have powerful indirect effects, by spreading parasites and diseases (Dunn and Hatcher, Reference Dunn and Hatcher2015). Each introduced species brings an average of three parasite species with them from their native range (Torchin et al. Reference Torchin, Lafferty, Dobson, McKenzie and Kuris2003), and these parasites can have serious impacts on native species (Andreou et al. Reference Andreou, Arkush, Guégan and Gozlan2012). The impact of parasites during biological invasions is challenging to unravel, involving interactions between native and introduced hosts and their respective parasite communities (Dunn et al. Reference Dunn, Torchin, Hatcher, Kotanen, Blumenthal, Byers, Coon, Frankel, Holt, Hufbauer, Kanarek, Schierenbeck, Wolfe and Perkins2012; Telfer and Bown, Reference Telfer and Bown2012; Perkins et al. Reference Perkins, White, Pascoe and Gillingham2017).
Introduced hosts may affect parasite prevalence in native hosts in three ways (Telfer and Bown, Reference Telfer and Bown2012). First, introduced parasites may switch to native hosts, causing a ‘spillover’ of parasites (Andreou et al. Reference Andreou, Arkush, Guégan and Gozlan2012; Miller et al. Reference Miller, Kinsella, Snow, Hayes, Falk, Reed, Mazzotti, Guyer and Romagosa2017). Naïve native species are often highly susceptible to introduced parasites, while invasive species, which have co-evolved with these parasites, may not be obviously affected (Andreou et al. Reference Andreou, Arkush, Guégan and Gozlan2012). Thus, spillover may be a primary mechanism of impact on native hosts (Hudson and Greenman, Reference Hudson and Greenman1998). Second, invasive hosts acquire native parasites in the new range (Torchin and Mitchell, Reference Torchin and Mitchell2004). The presence of this new (often highly abundant) host, may cause a dramatic increase in native parasite abundance, which may affect native hosts through parasite ‘spillback’ (Kelly et al. Reference Kelly, Paterson, Townsend, Poulin and Tompkins2009; Hartigan et al. Reference Hartigan, Fiala, Dyková, Jirků, Okimoto, Rose, Phalen and Šlapeta2011; Kelehear et al. Reference Kelehear, Brown and Shine2013). Third, the opposite may happen if the introduced host acquires native parasites but the native parasite is not competent in this new host. In this case, an introduced host potentially reduces parasite prevalence by diluting parasite infection in native hosts (Telfer and Bown, Reference Telfer and Bown2012). A first step in resolving these possibilities is, of course, to assess parasite transmission from introduced to native hosts.
Conversely, if invasive species are susceptible to native parasites, acquisition of these parasites may impact the fitness of the invasive host (Krakau et al. Reference Krakau, Thieltges and Reise2006; Telfer and Bown, Reference Telfer and Bown2012). In such cases, native parasites might affect the establishment, impact, or range expansion of an invasive species (Case and Taper, Reference Case and Taper2000; Dunn, Reference Dunn2009; Perkins, Reference Perkins2012). Indeed, negative impacts of native parasites on invasive species have been recorded and likely affect the competitive ability of the invader (Krakau et al. Reference Krakau, Thieltges and Reise2006; Dunn, Reference Dunn2009; Gendron et al. Reference Gendron, Marcogliese and Thomas2012). Again, a first step in resolving these possibilities is to assess parasite transmission, this time from native to introduced hosts.
While invasive species do bring novel parasites and pathogens with them, they generally leave many of their natural parasites behind when they establish in their new range (MacLeod et al. Reference MacLeod, Paterson, Tompkins and Duncan2010). This ‘parasite release’ – driven by founder events or a lack of intermediate hosts in the new range – may facilitate establishment and range expansion of invasive species (Torchin et al. Reference Torchin, Lafferty, Dobson, McKenzie and Kuris2003; Prenter et al. Reference Prenter, Macneil, Dick and Dunn2004; Dunn and Hatcher, Reference Dunn and Hatcher2015). As well as this loss of parasites at the establishment, parasites often lag behind during subsequent range expansion, and populations on the leading edge of the invasion may be exposed to a lower prevalence of parasites than longer established populations (Phillips et al. Reference Phillips, Kelehear, Pizzatto, Brown, Barton and Shine2010). This may give invasion front populations an advantage and facilitate the growth of dense populations in newly colonized areas (Phillips et al. Reference Phillips, Kelehear, Pizzatto, Brown, Barton and Shine2010).
Clearly, host–parasite dynamics can play a crucial role in both the success of an invasion and the impact the invader has on recipient communities. The possible outcomes are manifold and complex, but the list of possible outcomes can be rapidly shortened with observational data on host-switching and impacts on host fitness. Issues of parasite loss during ongoing invasion can also be assessed by examining changes in prevalence through space. Here we address these questions for an invasive gecko spreading into the gecko-rich woodlands of northern Australia.
The Asian house gecko, Hemidactylus frenatus, is a human-commensal species that has been accidentally introduced to many tropical and subtropical areas globally (Lever, Reference Lever2003; Hoskin, Reference Hoskin2011). These geckos often establish large populations where they are introduced, and in some areas have caused declines and local extinctions of native species (Cole et al. Reference Cole, Jones and Harris2005; Dame and Petren, Reference Dame and Petren2006). Despite documented impacts in some areas, H. frenatus is typically considered a benign invader due to the belief that it is restricted to built areas (Vanderduys and Kutt, Reference Vanderduys and Kutt2013). In northern Australia, however, H. frenatus are spreading from urban areas into bushland (Hoskin, Reference Hoskin2011; Barnett et al. Reference Barnett, Phillips and Hoskin2017). This range expansion is bringing H. frenatus into contact with a number of ecologically similar native species (Hoskin, Reference Hoskin2011; Barnett et al. Reference Barnett, Phillips and Hoskin2017). Are these native species acquiring the parasites of H. frenatus? Are H. frenatus acquiring native parasites? And, if so, does such host switching affect host fitness?
In this study, we focussed on mites (Geckobia and Neotrombicula) and pentastomes of the genus Waddycephalus because they are visible externally on geckos in the field. At least seven species of Geckobia mites have been recorded on H. frenatus in other parts of its introduced range (Heath and Whitaker, Reference Heath and Whitaker2015) but only Geckobia bataviensis is previously known to have been introduced with H. frenatus into Australia (Domrow, Reference Domrow1992; Hoskin, Reference Hoskin2011). There are many native gecko mites in Australia, including Geckobia and Trombiculid mites of the genera Ascoschoengastia, Neotrombicula and Trombicula (Domrow and Lester, Reference Domrow and Lester1985). There are no records of H. frenatus hosting native Australian gecko mites, nor of native Australian geckos hosting introduced mites, but this has not been assessed systematically. Geckobia mites live their entire lives on geckos and are likely transmitted through direct contact between individuals (Bauer et al. Reference Bauer, Russell and Dollahon1990; Rivera et al. Reference Rivera, Negrón and Bertrand2003), whereas the Trombiculid gecko mites have a free-living stage and geckos likely pick them up from their environment (Domrow and Lester, Reference Domrow and Lester1985). The impacts of haematophagous mites on the health and fitness of geckos are largely unknown (Hanley et al. Reference Hanley, Fisher and Case1995).
Waddycephalus are endoparasitic pentastomes that have been detected in many Australian snakes (definitive hosts) and lizards (one of the intermediate hosts) (Riley and Self, Reference Riley and Self1981; Riley et al. Reference Riley, Spratt and Presidente1985; Barton, Reference Barton2007; Paré, Reference Paré2008; Kelehear et al. Reference Kelehear, Saltonstall and Torchin2014b). Most species are described from Australia but the genus is also present in South-east Asia and Fiji (Riley and Self, 1981). Waddycephalus have a complex multiple host lifecycle: adults parasitize the lungs of snakes, and the two intermediate hosts are likely coprophagous insects (e.g. cockroaches) and insectivorous lizards, frogs and small mammals (Riley and Self, 1981; Paré, Reference Paré2008; Kelehear et al. Reference Kelehear, Saltonstall and Torchin2014b). Waddycephalus nymphs encyst subcutaneously in geckos (Fig. 1A) and may excyst when the host is sick or stressed (Fig. 1B) (Paré, Reference Paré2008). Infection with nymphal pentastomes can significantly affect the host, with migration and moulting of nymphs being associated with host morbidity (Paré, Reference Paré2008). Hemidactylus frenatus have been recorded to host nymphs of one or more species of Waddycephalus in Australia (Barton, Reference Barton2007; Coates et al. Reference Coates, Barnett, Hoskin and Phillips2017). These are assumed to be native Waddycephalus given the diversity and prevalence of the genus in Australian reptiles, and given that Waddycephalus infection is only seen in bushland populations of H. frenatus and not in urban populations (Barton, Reference Barton2007; Coates et al. Reference Coates, Barnett, Hoskin and Phillips2017).

Fig. 1. (A) Waddycephalus nymphs encysted subcutaneously are visible as protrusions on the mid-body of an adult H emidactylus frenatus (an example is highlighted by the black arrow) and (B) a Waddycephalus nymph beginning to excyst after the gecko was captured. Photos: Matthew McIntosh
There has been one study of host–parasite dynamics in H. frenatus in Australia. Coates et al. (Reference Coates, Barnett, Hoskin and Phillips2017) assessed the prevalence of Geckobia mites (not identified to species), Waddycephalus (not identified to species) and Raillietiella frenata, an endoparasitic pentastome known to have been introduced with H. frenatus into Australia (Barton, Reference Barton2007). They observed abrupt changes in parasite prevalence across the H. frenatus range edge (Coates et al. Reference Coates, Barnett, Hoskin and Phillips2017). The prevalence of Geckobia mites declined past the urban edge, while R. frenata were completely absent outside of inner urban areas. In contrast, native Waddycephalus nymphs were found on H. frenatus in woodland environments and up to the urban edge (i.e. at the urban–woodland interface), but were absent in inner urban areas (Coates et al. Reference Coates, Barnett, Hoskin and Phillips2017). The study concluded that during range expansion from urban to natural areas, H. frenatus may experience release from co-evolved parasites (Geckobia and R. frenata) but are exposed to novel native parasites (Waddycephalus).
In the current study, we document the abundance of both native and invasive gecko hosts in bushland habitat and investigate host specificity of mites and Waddycephalus where invasive and native geckos co-occur. We then investigate changes in parasite prevalence at the invasion front of H. frenatus, and assess factors affecting individual infection probability and intensity. Finally, we investigate the effects of these parasites on H. frenatus body condition and draw inferences on impacts on survival.
Methods
The parasites
We focused on mites and Waddycephalus nymphs in this study because they are visible externally on geckos in the field. Mites are visible as minute red or orange dots on the skin, often on the feet of geckos (Heath and Whitaker, Reference Heath and Whitaker2015), while Waddycephalus nymphs are visible as small protrusions under the skin of geckos (e.g., Fig. 1A). We dissected three nymphs from one H. frenatus specimen to ensure that the protrusions under the skin were Waddycephalus nymphs. These nymphs were weighed to determine a representative weight to deduct in tests of host body condition.
Fieldwork
This study was conducted around the city of Townsville in North-east Australia, where H. frenatus have established large populations in dry sclerophyll woodland surrounding urban areas (Barnett et al. Reference Barnett, Phillips and Hoskin2017). We used the same ten transects as Barnett et al. (Reference Barnett, Phillips and Hoskin2017), which each consisted of five survey sites, with site 1 on the urban edge and subsequent sites 500 m apart out to Site 5 at 2 km (in a straight line) from the urban edge (Fig. 2). Each site consisted of a surveyed area approximately 200 m long by 15 m wide. The habitat at Site 1 was urban housing and gardens, and the other four sites were in adjacent woodland. Each site was surveyed for H. frenatus and native geckos once a month between May 2013 and April 2014, giving a total of 12 surveys per site, except for one site (Yabulu, YA) where ten surveys were conducted. We, therefore, conducted a total of 590 site surveys over 12 months. Sites on the same transect were surveyed on the same night but the order of surveying these sites was randomized for each visit.
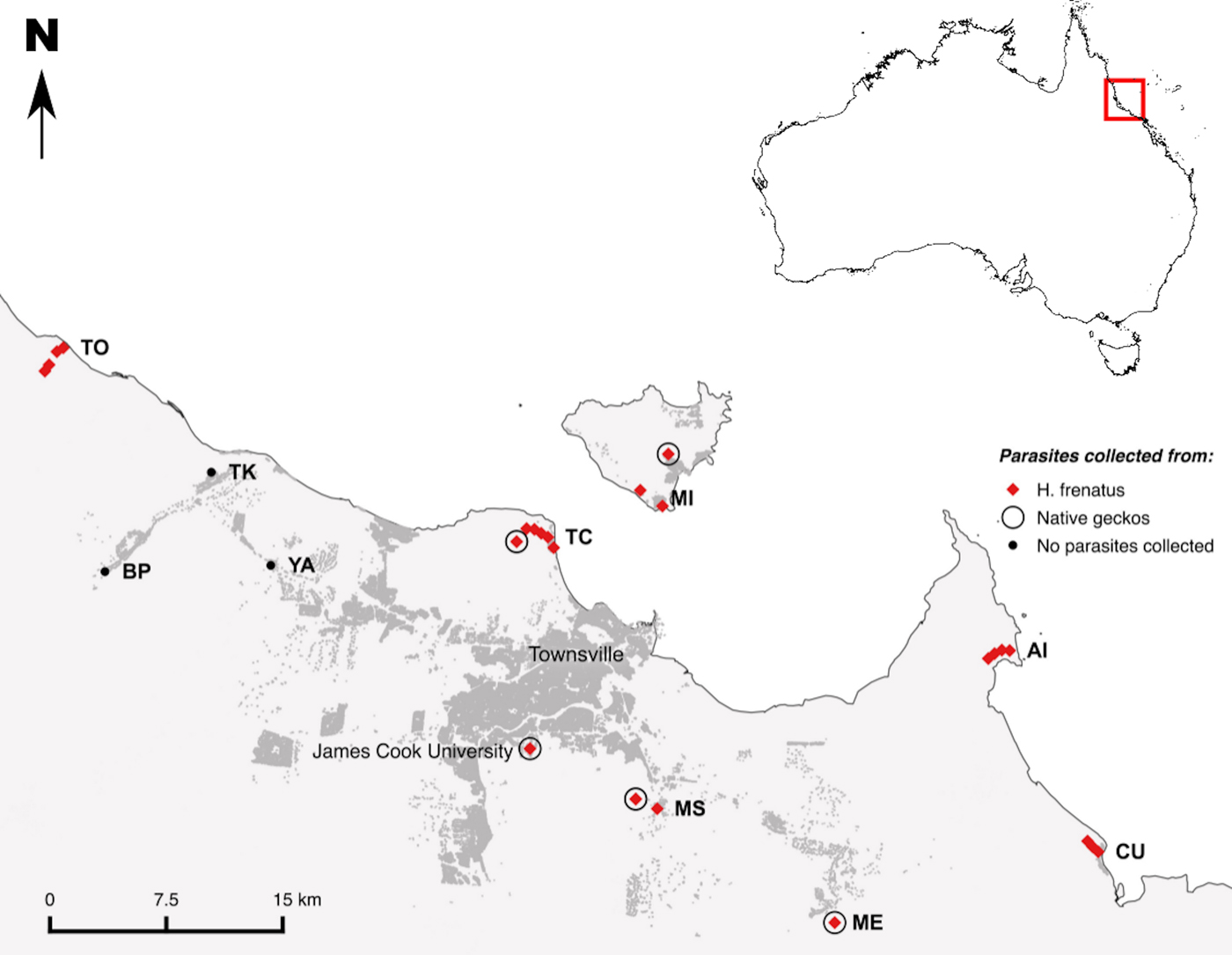
Fig. 2. Map of the Townsville region, showing transects (two-letter codes) where parasite prevalence and intensity data for H emidactylus frenatus was collected. Transects were located at Mount Stuart (MS), Cungulla (CU), Mount Elliott (ME), the Australian Institute of Marine Science (AI), the Town Common (TC), Magnetic Island (MI), Yabulu (YA), Bluewater Park (BP), Toolakea (TK) and Toomulla (TO). Hemidactylus frenatus and native geckos occur at all ten transects. Mites were collected for identification at a sub-set of these transects, from either H. frenatus (red diamonds) or native geckos (open circles). Additional mites were collected from geckos at a site close to the Magnetic Island (MI) transect and from the James Cook University campus. Buildings are shaded in dark grey
At each site, we conducted a 5-min auditory survey, where we counted each time we heard the distinctive ‘chuck chuck chuck…’ vocalization of H. frenatus, and a 15-min visual search, where we counted each H. frenatus or native gecko we found. During the 15-min visual survey, we walked slowly from a starting point, using head torches to locate geckos by their eye-shine. We also caught up to five H. frenatus on each survey to assess external parasites and to take measurements for body condition analyses. These geckos were placed inside a small snap-lock bag and weighed using a 10 g Pesola spring balance, and then measured for snout–vent length (SVL) using a small plastic ruler. Sex was determined by visually checking for testes bulges on males.
We began inspecting the captured H. frenatus for parasites during these surveys in September 2013 (the fifth month of surveys) and continued parasite screening through all surveys for the next eight months (a total of 400 surveys). Captured geckos (N = 617) were examined visually for Waddycephalus nymphs and mites. These mites were not collected for identification to species and therefore in this section, we generalize all mites as ‘Geckobia’. The numbers of Waddycephalus nymphs and Geckobia mites on each gecko were counted. The 15-min survey time was paused while geckos were being measured and examined for parasites. After examination, geckos were released at the point of capture. Parasites were not quantified on native geckos because time and permit constraints meant that native geckos were sighted but not handled during these surveys. However, native geckos were often seen at close proximity and anecdotal observations of Waddycephalus nymphs and mites were made.
Targeted mite collection
To assess the potential for host-switching we collected mites from H. frenatus (N = 95) and native geckos (N = 26). Mite collection was targeted to areas where native geckos and H. frenatus co-occur. This included sites at seven of the permanent transects as well as some nearby areas where H. frenatus and native geckos coexist (Fig. 2). We also collected mites from geckos at the James Cook University campus (Fig. 2), an additional site where H. frenatus and native geckos co-occur on the urban–woodland interface. Mites were collected from the following native gecko species: Gehyra dubia (N = 20), Amalosia rhombifer (N = 4) and Heteronotia binoei (N = 2). All mites were identified morphologically to species by a specialist (AH).
Statistical analyses
All analyses were conducted in R version 3.1.2 (R Core Team, 2017). All variables were normalized (converted to the same scale) with a mean of zero and a standard deviation of one, which aids in model fitting and the interpretation of results (Zuur et al. Reference Zuur, Ieno, Walker, Saveliev and Smith2009). We used either linear mixed effects models (LMMs) or generalized linear mixed effects models (GLMMs), within the lme4 package (Bates et al. Reference Bates, Mächler, Bolker and Walker2015), depending on the error structure of the response variable. To assess which factors affect parasite prevalence and the intensity of parasite infection, we analysed data for Geckobia mites and Waddycephalus nymphs in separate models (outlined below).
We chose parameters to include in analyses a priori based on current ecological knowledge of parasite–host relationships and specific questions we had about this study system (Crawley, Reference Crawley2007).
Which factors affect population-level parasite prevalence?
We used parasite prevalence data spanning 8 months, 10 transects and 617 individual geckos to build on the results of Coates et al. (Reference Coates, Barnett, Hoskin and Phillips2017), who assessed population-level parasite prevalence in the same region over one month, using seven transects and 231 individual geckos. Data for these studies were collected separately, and our approach allowed us to assess the impact of seasonal effects on parasite prevalence.
We calculated the first-principle component of H. frenatus seen in visual surveys and H. frenatus heard in auditory surveys (using the correlation matrix), and used this as an estimation of relative abundance. This was necessary because environmental conditions affect the detection probability of geckos differently for each survey technique (Barnett et al. in preparation), and conditions varied temporally and spatially throughout surveys. Across sites, we found relative abundance and distance to the urban edge were not highly correlated (Pearson's correlation coefficient = −0.31), so we included both as independent variables in the following models.
We used GLMMs with binomial error structures and logit link functions (Bolker et al. Reference Bolker, Brooks, Clark, Geange, Poulsen, Stevens and White2009) to assess whether the distance from the urban edge, relative abundance, or season affected: (1) the number of infected/uninfected geckos with respect to Geckobia mites per survey, and (2) the number of infected/uninfected geckos with respect to Waddycephalus per survey. Seasonal changes were assessed by including a first-order Fourier function: Sine(day of the year) and Cosine(day of the year) as predictors in each model, where ‘day of the year’ was transformed to fall between 0 and 2π. This approach allows a seasonal effect to be fitted as a sinusoidal curve across the course of the year (Stolwijk et al. Reference Stolwijk, Straatman and Zielhuis1999; Cox, Reference Cox2006), which is suitable for this system because we expect temporal autocorrelation and annual cycles. In both models, we initially included the interaction between the distance from the urban edge and relative abundance but excluded this parameter in the final model if the interaction was not significant (Crawley, Reference Crawley2007). The transect was included as a random effect in both models to account for broad variation in prevalence between transects.
Which factors affect infection probability and infection intensity on individual hosts?
GLMMs with binomial error structures were used to assess whether H. frenatus sex or body size (SVL) affected the likelihood of infection with (1) Geckobia mites or (2) Waddycephalus nymphs. In these analyses, we were less interested in the across the population and temporal effects, so we treated site and survey month as random effects such that our fixed (individual-level) effects were conditioned on the mean prevalence at each site.time.
To assess whether sex or body size (SVL) of individuals affected the intensity of infection with Geckobia mites or Waddycephlaus nymphs we used zero-truncated GLMMs with negative binomial error distributions to account for overdispersion (Zuur et al. Reference Zuur, Leno and Smith2007; Bolker et al. Reference Bolker, Brooks, Gardner, Lennert and Minami2012). Zero-truncated distributions were necessary because we only included infected individuals in these analyses: intensity of infection could not equal zero. Site and survey month were included as random effects in both models.
Do parasites affect body condition?
To investigate whether parasites affect body condition of H. frenatus we used LMMs with the natural log of gecko mass as the response variable. Here, we included Waddycephalus and Geckobia mites in the same models. First, we assessed whether the presence or absence of either parasite affected condition by including the following predictor variables: (1) the natural log of SVL, (2) the presence/absence of Geckobia mites and (3) the presence/absence of Waddycephalus. We then investigated whether the intensity of infection affected body condition. In this model, the predictor variables were: (1) the natural log of SVL, (2) the intensity of mite infection, and (3) the intensity of Waddycephalus infection. For individuals infected with Waddycephalus, gecko mass was first corrected by subtracting 0.005 g per nymph: the estimated mean weight of a nymph (calculated from weighing a subset of nymphs dissected out of a gecko). In both body condition analyses, we included site and survey month as random effects.
Results
Co-occurrence and relative abundance of hosts
Hemidactylus frenatus was detected at 46 of the 50 transect sites over the survey period. Hemidactylus frenatus was most common at the urban edge sites (site 1) but was present out to the furthest sites (site 5) at 2 km from the urban edge on nine out of ten transects. Native geckos were detected at 47 of the 50 sites, including 43 of the sites where H. frenatus was detected. The mean number of H. frenatus was higher than native geckos at all distances from the urban edge (all transects combined; Fig. 3), and, summed for all the woodland sites (Site 2–Site 5), H. frenatus was much more common than all six native gecko species combined (Fig. 4).
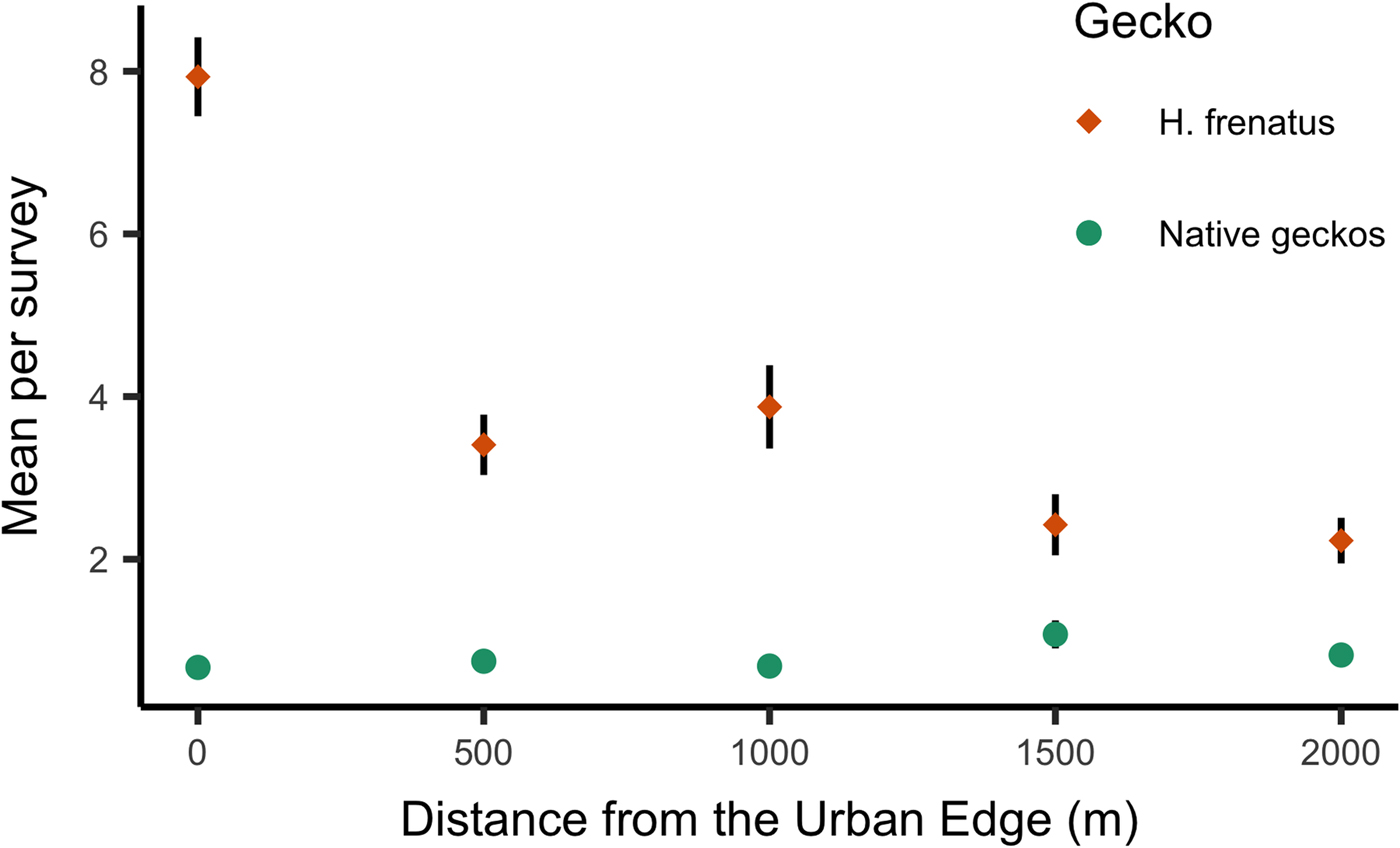
Fig. 3. The mean number of H emidactylus frenatus and native geckos seen per survey plotted for all transects combined.

Fig. 4. The total number of each gecko species (on a log10 scale) observed in visual surveys at woodland sites (>500 m from the urban edge) throughout the 12-month survey period.
Host specificity
Targeted mite collections for identification revealed that H. frenatus was infected exclusively with the introduced Geckobia mites, G. bataviensis and G. keegani (Table 1). This was the first record of G. keegani in Australia. Conversely, only native mite species were collected from three co-occurring native gecko species, G. dubia, A. rhombifer and H. binoei (Table 1). Both G. dubia and A. rhombifer were new host records for G. gymnodactyli. This was also the first record of A. rhombifer hosting Neotrombicula greenlyi.
Table 1. Geckobia and Neotrombicula mites collected on H emidactylus frenatus and native geckos. Geckobia sp. in the final two columns refers to mites that could not be identified to species due to preservation condition

Waddycephalus nymphs were not identified to species so it was not possible to assess host specificity in detail. The nymph bulges were also observed multiple times on the native gecko Gehyra dubia during the transect surveys (authors, personal observation). Infection of this introduced host is taken to represent host switching, as the Waddycephalus found in H. frenatus are deemed to be one or more native species (Barton, Reference Barton2007; Coates et al. Reference Coates, Barnett, Hoskin and Phillips2017).
General patterns of parasite infection in H. frenatus
Mites were detected at high prevalence on H. frenatus on all transects (Table 2), and up to 2 km from the urban edge at five transects (TO, MI, MS, YA, TK). Waddycephalus nymphs were detected on H. frenatus on eight of the ten transects (Table 2), generally at low prevalence (Table 2). The maximum infection intensity for Waddycephalus was ten nymphs per gecko, observed in two individuals.
Table 2. Prevalence of parasites and mean intensity of infection in H emidactylus frenatus assessed during transect surveys in 2013–2014

Which factors affect population-level parasite prevalence in H. frenatus populations?
Distance from the urban edge significantly affected the prevalence of Geckobia mites in H. frenatus populations, with lower proportions of infected individuals found farther from the urban edge (P < 0.0001, t = −4.53, Fig. 5A, Table 3). Relative abundance of H. frenatus did not affect the prevalence of mites directly (P = 0.59, t = −0.54), but there was a negative interaction between distance and abundance, with a steeper cline in mite prevalence in relatively larger host populations (P = 0.04, t = −2.05; Supplementary Fig. S1). In Supplementary Fig. S1, we show how the interaction between the distance from the urban edge and relative abundance affects Geckobia prevalence. Season also significantly affected the prevalence of Geckobia mites [Sine(day): P < 0.0001, t = 3.88; Cosine(day): P < 0.0001 t = 6.95; Supplementary Fig. S2A], with higher prevalence in the warmer summer months.

Fig. 5. (A) The prevalence of Geckobia mite infection in H emidactylus frenatus per survey with distance from the urban edge. (B) The prevalence of Waddycephalus sp. infection with distance from the urban edge. Size of the circles indicates sample size.
Table 3. Factors that affect prevalence of (A) Geckobia mites, and (B) Waddycephalus nymphs in H emidactylus frenatus populations

*P < 0.05.
***P < 0.0001.
Prevalence of Waddycephalus nymphs was not affected by distance from the urban edge (P = 0.41, t = 0.82; Fig. 5B), or by relative abundance (P = 0.42, t = 0.80). The interaction between these two covariates was non-significant and we therefore dropped it from the model. This had no discernible effect on model fit (AIC without interaction = 181.62, AIC with interaction = 183.60, ΔAIC = 1.98). Season significantly affected the prevalence of Waddycephalus [Sine(day): P = 0.03, t = 2.11; Cosine(day): P = 0.02, t = 2.35; Supplementary Fig. S2B, Table 3], with higher prevalence in the warmer summer months.
Which factors affect individual infection?
The likelihood of Geckobia mite infection (i.e. presence/absence of mites) was not significantly affected by either the sex (P = 0.94, t = 0.08) or SVL (P = 0.81, t = 0.24 Table 4; Fig. 6A) of geckos. Likelihood of infection with Waddycephalus sp. was similarly unaffected by the sex of individuals (P = 0.72, t = 0.36), but larger geckos were more likely to be infected with at least one Waddycephalus nymph (P < 0.01, t = 2.93, Table 4; Fig. 6B).

Fig. 6. The predicted relationship (± standard error) between the snout–vent length of H emidactylus frenatus and the presence/absence of (A) Geckobia mites and (B) Waddycephalus nymphs; and intensity of infection with (C) Geckobia mites and (D) Waddycephalus nymphs, from our generalized linear mixed effects models. In these models we incorporated Site and Survey Number as random effects such that the fixed (individual-level) effects shown here are conditioned on the mean prevalence/intensity at each site.time.
Table 4. Factors that affect the presence and infection intensity of Geckobia mites [models (A) and (B)] and Waddycephalus nymphs [models (C) and (D)] in individual H emidactylus frenatus

*P < 0.05.
**P < 0.01.
When looking only at infected individuals, sex affected neither the intensity of mite (P = 0.79, t = 0.27, Table 4), nor Waddycephalus infection (P = 0.32, t = 1.00, Table 4). Gecko size positively affected the intensity of mite infection (P < 0.01, t = 2.83, Table 4; Fig. 6C) but negatively affected the intensity of Waddycephalus infection, with smaller geckos having more Waddycephalus nymphs than larger geckos (P = 0.03, t = −2.12, Table 4; Fig. 6D).
Do parasites affect body condition?
Body condition of geckos was not affected by infection with Geckobia (P = 0.15, t = −1.44) or Waddycephalus (P = 0.94, t = −0.07). There was also no significant effect of the intensity of Geckobia (P = 0.71, t = −0.37) or Waddycephalus infections on body condition (P = 0.29, t = 1.07).
Discussion
Host specificity and potential impact
We found large populations of H. frenatus out to the furthest sites at 2 km from the urban edge on some transects. Their relative abundance was, on average, over three times higher than co-occurring native gecko species (Figs 3 and 4). This suggests that H. frenatus are achieving higher density in natural environments than are native geckos. The presence of comparatively large H. frenatus populations in natural environments gives the potential for both parasite spillover and spillback, as well as dilution, to be occurring in this system.
We found no evidence for spillover of mites – the invasive gecko had introduced mites and the native geckos had native mites (Table 1). This was surprising, given the apparently ample opportunity for spillover. Hemidactylus frenatus co-occurs with native geckos at many of these sites, and is regularly found side-by-side with natives. Additionally, this invasive species has been present at some of these sites for more than 20 years (Barnett et al. Reference Barnett, Phillips and Hoskin2017). Prevalence of mites on H. frenatus was high, and infected H. frenatus were present at most sites (Table 2). The lack of evidence of host switching was unexpected because elsewhere in their introduced range, there are records of H. frenatus hosting Geckobia mites that are not present in their native range (Heath and Whitaker, Reference Heath and Whitaker2015). It was particularly unexpected given the relative abundance of H. frenatus – spillover from invasive to native species is predicted to be more common in areas where invasive hosts are higher density than native species (Kelehear et al. Reference Kelehear, Saltonstall and Torchin2014b).
There are three potential reasons for the absence of mite transmission between invasive and native geckos in this system. First, in natural environments, individual Geckobia mites may rarely switch hosts. These (Pterygosomatid) mites spend their entire lives on geckos, and may only be transmitted during close and prolonged contact, such as mating or fighting (Bauer et al. Reference Bauer, Russell and Dollahon1990; Rivera et al. Reference Rivera, Negrón and Bertrand2003). Indeed, Hanley et al. (Reference Hanley, Fisher and Case1995) found no evidence of mite transfer between geckos (Lepidodactylus spp.) even after keeping them confined together for 48 h (Hanley et al. Reference Hanley, Fisher and Case1995). While mites that have co-evolved with H. frenatus (i.e. G. keegani and G. bataviensis) have previously been recorded infecting other gecko species, including two members of the Gehyra genus (G. oceanica and G. mutilata), competency on these hosts has not been assessed (Heath and Whitaker, Reference Heath and Whitaker2015). Second, although H. frenatus and native geckos co-occur in many of the areas where we collected mites, they may generally occupy different microhabitats or actively avoid encounters with each other. Elsewhere in their invasive range H. frenatus exclude native Nactus geckos from retreat sites, resulting in fewer encounters between the two species (Cole et al. Reference Cole, Jones and Harris2005). Third, it is possible that our sample of mites from native geckos (N = 26) was too small to detect transmission to native species. However, if that is the case, transmission frequency must be very low given the ample opportunity for transfer outlined above. Laboratory studies would further our understanding of host specificity of the introduced and native mites in this system.
We found H. frenatus infected with Waddycephalus nymphs on eight of the ten transects (Table 2), and we recorded Waddycephalus infections at all distances from the urban edge (Fig. 5B). Prevalence was generally low; a result echoed in the scarcity of Waddycephalus records from museum specimens (Barton, Reference Barton2007), but prevalence also appears low in native geckos in this system (authors pers. obs.). Despite this low prevalence, the high abundance of H. frenatus may increase the prevalence of Waddycephalus in the final hosts (native snakes) and in turn other intermediate hosts (coprophagous insects and native geckos). We witnessed native snakes (Boiga irregularis and Morelia spilota) preying on H. frenatus on multiple occasions during the study (authors, pers. obs.). It seems likely, therefore, that H. frenatus provide the same trophic link as native geckos, enabling Waddycephalus to complete its life cycle and potentially increasing its prevalence in native hosts through parasite spillback.
Do parasites affect the range expansion of H. frenatus?
Prevalence of mites in H. frenatus populations decreased with distance from the urban edge, which is consistent with Coates et al. (Reference Coates, Barnett, Hoskin and Phillips2017). There was also a significant negative interaction between relative abundance and distance, with larger H. frenatus populations having steeper declines in mite prevalence with distance from the urban edge (Supplementary Fig. S1). This pattern is driven by both higher prevalence at the urban edge and lower prevalence deeper into bushland. This is an intriguing pattern that requires further investigation. Higher density bushland populations of H. frenatus are generally also those that have been in the bushland the longest (Barnett et al. Reference Barnett, Phillips and Hoskin2017). Therefore, a potential explanation is that parasite release occurs when H. frenatus establishes in bushland at low density and then as populations increase rapidly to high density, mite increase lags behind. Another explanation could be differences in behaviour between urban and woodland geckos. If, for example, woodland geckos move more and are less territorial than urban geckos, they may be less likely to acquire mites (especially if this difference is magnified at high densities).
The prevalence of Geckobia mites and Waddycephalus nymphs was also affected by season, with higher prevalence in the warmer summer months (i.e. at the start and end of the year; Supplementary. Fig. S2). This could be explained by a greater proportion of juvenile geckos being found towards the middle of the year (Supplementary Fig. S2), as larger geckos are more likely to be infected with Waddycephalus. However, for Geckobia mites, there was no relationship between individual-level prevalence and gecko size (discussed below). Therefore, seasonal patterns in prevalence may be driven by increased contact between geckos during the peak breeding season (i.e. summer in north Queensland; Barnett et al. Reference Barnett, Phillips and Hoskin2017), which may lead to higher mite transmission rates. Future work should address the effects of season on these parasites more thoroughly by assessing prevalence over the entire year, as our data spanned only 8 months.
In terms of individual-level determinants of infection, we found that larger (and hence generally older) H. frenatus had higher intensity mite infections, suggesting that geckos steadily acquire mites through life. There was, however, no detectible impact of mite presence, or intensity of mite infection, on host body condition. Haematophagous mites can cause ulcerative dermatitis and inhibit skin sloughing in other lizards (Goldberg and Bursey, Reference Goldberg and Bursey1991; Goldberg and Holshuh, Reference Goldberg and Holshuh1992; Walter and Shaw, Reference Walter and Shaw2002), but to date there is no evidence of gecko mites affecting the condition in wild populations (Hanley et al. Reference Hanley, Fisher and Case1995). Here, we found significantly more mites on larger geckos. If mites affected the lifespan of geckos, one would expect to see fewer highly infected large (i.e. older) individuals. Together with the lack of effect on body condition, our results suggest that the impact of mites on host fitness may be negligible. It is, therefore, unlikely that release from mites increases the rate of range expansion in this system, despite an apparently lower prevalence of mites on the invasion front.
The prevalence of Waddycephalus was not affected by distance from the urban edge or relative abundance of H. frenatus. Predictions regarding the prevalence of Waddycephalus in H. frenatus are complicated by the fact that Waddycephalus have a complex lifecycle, and so are limited by the abundance of their final hosts (native snakes) and their first intermediate hosts (likely coprophagous insects) (Ali and Riley, Reference Ali and Riley1983; Kelehear et al. Reference Kelehear, Spratt, O'Meally and Shine2014a). The Waddycephalus that infect H. frenatus are most likely a native species (or multiple native species) and H. frenatus are infected at low prevalence at most of our woodland sites. In contrast, the parasite has been found to be completely absent in adjacent inner urban sites, probably due to smaller snake populations (Coates et al. Reference Coates, Barnett, Hoskin and Phillips2017).
Due to their presence in natural areas but absence in inner urban environments, Waddycephalus could potentially affect range expansion of H. frenatus in natural habitats if infection impacts body condition or survival. We found no effect of presence or intensity of Waddycephalus nymphs on body condition of H. frenatus. In infected individuals, however, smaller geckos had a greater intensity of infection than larger geckos (Table 4). One explanation for this pattern is that high-intensity Waddycephalus infection increases mortality with time, so adult geckos with high-intensity infection are missing from the population. While data on the effects of nymphal pentastomes on H. frenatus are lacking, they can kill reptile hosts when they migrate or moult, particularly when they burrow from the stomach to the body wall (Paré, Reference Paré2008), or affect the likelihood of predation through changes to host behaviour (Lefèvre et al. Reference Lefèvre, Lebarbenchon, Gauthier-Clerc, Missé, Poulin and Thomas2009). Another explanation is that some nymphs are lost through time, either because they excyst or because adult geckos can somehow shed them. Future studies should further explore the impact of Waddycephalus nymphs on geckos.
Concluding remark
This study examined the potential impacts of an invasive gecko on native species through parasite transmission and the potential effect of parasites on range expansion of the invasive gecko. We found no evidence of invasive mites infecting native geckos in this system, or vice versa, despite apparently ample opportunity in terms of time and fine-scale co-occurrence. Hemidactylus frenatus are, however, susceptible to infection by native Waddycephalus nymphs, and high-intensity infections may reduce the survival of individual H. frenatus. We explored the complex interactions between parasites and range expansion of this invasive gecko and found that range expansion into natural environments means both release from co-evolved mites and exposure to novel Waddycephalus nymphs. The relatively high density of H. frenatus makes parasite spillback (of Waddycephalus) to native host species a concern. Future work should investigate parasite spillback by assessing whether the prevalence of Waddycephalus nymphs in native gecko and snake populations is higher where they co-occur with H. frenatus.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003118201800015X
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.













