Introduction
A plethora of experimental evidence supports a key role of infections by gastrointestinal (GI) helminth parasites in shaping the composition of the vertebrate gut microbiota, with significant implications for local and systemic host immunity (reviewed by Brosschot and Reynolds, Reference Brosschot and Reynolds2018). For instance, recent studies have partly attributed parasite-associated qualitative and/or quantitative alterations to host GI microbial profiles to the ability of GI helminths to stimulate the initial onset of T-regulatory (Treg) immune responses (cf. Reynolds et al., Reference Reynolds, Smith, Filbey, Harcus, Hewitson, Redpath, Valdez, Yebra, Finlay and Maizels2014; Giacomin et al., Reference Giacomin, Zakrzewski, Croese, Su, Sotillo, McCann, Navarro, Mitreva, Krause, Loukas and Cantacessi2015, Reference Giacomin, Zakrzewski, Jenkins, Su, Al-Hallaf, Croese, de Vries, Grant, Mitreva, Loukas, Krause and Cantacessi2016; Zaiss et al., Reference Zaiss, Rapin, Lebon, Dubey, Mosconi, Sarter, Piersigilli, Menin, Walker, Rougemont, Paerewijck, Geldhof, McCoy, Macpherson, Croese, Giacomin, Loukas, Junt, Marsland and Harris2015). On the other hand, other studies have reported associations between acute helminth infections and gut microbiota imbalances (= dysbiosis) characterised by significant expansion of populations of putative pro-inflammatory bacteria (e.g. Rausch et al., Reference Rausch, Held, Fischer, Heimesaat, Kühl, Bereswill and Hartmann2013; Jenkins et al., Reference Jenkins, Peachey, Ajami, MacDonald, Hsieh, Brindley, Cantacessi and Rinaldi2018a; Schneeberger et al., Reference Schneeberger, Coulibaly, Panic, Daubenberger, Gueuning, Frey and Keiser2018a); these observations have lent credit to the hypothesis that helminth-associated alterations of gut microbiota composition may lead to both localised and systemic consequences for the host organism, that include immunopathology and exacerbated malnutrition in at-risk subjects from parasite-endemic areas (reviewed by Glendinning et al., Reference Glendinning, Nausch, Free, Taylor and Mutapi2014; Houlden et al., Reference Houlden, Hayes, Bancroft, Worthington, Wang, Grencis and Roberts2015; Cattadori et al., Reference Cattadori, Sebastian, Hao, Katani, Albert, Eilertson, Kapur, Pathak and Mitchell2016).
Over the past decade, newly acquired knowledge of the impact that GI helminth infections exert on the vertebrate gut microbial composition and metabolism has contributed to a better understanding of parasite systems biology and host-pathogen interactions (reviewed by Peachey et al., Reference Peachey, Jenkins and Cantacessi2017; Leung et al., Reference Leung, Graham and Knowles2018; Rapin and Harris, Reference Rapin and Harris2018), and has been proposed as a first step towards the identification and development of novel strategies of parasite control based on the targeted manipulation of the host gut microbiota (cf. Peachey et al., Reference Peachey, Jenkins and Cantacessi2017). Nevertheless, for humans in particular, progress in this field of research is greatly impaired by the impact of several confounding factors that inevitably affect studies conducted in naturally infected individuals (Mutapi, Reference Mutapi2015; Chabé et al., Reference Chabé, Lokmer and Segurel2017). In this review, we summarise current knowledge of GI helminth-microbiome interactions in humans under natural conditions of infection, identify similarities and differences between datasets and provide an overview of the confounding factors that may affect the interpretation of findings.
Helminth-gut microbiota interactions in real-world scenarios
In endemic areas for helminthiases, the vast majority of infected individuals harbour multiple helminth species, often occupying different niches of the host organism (Hotez et al., Reference Hotez, Alvarado, Basanez, Bolliger, Bourne, Boussinesq, Brooker, Brown, Buckle, Budke, Carabin, Coffeng, Fevre, Furst, Halasa, Jasrasaria, Johns, Keiser, King, Lozano, Murdoch, O'Hanlon, Pion, Pullan, Ramaiah, Roberts, Shepard, Smith, Stolk, Undurraga, Utzinger, Wang, Murray and Naghavi2014). Whilst polyparasitism is often regarded as a major confounding factor in investigations of parasite-microbiota interactions conducted in humans under natural conditions of infection (Cooper et al., Reference Cooper, Walker, Reyes, Chico, Salter, Vaca and Parkhill2013; Jenkins et al., Reference Jenkins, Rathnayaka, Perera, Peachey, Nolan, Krause, Rajakaruna and Cantacessi2017; Martin et al., Reference Martin, Djuardi, Sartono, Rosa, Supali, Mitreva, Houwing-Duistermaat and Yazdanbakhsh2018; Rosa et al., Reference Rosa, Supali, Gankpala, Djuardi, Sartono, Zhou, Fischer, Martin, Tyagi, Bolay, Fischer, Yazdanbakhsh and Mitreva2018), findings from these studies are key to assessing the impact that GI helminths exert on gut microbiota homeostasis in a ‘real-world’ scenario. Nevertheless, several factors should be considered when interpreting results obtained from individuals infected by multiple helminth species. First, anthropometric (e.g. age and gender) and anthropologic variables (e.g. ethnicity, diet and occupation) are well known to profoundly impact the ‘baseline’ composition of the human gut microbiota (Sekirov et al., Reference Sekirov, Russell, Antunes and Finlay2010; Yatsunenko et al., Reference Yatsunenko, Rey, Manary, Trehan, Domínguez-Bello, Contreras, Magris, Hidalgo, Baldassano, Anokhin, Heath, Warner, Reeder, Kuczynski, Caporaso, Lozupone, Lauber, Clemente, Knights, Knight and Gordon2012) (cf. Fig. 1); therefore, the enrolment of large cohorts of individuals is often necessary in order to achieve sufficient statistical power and avoid uninformative and/or misleading results (Kelly et al., Reference Kelly, Gross, Bittinger, Sherrill-Mix, Lewis, Collman, Bushman and Li2015). However, in many studies, the number of individuals enrolled and samples analysed is inevitably dictated by logistical and financial constraints. In these instances, population-related variables that impact gut microbiota composition may contribute substantially to inconsistencies among findings from different studies (cf. Fig. 1). For instance, a negative association between colonisation by the whipworm Trichuris trichiura and the abundance of bacteria belonging to the genus Prevotella in the faeces of infected individuals has been reported in two separate studies conducted in Malaysia (Lee et al., Reference Lee, Tang, Lim, Choy, Kurtz, Cox, Gundra, Cho, Bonneau, Blaser, Chua and Loke2014; Ramanan et al., Reference Ramanan, Bowcutt, Lee, Tang, Kurtz, Ding, Honda, Gause, Blaser, Bonneau, Lim, Loke and Cadwell2016), while other studies conducted in Ecuador, and Liberia and Indonesia, respectively, have failed to identify significant variations in faecal populations of Prevotella in individuals either solely infected by T. trichiura or co-infected with other species of soil-transmitted helminths (STHs) (Cooper et al., Reference Cooper, Walker, Reyes, Chico, Salter, Vaca and Parkhill2013; Martin et al., Reference Martin, Djuardi, Sartono, Rosa, Supali, Mitreva, Houwing-Duistermaat and Yazdanbakhsh2018; Rosa et al., Reference Rosa, Supali, Gankpala, Djuardi, Sartono, Zhou, Fischer, Martin, Tyagi, Bolay, Fischer, Yazdanbakhsh and Mitreva2018).
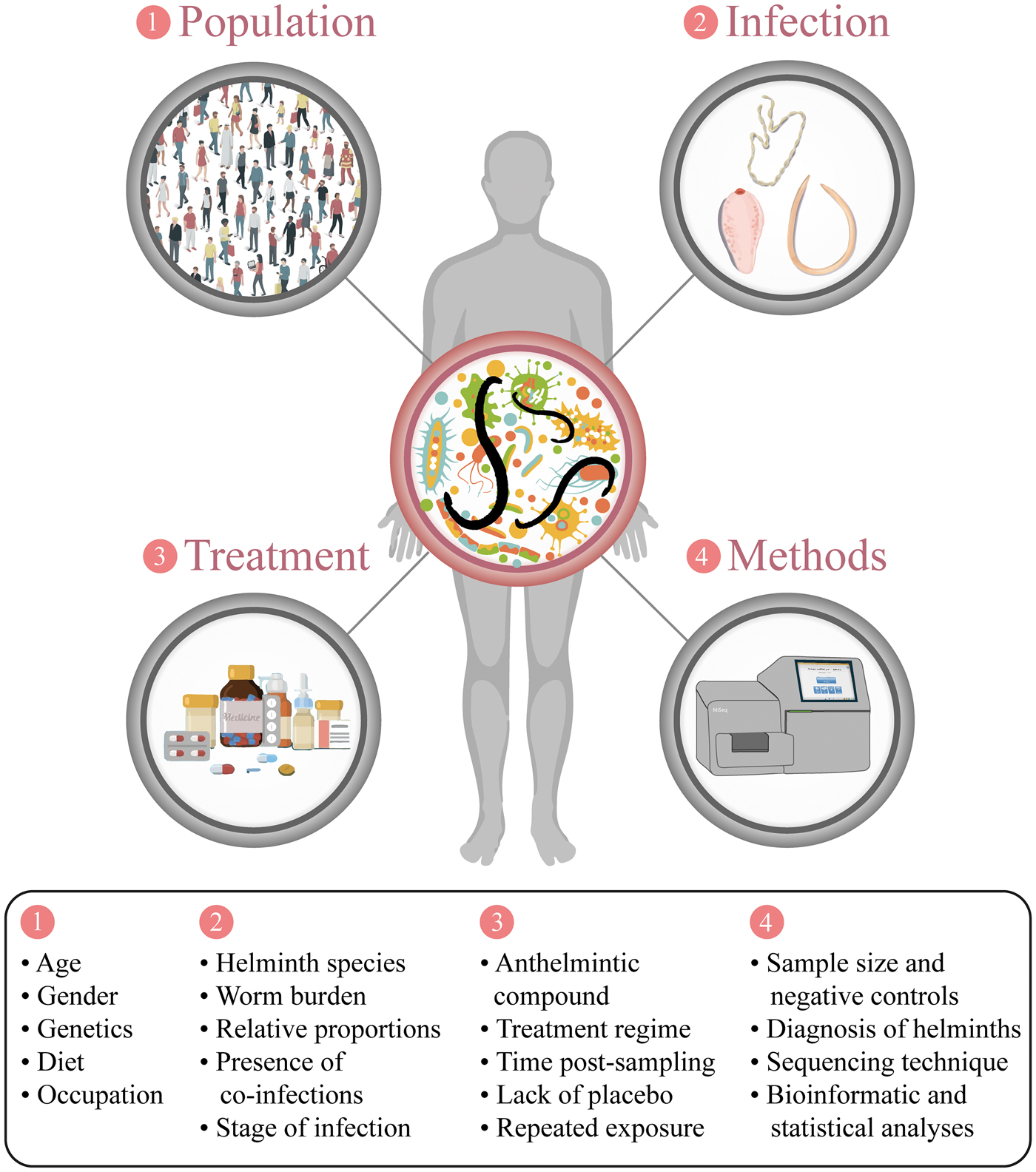
Fig. 1. Sources of variation and confounding factors potentially impacting on the outcome of studies of helminth-gut microbiota interactions in parasite-endemic regions.
In addition, whilst Rosa et al. (Reference Rosa, Supali, Gankpala, Djuardi, Sartono, Zhou, Fischer, Martin, Tyagi, Bolay, Fischer, Yazdanbakhsh and Mitreva2018) detected several distinctive features in the gut microbial profiles of helminth-harbouring individuals that were specifically associated to single infections with the hookworm Necator americanus, the roundworm Ascaris lumbricoides or T. trichiura, such features were inconsistent between two independent cohorts of helminth-infected volunteers from Liberia and Indonesia; this discrepancy suggests that other yet undetermined environmental factors may contribute to qualitative and quantitative alterations of the gut microbial profiles of helminth-infected individuals from different geographical areas. In contrast, an association between the abundance of selected bacterial taxa and infections by one or more STHs could be consistently detected in samples from both Liberian and Indonesian cohorts (Rosa et al., Reference Rosa, Supali, Gankpala, Djuardi, Sartono, Zhou, Fischer, Martin, Tyagi, Bolay, Fischer, Yazdanbakhsh and Mitreva2018). These taxa included bacteria belonging to the genera Olsenella and Allobaculum, which were expanded in the gut microbiota of helminth-infected individuals when compared to that of uninfected controls. To the best of our knowledge, the study by Rosa et al. (Reference Rosa, Supali, Gankpala, Djuardi, Sartono, Zhou, Fischer, Martin, Tyagi, Bolay, Fischer, Yazdanbakhsh and Mitreva2018) was the first to report a link between infections by STHs and the abundance of these bacterial genera in the human gut. Interestingly, in mice suffering from metabolic syndrome, administration of probiotics was followed by the expansion of populations of Olsenella and/or Allobaculum, and a reduction in systemic and/or local gut inflammatory responses (Wang et al., Reference Wang, Tang, Zhang, Zhao, Derrien, Rocher, van-Hylckama Vlieg, Strissel, Zhao, Obin and Shen2015a). Moreover, Allobaculum spp. are putative producers of anti-inflammatory short-chain fatty acids (Greetham et al., Reference Greetham, Gibson, Giffard, Hippe, Merkhoffer, Steiner, Falsen and Collins2004), and are severely reduced in the gut of mice genetically predisposed to spontaneous colitis (Pérez-Muñoz et al., Reference Pérez-Muñoz, Bergstrom, Peng, Schmaltz, Jiménez-Cardona, Marsteller, McGee, Clavel, Ley, Fu, Xia and Peterson2014). This knowledge led Rosa et al. (Reference Rosa, Supali, Gankpala, Djuardi, Sartono, Zhou, Fischer, Martin, Tyagi, Bolay, Fischer, Yazdanbakhsh and Mitreva2018) to hypothesize that these bacteria may play a yet undetermined role in the anti-inflammatory properties of parasitic helminths, and reinforce the proposition that the interactions between hosts, parasites and gut microbiota are multidirectional and should be approached in a holistic manner (cf. Cortés et al., Reference Cortés, Toledo and Cantacessi2018; Leung et al., Reference Leung, Graham and Knowles2018). Interestingly, in contrast to evidence acquired in human hosts, a negative association between Allobaculum abundance and colonisation by GI helminths has been observed in a mouse model of chronic trichuriasis (Holm et al., Reference Holm, Sorobetea, Kiilerich, Ramayo-Caldas, Estelle, Ma, Madsen, Kristiansen and Svensson-Frej2015), in which Th1-mediated immune responses are dominant (reviewed by Cliffe and Grencis, Reference Cliffe and Grencis2004), as well as in mice with patent infection by the blood fluke Schistosoma mansoni (Jenkins et al., Reference Jenkins, Peachey, Ajami, MacDonald, Hsieh, Brindley, Cantacessi and Rinaldi2018a), in which migrating eggs are responsible for the onset of marked Th2-mediated inflammatory responses (reviewed by Pearce and MacDonald, Reference Pearce and MacDonald2002). The immune-molecular mechanisms via which members of the genus Allobaculum may regulate local and systemic inflammation are still unclear (Greetham et al., Reference Greetham, Gibson, Giffard, Hippe, Merkhoffer, Steiner, Falsen and Collins2004; Pérez-Muñoz et al., Reference Pérez-Muñoz, Bergstrom, Peng, Schmaltz, Jiménez-Cardona, Marsteller, McGee, Clavel, Ley, Fu, Xia and Peterson2014; Wang et al., Reference Wang, Tang, Zhang, Zhao, Derrien, Rocher, van-Hylckama Vlieg, Strissel, Zhao, Obin and Shen2015a). Nonetheless, current data reporting reductions in populations of Allobaculum alongside helminth-associated gut inflammation supports the hypothesis raised by Rosa et al. (Reference Rosa, Supali, Gankpala, Djuardi, Sartono, Zhou, Fischer, Martin, Tyagi, Bolay, Fischer, Yazdanbakhsh and Mitreva2018); in the future, rodent models of GI helminth infections whose gut microbiota is deprived of, and subsequently recolonised with, the genus Allobaculum could be exploited to investigate the potential role(s) of these bacteria in parasite-mediated immunomodulation.
Beside the intrinsic variability of the human gut microbiota, studies conducted under natural conditions of helminth colonisation are likely to be affected by factors linked to the different combinations of infecting species and their relative abundances. For instance, in a study conducted in a cohort of Ecuadorian children, the specific features detected in the gut microbial profiles of subjects co-infected with T. trichiura and A. lumbricoides could not be identified in the microbiota of Trichuris-only infected individuals (Cooper et al., Reference Cooper, Walker, Reyes, Chico, Salter, Vaca and Parkhill2013). Similarly, selected microbial features that were observed in studies conducted in human volunteers with mono-specific infections with, for instance, A. lumbricoides, could not be detected in the gut microbiota of subjects harbouring the same parasite alongside other helminth species (e.g. T. trichiura and N. americanus) (Rosa et al., Reference Rosa, Supali, Gankpala, Djuardi, Sartono, Zhou, Fischer, Martin, Tyagi, Bolay, Fischer, Yazdanbakhsh and Mitreva2018), thus suggesting that a complex interplay exists between the host gut and its macro- and microbiota, that might be difficult to replicate in experimental settings. Furthermore, current evidence obtained from animal models of helminth infections indicates that worm burdens can impact the nature and/or the magnitude of parasite-associated alterations in gut microbial composition (Wu et al., Reference Wu, Li, Li, Beshah, Dawson and Urban2012; Peachey et al., Reference Peachey, Molena, Jenkins, Di Cesare, Traversa, Hodgkinson and Cantacessi2018). Nevertheless, such evidence is not yet available for human infections, in which parasite burdens may range from low to very high in endemic areas (Barbour and Kafetzaki, Reference Barbour and Kafetzaki1991; Churcher et al., Reference Churcher, Ferguson and Basáñez2005).
Another frequent constraint of investigations conducted in cohorts of human subjects with natural helminth infections is the limited availability of ‘genuine’ negative controls, i.e. individuals from the same communities of parasite-infected subjects who lack previous exposure to infections by parasitic helminths. Instead, individuals with no evidence of patent helminth infections are inevitably enrolled as control subjects (e.g. Cooper et al., Reference Cooper, Walker, Reyes, Chico, Salter, Vaca and Parkhill2013; Lee et al., Reference Lee, Tang, Lim, Choy, Kurtz, Cox, Gundra, Cho, Bonneau, Blaser, Chua and Loke2014; Jenkins et al., Reference Jenkins, Rathnayaka, Perera, Peachey, Nolan, Krause, Rajakaruna and Cantacessi2017; Rosa et al., Reference Rosa, Supali, Gankpala, Djuardi, Sartono, Zhou, Fischer, Martin, Tyagi, Bolay, Fischer, Yazdanbakhsh and Mitreva2018); nevertheless, studies in helminth-infected individuals subjected to anthelmintic treatment, as well as in primates and pigs exposed to Trichuris spp., have shown that parasite-associated alterations in gut microbial communities can persist, at least partly, in absence of active infections (Broadhurst et al., Reference Broadhurst, Ardeshir, Kanwar, Mirpuri, Gundra, Leung, Wiens, Vujkovic-Cvijin, Kim, Yarovinsky, Lerche, McCune and Loke2012; Wu et al., Reference Wu, Li, Li, Beshah, Dawson and Urban2012; Cooper et al., Reference Cooper, Walker, Reyes, Chico, Salter, Vaca and Parkhill2013; Kay et al., Reference Kay, Millard, Sergeant, Midzi, Gwisai, Mduluza, Ivens, Nausch, Mutapi and Pallen2015; Schneeberger et al., Reference Schneeberger, Coulibaly, Panic, Daubenberger, Gueuning, Frey and Keiser2018a). These data call for caution when interpreting the biological meaning of differences between the gut microbial profiles of helminth-infected and uninfected volunteers from the same communities. In addition, patent infections are often diagnosed using stool-based microscopic methods, that are known for their relatively low sensitivity and that may yield false negative results, e.g. in case of intermittent shedding of eggs and/or larvae (O'Connell and Nutman, Reference O'Connell and Nutman2016). Recently, Rosa et al. (Reference Rosa, Supali, Gankpala, Djuardi, Sartono, Zhou, Fischer, Martin, Tyagi, Bolay, Fischer, Yazdanbakhsh and Mitreva2018) used quantitative real-time PCR to diagnose STH infections in individuals subjected to gut microbiota profiling, indicating that this technique may represent a robust and sensitive alternative to microscopic methods, since it provides users with the ability to semi-quantify burdens of different helminth species from minute amounts of DNA template. However, in spite of their higher sensitivity, molecular methods rely on the use of primers that selectively target the parasite species of interest, thus impairing the simultaneous detection of potential (asymptomatic or subclinical) co-infections with other helminth and/or non-helminth pathogens (O'Connell and Nutman, Reference O'Connell and Nutman2016). Indeed, the impact of protozoa on the gut microbial diversity and composition has been clearly demonstrated in humans and other vertebrates (reviewed by Chabé et al., Reference Chabé, Lokmer and Segurel2017; Stensvold and van der Giezen, Reference Stensvold and van der Giezen2018). Furthermore, a recent study conducted in a cohort of Colombian schoolchildren reported common features in the faecal microbial composition of subjects co-infected with helminths and protozoans and mono-parasitized with the flagellate Giardia intestinalis compared to uninfected individuals (Toro-Londono et al., Reference Toro-Londono, Bedoya-Urrego, Garcia-Montoya, Galvan-Diaz and Alzate2019). Whilst the mechanisms via which each group of parasites alters the host gut flora, as well as the nature of such alterations, are yet to be determined, these findings support the need to conduct additional diagnostic tests on stool samples from helminth-infected cohorts, as well as the corresponding uninfected subjects, in order to rule out the influence of concomitant bacterial, viral and/or protozoan infections that may be responsible for the changing gut microbial profiles of these individuals (cf. Chabé et al., Reference Chabé, Lokmer and Segurel2017).
Nevertheless, in spite of the several confounding factors outlined above (cf. Fig. 1), observational studies in helminth endemic areas have proven useful for the identification of significant associations between parasite colonisation and the gut microbial profiles of humans under natural conditions of infection. Importantly, studies conducted in these communities provide excellent opportunities to evaluate the effect(s) that parasite removal (e.g. via the administration of broad-spectrum anthelmintics) exert(s) on the gut microbiota of previously infected individuals, thus contributing cues to understand the causality of helminth-microbiota relationships.
Impact of deworming on the human gut microbiota
The implementation of mass drug administration programmes in endemic areas for STHs and schistosomiasis offers opportunities to elucidate potential mechanisms via which parasitic helminths modulate the host gut microbiota. For instance, qualitative and quantitative changes in gut microbial profiles that are caused by direct interactions between parasites and gut bacteria may be expected to rapidly reverse following parasite removal, whilst long-lasting alterations are likely to result from indirect interplay mediated by the host immune system (Houlden et al., Reference Houlden, Hayes, Bancroft, Worthington, Wang, Grencis and Roberts2015; Su et al., Reference Su, Su, Li, Long, Chang, Zhang, Walker, Xavier, Cherayil and Shi2018). Nevertheless, such investigations are also generally constrained by the presence of several confounding factors that include not only the host- and parasite-dependent variables outlined above, but also variations linked to the use of different drugs and treatment regimes (Schneeberger et al., Reference Schneeberger, Coulibaly, Gueuning, Moser, Coburn, Frey and Keiser2018b), as well as time of sampling post-anthelmintic treatment (Houlden et al., Reference Houlden, Hayes, Bancroft, Worthington, Wang, Grencis and Roberts2015) (Fig. 1). The latter in particular may profoundly affect findings from these studies, as the presence of tissue lesions caused by, for instance, parasite feeding activity and location (e.g. blood-feeders vs. non blood-feeders and luminal vs. tissue dwellers) are likely to influence the timespan between helminth removal and microbiome recovery (reviewed by Leung et al., Reference Leung, Graham and Knowles2018). Moreover, for ethical reasons, data from these experiments is often biased by the lack of placebo-treated control groups. These limitations may be at least partially responsible for the differences between findings from studies aimed at elucidating the effect of anthelmintic treatment on the gut microbiota of helminth-infected volunteers; notwithstanding, it is worth noting that, in instances where deworming-associated changes in human gut microbial profiles were detected, these were generally moderate (Ramanan et al., Reference Ramanan, Bowcutt, Lee, Tang, Kurtz, Ding, Honda, Gause, Blaser, Bonneau, Lim, Loke and Cadwell2016; Martin et al., Reference Martin, Djuardi, Sartono, Rosa, Supali, Mitreva, Houwing-Duistermaat and Yazdanbakhsh2018; Schneeberger et al., Reference Schneeberger, Coulibaly, Gueuning, Moser, Coburn, Frey and Keiser2018b).
Consistent with this, a recent study conducted on faecal samples collected from a rural community in Indonesia reported that the composition of the gut microbiota of individuals repeatedly treated with either albendazole or placebo (for 21 months) resembled that of samples collected from the same subjects prior to treatment, rather than that of uninfected controls (Rosa et al., Reference Rosa, Supali, Gankpala, Djuardi, Sartono, Zhou, Fischer, Martin, Tyagi, Bolay, Fischer, Yazdanbakhsh and Mitreva2018). Moreover, a parallel investigation conducted on the same cohort of individuals detected reduced populations of Prevotella in albendazole-treated subjects in which complete deworming did not occur, compared to placebo-treated individuals with patent helminth infections (Martin et al., Reference Martin, Djuardi, Sartono, Rosa, Supali, Mitreva, Houwing-Duistermaat and Yazdanbakhsh2018). Intriguingly, failure of albendazole treatment was accompanied by a dominance of T. trichiura (over other helminth species) in these subjects, while placebo-treated individuals maintained a diverse macrobiota (i.e. infections by multiple helminths); hence, differences in the composition of the GI macrobiota (i.e. species present and their relative abundances) between albendazole- and placebo-treated individuals could account for variations in the composition of the intestinal microflora of these subjects (Martin et al., Reference Martin, Djuardi, Sartono, Rosa, Supali, Mitreva, Houwing-Duistermaat and Yazdanbakhsh2018). Significant associations between colonisation by T. trichiura and Prevotella abundance were not observed in the Indonesian cohort (Martin et al., Reference Martin, Djuardi, Sartono, Rosa, Supali, Mitreva, Houwing-Duistermaat and Yazdanbakhsh2018; Rosa et al., Reference Rosa, Supali, Gankpala, Djuardi, Sartono, Zhou, Fischer, Martin, Tyagi, Bolay, Fischer, Yazdanbakhsh and Mitreva2018). However, negative associations between whipworm infections and Prevotella abundance had been detected previously in two independent studies conducted in Malaysia (Lee et al., Reference Lee, Tang, Lim, Choy, Kurtz, Cox, Gundra, Cho, Bonneau, Blaser, Chua and Loke2014; Ramanan et al., Reference Ramanan, Bowcutt, Lee, Tang, Kurtz, Ding, Honda, Gause, Blaser, Bonneau, Lim, Loke and Cadwell2016). In particular, Ramanan and co-authors (2016) observed that, following albendazole treatment, expansion of Prevotella populations in the human faecal microbiota was related to reduced T. trichiura faecal egg counts. In contrast, no significant associations between helminth infection and abundance of Prevotella was reported in a study investigating the impact of parasite colonisation and successful treatment with a combination of albendazole and ivermectin on the faecal microbial profiles of a cohort of Trichuris-infected children from Ecuador (Cooper et al., Reference Cooper, Walker, Reyes, Chico, Salter, Vaca and Parkhill2013), nor in a group of helminth-infected adults from Sri Lanka treated with pyrantel pamoate (Jenkins et al., Reference Jenkins, Rathnayaka, Perera, Peachey, Nolan, Krause, Rajakaruna and Cantacessi2017). Similarly, no qualitative or quantitative changes to faecal microbial composition were observed in two cohorts of schoolchildren from Côte d'Ivoire and Zimbabwe infected by S. mansoni and S. haematobium, respectively, following treatment with praziquantel (Kay et al., Reference Kay, Millard, Sergeant, Midzi, Gwisai, Mduluza, Ivens, Nausch, Mutapi and Pallen2015; Schneeberger et al., Reference Schneeberger, Coulibaly, Panic, Daubenberger, Gueuning, Frey and Keiser2018a). However, successful elimination of S. mansoni was associated with a higher abundance of Fusobacterium spp. pre-treatment, as well as 24 h post-treatment (Schneeberger et al., Reference Schneeberger, Coulibaly, Panic, Daubenberger, Gueuning, Frey and Keiser2018a).
Whilst drug administration in endemic regions may result in effective elimination of helminth infections, potential co-infecting protozoan parasites are not susceptible to anthelmintic treatment; this, together with the sub-standard hygienic and sanitary conditions that generally characterise these areas and that result in continuous re-exposure to infective helminth developmental stages (Campbell et al., Reference Campbell, Biritwum, Woods, Velleman, Fleming and Stothard2018), impairs the full assessment of the consequences of helminth removal on the composition of the human gut microbiota. To the best of our knowledge, thus far, a single study has investigated the effects of chronic infections by a GI helminth, Strongyloides stercoralis, and anthelmintic treatment on the composition of the faecal microbiota and metabolome of humans from a non-endemic area of Europe, where parasite transmission had been interrupted (Jenkins et al., Reference Jenkins, Formenti, Castro, Piubelli, Perandin, Buonfrate, Otranto, Griffin, Krause, Bisoffi and Cantacessi2018b). Treatment with ivermectin resulted in compositional changes of the faecal microbiota (analysed 6 months post-treatment), which partially resembled that of uninfected control subjects (Jenkins et al., Reference Jenkins, Formenti, Castro, Piubelli, Perandin, Buonfrate, Otranto, Griffin, Krause, Bisoffi and Cantacessi2018b); in particular, alpha diversity [= a measure of the number of bacterial species present in a given microbial community (richness) and their relative abundance (evenness)] was reduced in the microbiota of individuals post-treatment (although statistical significance was not achieved) and accompanied by expanded populations of potentially pathogenic bacteria (Jenkins et al., Reference Jenkins, Formenti, Castro, Piubelli, Perandin, Buonfrate, Otranto, Griffin, Krause, Bisoffi and Cantacessi2018b). In addition, the faecal metabolic profiles obtained from samples collected post-ivermectin treatment shared features with both those obtained from samples collected pre-treatment and from uninfected controls (Jenkins et al., Reference Jenkins, Formenti, Castro, Piubelli, Perandin, Buonfrate, Otranto, Griffin, Krause, Bisoffi and Cantacessi2018b); this observation led Jenkins et al. (Reference Jenkins, Formenti, Castro, Piubelli, Perandin, Buonfrate, Otranto, Griffin, Krause, Bisoffi and Cantacessi2018b) to hypothesise that, following parasite removal and over time, both gut microbiota and metabolome may revert to the original pre-infection state. Multiple factors, including but not limited to those outlined above, may contribute to the discrepancies observed between the findings from this work and those that reported no or minor effects of anthelmintic treatment on the gut microbiome of helminth-infected humans (Cooper et al., Reference Cooper, Walker, Reyes, Chico, Salter, Vaca and Parkhill2013; Ramanan et al., Reference Ramanan, Bowcutt, Lee, Tang, Kurtz, Ding, Honda, Gause, Blaser, Bonneau, Lim, Loke and Cadwell2016; Martin et al., Reference Martin, Djuardi, Sartono, Rosa, Supali, Mitreva, Houwing-Duistermaat and Yazdanbakhsh2018; Rosa et al., Reference Rosa, Supali, Gankpala, Djuardi, Sartono, Zhou, Fischer, Martin, Tyagi, Bolay, Fischer, Yazdanbakhsh and Mitreva2018; Schneeberger et al., Reference Schneeberger, Coulibaly, Panic, Daubenberger, Gueuning, Frey and Keiser2018a, Reference Schneeberger, Coulibaly, Gueuning, Moser, Coburn, Frey and Keiser2018b).
Despite the limitations outlined above, studies of GI helminth-microbiota relationships conducted in endemic areas for helminthiases have provided repeated evidence of the perturbations that parasites and anthelmintic treatment exert on the equilibrium of resident populations of bacteria and on gut homeostasis. However, the identification of common signatures across studies remains key to designing future experiments, e.g. in animal models of helminth infections, that may assist the elucidation of the mechanisms that underpin the interactions between GI helminths, the gut microbiota and the host immune system.
Do common signatures exist across studies of host-helminth-microbiota interactions?
The identification of gut microbial signatures that occur reproducibly across several host-GI helminth systems is crucial for designing novel anti-helminth intervention strategies based on the manipulation of the gut microbiota (Peachey et al., Reference Peachey, Jenkins and Cantacessi2017). Studies conducted in animal models of helminth infections are expected to assist the identification of such signatures, as well as the direct (i.e. parasite-mediated) and/or indirect (i.e. immune-mediated) mechanisms that govern helminth-microbiota interactions (Cortés et al., Reference Cortés, Toledo and Cantacessi2018); nevertheless, the inconsistencies that characterise studies of helminth-microbiota relationships published to date make such a task highly challenging. Indeed, for patterns to be identified, fluctuations in selected populations of gut microbes must be interpreted in light of the physical and immunological alterations of the mucosal environment in which such alterations occur (Leung et al., Reference Leung, Graham and Knowles2018). For instance, expanded populations of Lactobacillaceae have been repeatedly detected following infection with several species of parasitic helminths in multiple host species (Reynolds et al., Reference Reynolds, Smith, Filbey, Harcus, Hewitson, Redpath, Valdez, Yebra, Finlay and Maizels2014; Holm et al., Reference Holm, Sorobetea, Kiilerich, Ramayo-Caldas, Estelle, Ma, Madsen, Kristiansen and Svensson-Frej2015; Houlden et al., Reference Houlden, Hayes, Bancroft, Worthington, Wang, Grencis and Roberts2015; Cattadori et al., Reference Cattadori, Sebastian, Hao, Katani, Albert, Eilertson, Kapur, Pathak and Mitchell2016; Duarte et al., Reference Duarte, Jenkins, Latrofa, Giannelli, Papadopoulos, de Carvalho, Nolan, Otranto and Cantacessi2016; Jenkins et al., Reference Jenkins, Peachey, Ajami, MacDonald, Hsieh, Brindley, Cantacessi and Rinaldi2018a; Kim et al., Reference Kim, Kim, Yi, Lee, Lee, Hwang, Yong, Sohn and Yong2018), and could thus be considered as a ‘consistent alteration’ in gut microbiota composition upon helminth colonisation. However, key differences exist between host-parasite pairs investigated in the studies that have reported such an outcome. Indeed, whilst populations of Lactobacillaceae promote regulatory responses in mice infected by Heligmosomoides polygyrus bakeri (Reynolds et al., Reference Reynolds, Smith, Filbey, Harcus, Hewitson, Redpath, Valdez, Yebra, Finlay and Maizels2014), a lack of correlation between Lactobacillaceae abundance and Treg populations has been observed in other host-parasites systems, such as mice chronically infected with T. muris and rabbits infected with Trichostrongylus retortaeformis, in which the expansion of populations of gut Lactobacillaceae upon helminth infection occurs in an environment dominated by Th1-mediated immune responses (Holm et al., Reference Holm, Sorobetea, Kiilerich, Ramayo-Caldas, Estelle, Ma, Madsen, Kristiansen and Svensson-Frej2015; Houlden et al., Reference Houlden, Hayes, Bancroft, Worthington, Wang, Grencis and Roberts2015; Cattadori et al., Reference Cattadori, Sebastian, Hao, Katani, Albert, Eilertson, Kapur, Pathak and Mitchell2016). These differences suggest that alternative mechanisms may regulate the differentiation and development of adaptive immune responses in each host-parasite system (Houlden et al., Reference Houlden, Hayes, Bancroft, Worthington, Wang, Grencis and Roberts2015), and thus that similar alterations in gut microbiota composition may result in different consequences that are dependent on the microenvironment where these changes occur. Notwithstanding, the interactions between hosts, helminths and the gut microbiota are likely multifaceted and multidirectional, and therefore the potential consequences that selected compositional changes in gut microbiota exert on host homeostasis are only one aspect of this complex interplay. For instance, a common yet unidentified mechanism may determine the expansion of Lactobacillaceae in the gut of helminth-infected hosts.
On the other hand, apparent ‘contradictory’ findings across studies may result from fundamental differences between gut compartments under investigation. For instance, Prevotella spp. was expanded in the abomasum and faeces of sheep infected by abomasal trichostrongyles (i.e. Haemonchus contortus and Teladorsagia circumcincta; Li et al., Reference Li, Li, Sun, Yu, Baldwin and Urban2016; Cortés et al., Reference Cortés, Wills, Su, Hewitt, Scotti, Robertson, Price, Bartley, McNeilly, Krause, Powell, Nisbet and Cantacessisubmitted), whilst the same taxa were reduced in the faeces of a range of host species, including mice, humans and horses, infected by nematodes residing in the large intestine, i.e. Trichuris spp. and cyathostomins, respectively (Lee et al., Reference Lee, Tang, Lim, Choy, Kurtz, Cox, Gundra, Cho, Bonneau, Blaser, Chua and Loke2014; Houlden et al., Reference Houlden, Hayes, Bancroft, Worthington, Wang, Grencis and Roberts2015; Peachey et al., Reference Peachey, Castro, Molena, Jenkins, Griffin and Cantacessiin press). It must be noted, however, that whilst increased abomasal pH favours Prevotella overgrowth in the abomasum (De Nardi et al., Reference De Nardi, Marchesini, Li, Khafipour, Plaizier, Gianesella, Ricci, Andrighetto and Segato2016; Li et al., Reference Li, Li, Sun, Yu, Baldwin and Urban2016), the same taxa are likely to be exposed to a dramatically different microenvironment in the large intestine that may determine the contraction of these bacterial groups. In addition, given the functional dissimilarities between the abomasal and colonic microbiota, such alterations are expected to result in fundamentally different outcomes for the homeostasis of each of these gut compartments (Ley et al., Reference Ley, Hamady, Lozupone, Turnbaugh, Ramey, Bircher, Schlegel, Tucker, Schrenzel, Knight and Gordon2008), and hence comparisons are, in our opinion, unwarranted.
In parallel to species of bacteria with functions that may vary depending on the gut compartment, multiple taxa share the same functions in different microenvironments (Lozupone et al., Reference Lozupone, Stombaugh, Gordon, Jansson and Knight2012); therefore, it is plausible that, even though inconsistencies are detected across studies, these may result in similar functional alterations in the host-parasite pairs being compared. For instance, recent studies in mouse and humans infected with S. mansoni have reported the expansion of different genera of bacteria with pro-inflammatory functions in the gut microbiota of the respective hosts (Jenkins et al., Reference Jenkins, Peachey, Ajami, MacDonald, Hsieh, Brindley, Cantacessi and Rinaldi2018a; Schneeberger et al., Reference Schneeberger, Coulibaly, Panic, Daubenberger, Gueuning, Frey and Keiser2018a). These observations lend credit to the hypothesis that the functional role of the gut microbiota in helminth infections could be far less ‘diverse’ than the taxonomic associations reported thus far. For this hypothesis to be confirmed or confuted, a better understanding of the function(s) of each bacterial taxon inhabiting the different gut compartments in a range of host species is needed. To this aim, the integration of metagenomic, metabolomic and metatranscriptomic technologies, alongside traditional microbiology and microscopy techniques, may assist to achieve a holistic picture of the impact of GI helminth infections on the functions of the human gut microbiota, and its significance for disease pathophysiology and overall host health (Wang et al., Reference Wang, Xu, Ren, Tao, Jiang and Zheng2015b).
Current needs and future directions
Understanding the complex interactions between GI helminths and their vertebrate hosts is pivotal for advancing our knowledge of the fundamental biology of these parasites and the diseases they cause (see Peachey et al., Reference Peachey, Jenkins and Cantacessi2017; Leung et al., Reference Leung, Graham and Knowles2018; Rapin and Harris, Reference Rapin and Harris2018 for reviews). Whilst the role of the gut microbiota in host-parasite relationships has long been overlooked, current knowledge of the key roles that resident bacteria play in host health and disease, together with recent technical advancements for microbiota profiling, have boosted research is this area. This is currently leading to increasing evidence of a role for the gut microbiota in the immune regulatory properties of helminth parasites (cf. Cantacessi et al., Reference Cantacessi, Giacomin, Croese, Zakrzewski, Sotillo, McCann, Nolan, Mitreva, Krause and Loukas2014; Reynolds et al., Reference Reynolds, Smith, Filbey, Harcus, Hewitson, Redpath, Valdez, Yebra, Finlay and Maizels2014; Giacomin et al., Reference Giacomin, Zakrzewski, Croese, Su, Sotillo, McCann, Navarro, Mitreva, Krause, Loukas and Cantacessi2015, Reference Giacomin, Zakrzewski, Jenkins, Su, Al-Hallaf, Croese, de Vries, Grant, Mitreva, Loukas, Krause and Cantacessi2016; Zaiss et al., Reference Zaiss, Rapin, Lebon, Dubey, Mosconi, Sarter, Piersigilli, Menin, Walker, Rougemont, Paerewijck, Geldhof, McCoy, Macpherson, Croese, Giacomin, Loukas, Junt, Marsland and Harris2015). In addition, data collected to date points towards a likely role of the gut microflora in the immunopathology of selected GI helminth infections that awaits experimental validation. Trying to untangle the relevance of particular fluctuations of specific bacterial taxa on infection outcome is challenging; nevertheless, currently available data suggest that low-intensity, chronic helminth infections are commonly linked to high microbial diversity and predominance of bacteria typically associated with gut health. Conversely, high-intensity, acute infections are often associated to gut dysbiosis, characterised by reduced alpha diversity and an increase in pro-inflammatory and often opportunistic pathogens (Peachey et al., Reference Peachey, Jenkins and Cantacessi2017). However, for this knowledge to be exploited in translational studies, further investigations in both natural and experimental settings are needed to distinguish spurious results from genuine helminth-microbiota associations (Peachey et al., Reference Peachey, Jenkins and Cantacessi2017), and mechanistic studies in animal models of helminth infections are necessary to dissect the causality of these relationships (cf. Cortés et al., Reference Cortés, Toledo and Cantacessi2018). Importantly, minimising variations between studies is crucial to warrant meaningful comparisons between datasets.
Whilst reducing the variability amongst samples collected from naturally helminth-infected humans may be difficult to achieve, the enormous impact that differences in technical and experimental approaches (from sample collection to bioinformatics and biostatistical analyses of high-throughput metagenomics sequence datasets) exert on the overall variation detected across studies can be reduced (Figs 1 and 2; Lindgreen et al., Reference Lindgreen, Adair and Gardner2016; Costea et al., Reference Costea, Zeller, Sunagawa, Pelletier, Alberti, Levenez, Tramontano, Driessen, Hercog, Jung, Kultima, Hayward, Coelho, Allen-Vercoe, Bertrand, Blaut, Brown, Carton, Cools-Portier, Daigneault, Derrien, Druesne, de Vos, Finlay, Flint, Guarner, Hattori, Heilig, Luna, van Hylckama Vlieg, Junick, Klymiuk, Langella, Le Chatelier, Mai, Manichanh, Martin, Mery, Morita, O'Toole, Orvain, Patil, Penders, Persson, Pons, Popova, Salonen, Saulnier, Scott, Singh, Slezak, Veiga, Versalovic, Zhao, Zoetendal, Ehrlich, Dore and Bork2017; Golob et al., Reference Golob, Margolis, Hoffman and Fredricks2017). In particular, a range of bioinformatics pipelines are available for the analysis of high-throughput amplicon and whole metagenome sequence datasets that include, e.g., different sequence-processing tools and reference databases for sequence annotation that could yield slightly different results (Lindgreen et al., Reference Lindgreen, Adair and Gardner2016; Golob et al., Reference Golob, Margolis, Hoffman and Fredricks2017). For instance, the use of validated open microbiome analysis packages such Multiplexed Analysis of Projections by Sequencing (MAPseq) (Matias Rodrigues et al., Reference Matias Rodrigues, Schmidt, Tackmann and von Mering2017) or QIIME2 (https://qiime2.org/) may assist accurate taxonomic classifications of bacterial 16S rRNA amplicon datasets; similarly, sequence annotation should rely on the use of regularly updated reference databases. Amongst these, SILVA (https://www.arb-silva.de/) (Quast et al., Reference Quast, Pruesse, Yilmaz, Gerken, Schweer, Yarza, Peplies and Glöckner2013) enables sensitive annotations of bacterial rRNA sequence data (Almeida et al., Reference Almeida, Mitchell, Tarkowska and Finn2018). The use of such standardized analysis workflows and reference databases for sequence annotation might prove extremely useful to increase consistency across studies and enable researchers to identify common and/or unique features between the gut microbiota of different host-parasite systems which, in turn, might assist to better understand the mechanisms that regulate helminth-microbiota relationships.
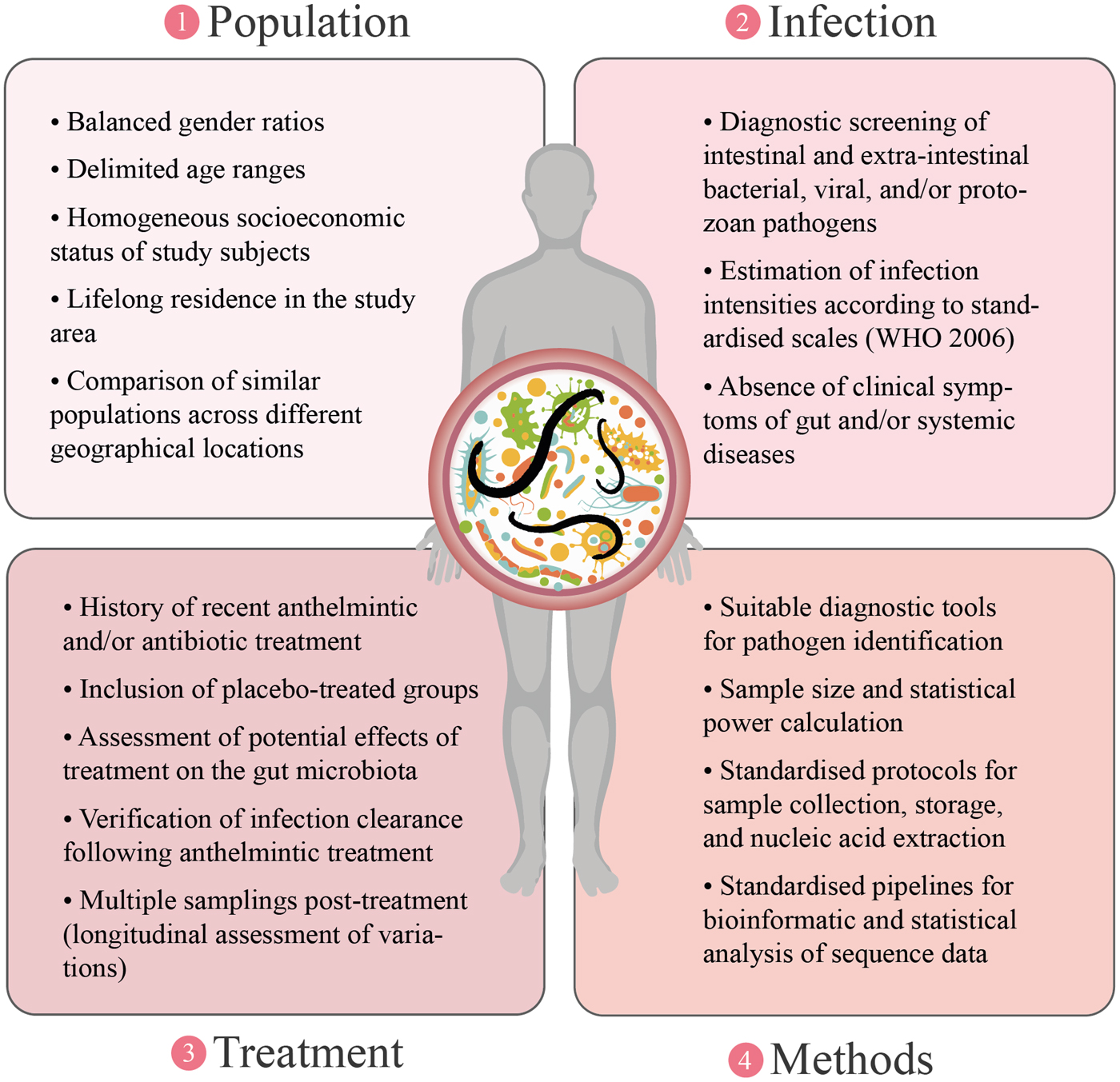
Fig. 2. Proposed approaches aimed at reducing the methodological sources of variation surrounding investigations of helminth-gut microbiota interactions.
The consequences that elucidating such mechanisms may exert on future strategies of parasite control are two-fold. First, disentangling the potential contribution of the gut flora to the pathogenesis of the infection is necessary in order to discover and develop new strategies to contrast helminth-associated pathology. Second, understanding the microbiota-dependent mechanisms by which parasitic helminths are able to modulate host immune responses and suppress inflammation may assist the discovery of novel immune-regulatory therapeutics against chronic inflammatory disorders of the GI tract that may act in synergy with helminth-based therapy (see Peachey et al., Reference Peachey, Jenkins and Cantacessi2017 and Rapin and Harris, Reference Rapin and Harris2018 for reviews). However, in order for this new knowledge to be fully exploited in translational research, further studies that thoroughly consider inclusion/exclusion criteria for the selection of participants, include appropriate controls, and follow standardised experimental and data analysis protocols are necessary, and will allow to disentangle the potential influence of parasite-, drug- and/or population-dependent variables in each setting (Fig. 2).
Acknowledgements
The authors would like to thank Professor R. Stephen Phillips for helpful suggestions on the draft manuscript.
Financial support
AC is supported by a postdoctoral fellowship from Fundación Alfonso Martín Escudero (Madrid, Spain). LEP is supported by funding from the Horserace Betting Levy Board (HBLB) and TPJ by scholarships by the Biotechnology and Biological Sciences Research Council (BBSRC) of the United Kingdom. Research in the CC laboratory is supported by grants by the Royal Society and the Isaac Newton Trust.
Conflict of interest
None.
Ethical standards
Not applicable.





