INTRODUCTION
Soil-transmitted helminth (STH) infections – ascariasis, trichuriasis and hookworm infections – are among the most common chronic infectious diseases worldwide, mainly affecting socio-economically deprived populations (WHO, 2012, 2016a ). STHs are main causes of disease burden in school-aged children in low- and middle-income countries, in severe cases resulting in anaemia, reduced school performance, stunting, malnutrition and death (Bethony et al. Reference Bethony, Brooker, Albonico, Geiger, Loukas, Diemert and Hotez2006; Hotez et al. Reference Hotez, Brindley, Bethony, King, Pearce and Jacobson2008; WHO, 2012, 2016a ).
About 1·5 billion individuals worldwide are estimated to be infected with at least one STH species (WHO, 2016a ), causing about 5 million DALYs (disability-adjusted life years) losts (Hotez et al. Reference Hotez, Alvarado, Basáñez, Bolliger, Bourne, Boussinesq, Brooker, Brown, Buckle and Budke2014; Pullan et al. Reference Pullan, Smith, Jasrasaria and Brooker2014). About 300 million people suffer from severe morbidity attributed to STHs, resulting in an estimation of 12 000–135 000 deaths annually (Bethony et al. Reference Bethony, Brooker, Albonico, Geiger, Loukas, Diemert and Hotez2006; WHO, 2016b ).
STHs have considerable public health importance in Brazil, occurring throughout the national territory. They are concentrated mainly in poor and vulnerable groups living in areas with inadequate water supply and sanitation, combined with lack of access to healthcare services and low levels of education (Fonseca et al. Reference Fonseca, Teixeira, Barreto, Carmo and Costa2010; Brazilian Ministry of Health, 2012; Scholte et al. Reference Scholte, Schur, Bavia, Carvalho, Chammartin, Utzinger and Vounatsou2013). As there is no specific national STH control programme, cases are usually detected in a passive manner by health facilities. STH carriers are also detected by the Schistosomiasis Surveillance Programme within the routine stool examinations, and treated (Brazilian Ministry of Health, 2012). The lack of a mandatory reporting system and an active surveillance programme throughout the national territory makes the true burden and the impact of disease unknown and probably underestimated in Brazil (Brazilian Ministry of Health, 2012; Couto et al. Reference Couto, Tibiriça, Pinheiro, Mitterofhe, Lima, Castro, Gonçalves, Silva, Guimarães, Rosa and Coimbra2014). There are few large-scale surveys and systematic studies on prevalence in the general population in the country (Scholte et al. Reference Scholte, Schur, Bavia, Carvalho, Chammartin, Utzinger and Vounatsou2013; Chammartin et al. Reference Chammartin, Guimarães, Scholte, Bavia, Utzinger and Vounatsou2014). It is estimated that 26·1–29·7 million Brazilian people are infected with Ascaris lumbricoides, 14·4–19·2 million with Trichuris trichiura and 4·7–11·3 million with hookworms (Scholte et al. Reference Scholte, Schur, Bavia, Carvalho, Chammartin, Utzinger and Vounatsou2013; Vos et al. Reference Vos, Barber, Bell, Bertozzi-Villa, Biryukov, Bolliger, Charlson, Davis, Degenhardt, Dicker, Duan, Erskine, Feigin, Ferrari, Fitzmaurice, Fleming, Graetz, Guinovart, Haagsma, Hansen, Hanson, Heuton, Higashi, Kassebaum, Kyu, Laurie, Liang, Lofgren, Lozano and MacIntyre2016). In 2014, an estimated 11·5 million Brazilian children (about 3 million preschool-age children and 8·5 million school-age children) were in need of treatment and preventive interventions from STH infections (WHO, 2016b ).
As STHs are considered diseases rarely leading to death, studies that evaluate the burden of STH-related mortality in endemic countries are limited (Silva et al. Reference Silva, Chan and Bundy1997; Pullan et al. Reference Pullan, Smith, Jasrasaria and Brooker2014). Here we present the epidemiological patterns of STH-related mortality in Brazil from 2000 to 2011.
MATERIALS AND METHODS
Study area and population
Brazil, the largest South American country, has a total territory of 8·5 million km2, and a population of about 205 million in 2015. The country is divided into five geographic regions (South, Southeast, Central-West, North and Northeast), 27 Federal Units (26 states and one Federal District), and 5570 municipalities (Instituto Brasileiro de Geografia e Estatística – IBGE; http://www.ibge.gov.br).
We analysed nationwide secondary mortality data from 2000 to 2011. We included all death certificates, in which any STH was mentioned as underlying or associated causes of death (the so-called multiple causes of death). We used the following ICD-10 codes: B77 (ascariasis), B76 (hookworm diseases) and B77 (trichuriasis) (WHO, 2014).
Data sources
Mortality data were obtained from the Mortality Information System (Sistema de Informações sobre Mortalidade – SIM) of the Brazilian Ministry of Health. SIM data are based on death certificates (a standardized form to be filled out by physicians). These data are public domain and freely available on the website of the IT Department of the Ministry of Health's Unified Health System (Departamento de Informática do Sistema Único de Saúde – DATASUS, http://www2.datasus.gov.br). We processed a total of 324 mortality datasets, with about 12·5 million entries. The detailed methods of downloading and consolidation of databases has been described previously (Martins-Melo et al. Reference Martins-Melo, Alencar, Ramos and Heukelbach2012a , Reference Martins-Melo, Ramos, Alencar, Lange and Heukelbach b , Reference Martins-Melo, Ramos, Alencar and Heukelbach2016a , Reference Martins-Melo, Ramos, Alencar and Heukelbach b ).
Population data were obtained from the Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística – IBGE), based on two demographic censuses (2000 and 2010) and population estimates for inter-census years (2001–2009 and 2011), and extracted from the DATASUS website (http://tabnet.datasus.gov.br/cgi/deftohtm.exe?ibge/cnv/popuf.def).
Statistical analysis
We present means and standard deviations (s.d.) for continuous variables (age), and absolute numbers and proportions with their respective 95% confidence intervals (95% CI) for categorical variables (sex, race/ethnicity, marital status, place of residence, year of occurrence, underlying and associated causes of death).
We calculated crude mortality rates by 1 000 000, stratified by sex, age group, race/ethnicity and place of residence (regions and states), using the number of STH-related deaths of each calendar year as numerator and the respective population at risk as denominator. Age-standardized rates were calculated by the direct standardization method, using the 2010 census population. Age categories used for standardization and calculation of age-specific mortality rates were: 0–9, 10–19, 20–29, 30–39, 40–49, 50–59, 60–69 and ⩾70 years. Based on the crude mortality rates, we estimated rate ratios (RR) and 95% CIs. To determine the differences among study groups we applied Pearson's chi-squared (χ 2) or Fisher's exact test, as applicable. We used the Student's t and Mann–Whitney tests to compare the mean and median age at death (in years) between groups, respectively.
Time trend analysis was performed by joinpoint regression models (Kim et al. Reference Kim, Fay, Feuer and Midthune2000). Joinpoints were identified via a log-linear method, where the direction or the magnitude of the trends changed significantly (Kim et al. Reference Kim, Fay, Feuer and Midthune2000). Statistical significance of up to three joinpoints was tested using the Monte Carlo permutation test, which chooses the best segment for each model (Kim et al. Reference Kim, Fay, Feuer and Midthune2000). The annual percent change (APC) and 95% CI were calculated for each segment to quantify the trend and to assess the statistical significance.
We analysed the spatial patterns of STH-related mortality using all 5565 Brazilian municipalities of residence as units of analysis (territorial division of 2010). We excluded deaths with unknown municipality. Overall crude STH-related mortality rates by municipality-level over the study period were calculated dividing the average number of STH-related deaths by the population size in the middle of the study period, expressed per 100 000 inhabitants. We also calculated smoothed mortality rates (per 100 000 inhabitants) using a local empirical Bayesian method to minimize random variations and provide greater stability of mortality rates in municipalities with small populations and rare events (Assunção et al. Reference Assunção, Barreto, Guerra and Sakurai1998; Martins-Melo et al. Reference Martins-Melo, Pinheiro, Ramos, Alencar, de Moraes Bezerra and Heukelbach2015).
We used Global Moran's I statistic (values ranging from −1 to + 1) to assess the extension of global spatial autocorrelation. Values close to zero indicate spatial randomness; positive values indicate positive spatial autocorrelation; and negative values indicate negative spatial autocorrelation (Cliff and Ord, Reference Cliff and Ord1981). Then, we evaluated the existence of local autocorrelation [local indicators of spatial association (LISA)], using Local Moran's I statistic (Anselin, Reference Anselin1995). LISA was used to identify significant hotspots (High–High: high values spatial clusters), coldspots (Low–Low: low values spatial clusters) and spatial outliers (High–Low: high values surrounded with low values or Low–High: low values surrounded with high values) of mortality rates (Anselin, Reference Anselin1995). For spatial representation of the Local Moran's index, Moran Maps were used including municipalities with statistically significant differences (P < 0·05).
We present other diseases and disorders mentioned on the death certificates that were associated with STH-related deaths. All causes reported on the death certificates were analysed, even ill-defined (classified in Chapter XVIII – R00-R99 of the ICD-10), and those characterized by the WHO as modes of death, such as cardiorespiratory arrest and multiple organ failure (Santo, Reference Santo2007; Martins-Melo et al. Reference Martins-Melo, Ramos, Alencar and Heukelbach2012c , Reference Martins-Melo, Ramos, Cavalcanti, Alencar and Heukelbach2016c ).
Statistical analysis was performed using Stata software version 11·2 (StataCorp LP, College Station, TX, USA). Joinpoint regression analyses were performed using Joinpoint Regression Program version 4.0·4 (United States National Cancer Institute, Bethesda, MD, USA). ArcGIS software version 9.3 (Esri, Redlands, CA, USA) and TerraView software version 4.2 (Instituto Nacional de Pesquisas Espaciais – INPE, São José dos Campos, SP, Brazil) were used for data entry, processing, spatial analysis, calculation of autocorrelation indicators and construction of thematic maps.
Ethics
The study was approved by the Ethical Review Board of the Federal University of Ceará (Fortaleza, Brazil). Analysis was based on publicly available secondary anonymized data, with no possibility of identification of individuals.
RESULTS
STH-related deaths
During the study period, a total of 12 491 280 deaths were recorded in Brazil. STHs were identified in 853 deaths (0·01%): 60·7% (518/853) as an underlying cause and 39·3% (335/853) as an associated cause of death. Ascariasis was responsible for 827 deaths (97·0%) [61·8% (511) as underlying cause and 38·2% (316) as associated cause], hookworm infections for 25 deaths (2·8%) [28·0% (7) as underlying cause and 72·0% (18) as associated cause], and trichuriasis for one death (0·1%, associated cause). The average number of deaths per year was 71·1 (95% CI: 61·1–81·0), ranging from 51 in 2011 to 94 in 2001.
Demographic characteristics
Predominant demographic characteristics of STH-related deaths were: female sex [51·8% (442/853)], age 0–9 years [72·8 (617/848)], brown population group [48·1% (362/752)], and residency in the Northeast region [40·1% (342/853)] (Table 1). The state of São Paulo had the largest proportion of deaths [14·9% (127/853)] (Supplementary Table 1).
Table 1. Epidemiological characteristics and STH-related mortality rates (per 1 000 000 inhabitants) by sex, age group, race/colour and region of residence in Brazil, 2000–2011

STH, Soil-transmitted helminths; CI, confidence intervals; RR, rate ratio; -, not calculated.
a Average annual crude- and age-adjusted mortality rates (per 1 000 000 inhabitants), calculated using the average number of STH-related deaths as a numerator and population size in the middle of the studied period as a denominator. Population data on race/colour were derived from the Brazilian National Censuses (2000 and 2010). Population size in relation to race/colour for the middle of the period was derived from an average of the 2000 and 2010 censuses.
b Age-standardized to the 2010 Brazilian population.
c Based on crude mortality rates.
d Data not available in all cases (age group, 5; and race/colour, 101).
Mean and median age at death for all STH-related deaths was 15·8 years (s.d.: ± 25·1) and 3·0 years (range: 0–104·9), respectively. The mean and median age at death was significantly lower in ascariasis-infected than in hookworm-infected individuals: mean 14·8 years (s.d.: ± 24·2 years) vs 48·5 (s.d. ± 31·9 years), P < 0·001; and median 2·9 (range: 0–104·9 years) vs 46·4 (range: 0·7–94·7 years), P < 0·001.
STH-related mortality rates
The average annual crude mortality rate for the period was 0·38 deaths/1 000 000 inhabitants (0·30–0·48 deaths/1 000 000 inhabitants), and the age-adjusted mortality rate 0·34 deaths/1 000 000 inhabitants (0·27–0·44 deaths/1 000 000 inhabitants) (Table 1). Mortality rates were slightly higher in females than males, with no significant difference (Table 1). Children aged <10 years had the highest age-specific mortality rates, with a significant difference when compared with young adults (aged 20–29 years), followed by advanced age (⩾70 years) (Table 1). Indigenous ethnic groups had a 6-fold higher rate than caucasians (Table 1).
The Northeast region showed highest mortality rates, but there were no statistically significant differences among regional rates, as compared with the South region (Table 1). The highest average annual mortality rates by state of residence were observed in the states of Paraná (0·84 deaths/1 000 000 inhabitants), Amazonas (0·74 deaths/1 000 000 inhabitants), Bahia (0·68 deaths/1 000 000 inhabitants), Maranhão (0·68 deaths/1 000 000 inhabitants) and Rio Grande do Norte (0·66 deaths/1 000 000 inhabitants) (Fig. 1; Supplementary Table 1).
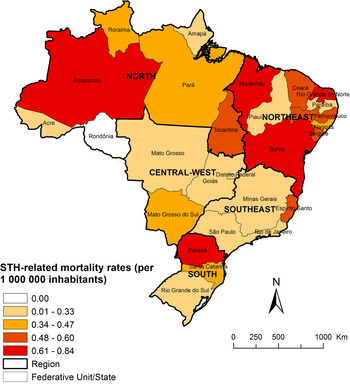
Fig. 1. Spatial distribution of average annual age-adjusted STH-related mortality rates (per 1 000 000 inhabitants) by state of residence in Brazil, 2000–2011.
Trends over time
Age-adjusted STH-related mortality rates decreased significantly at the national level over the entire period (APC: −5·7%; 95% CI: −6·9 to −4·4), with differences between regions. Similar to the nationwide pattern, there was a significant decrease in the Southeast (APC: −7·9%; 95% CI: −12·3 to −3·3) and South (APC: −14·3%; 95% CI: −19·4 to −8·8) regions (Fig. 2; Table 2). The North, Northeast and Central-West regions presented stability during the study period (Fig. 2; Table 2).

Fig. 2. Trends of age-adjusted STH-related mortality rates (per 1 000 000 inhabitants) in Brazil and regions, 2000–2011.
Table 2. Joinpoint regression analysis with corresponding APC of STH-related mortality rates (per 1 000 000 inhabitants) in Brazil, 2000–2011

APC, annual percent change; CI, confidence intervals.
* Significantly different from 0 (P < 0·05).
Both males and females presented significantly decreasing mortality trends over the period (Table 2). The age group 40–49 years presented an increase of mortality rates, whereas in all other age groups mortality decreased. This trend was statistically significant only in the <10-year-old group (Table 2).
Spatial patterns
In total, 8·9% (495/5565) of municipalities in 26 of the 27 Brazilian states recorded at least one STH-related death. The spatial distribution of the average annual crude and smoothed mortality rates by municipality of residence are presented in Figs 3 and 4, respectively. Crude STH-related mortality rates ranged from 0 to 5·54 deaths/100 000 inhabitants among Brazilian municipalities, and smoothed mortality rates from 0 to 1·24 deaths/100 000 inhabitants. We found municipalities with high STH mortality rates in all Brazilians regions, located mainly in the North, Northeast and South regions (Figs 3 and 4).

Fig. 3. Spatial distribution of average annual crude STH-related mortality rates (per 100 000 inhabitants) by municipality of residence in Brazil, 2000–2011.

Fig. 4. Spatial distribution of average annual average annual Bayesian-smoothed STH-related mortality rates (per 100 000 inhabitants) by municipality of residence in Brazil, 2000–2011.
Global Moran's I index showed a highly significant and slightly positive spatial autocorrelation (Moran's I: 0·116; z-score: 137·62; P < 0·001). Figure 5 presents the clusters of municipalities identified according to LISA analysis. During the study period, we identified high-risk clusters (High/High) covering areas in all Brazilian regions. There were concentric high-risk clusters in the states of Amazonas and Pará (North region), Maranhão and Bahia (Northeast region) and Paraná (South region) (Fig. 5). Clusters of municipalities with low mortality rates (Low/Low) covered large areas in states of Central-West, Southeast and Northeast regions (Fig. 5).

Fig. 5. LISA cluster analysis (Moran Map) of STH-related mortality rates by municipality of residence in Brazil, 2000–2011.
Associated causes of death
The main associated causes of death mentioned on death certificates with STHs as the underlying cause included diseases of the digestive system (45·2%), in particular paralytic ileus and intestinal obstruction without hernia and diseases of peritoneum (Supplementary Table 2). The second group was infectious and parasitic diseases (40·0%), particularly sepsis, and intestinal infectious diseases. Other associated causes frequently mentioned included: malnutrition (19·3%), respiratory arrest (13·7%), respiratory failure (12·2%) and pneumonia (11·4%) (Supplementary Table 2).
When STHs were mentioned as an associated cause, the most common underlying causes included the infectious and parasitic diseases (25·7%), diseases of the digestive system (22·7%) and diseases of the respiratory system (19·1%) (Supplementary Table 3). The intestinal infectious diseases (12·8%) were the principal specific underlying cause, followed by pneumonia (10·4%), malnutrition (9·9%) and paralytic ileus and intestinal obstruction without hernia (7·8%) (Supplementary Table 3).
DISCUSSION
To the best of our knowledge, this is the first nationwide study on STH-related mortality in Brazil. We covered a 12-year study period, highlighted epidemiological patterns and provided a comprehensive overview of deaths by these helminth infections. Ascariasis caused by far the highest death burden among STHs. Mortality was higher among females, children and indigenous populations. We identified a decreasing mortality trend at national level, and spatial high-risk clusters. Infectious/parasitic and digestive diseases/disorders were the most commonly associated causes of death recorded in the STH-related deaths as underlying or associated causes of death.
The higher STH-related mortality among children reflects the prevalence and morbidity pattern observed in Brazil and elsewhere, with highest STH prevalence and related complications in this age group (Fonseca et al. Reference Fonseca, Teixeira, Barreto, Carmo and Costa2010; Andrade et al. Reference Andrade, Leite, Vieira, Abramo, Tibiriçá and Silva2011; Visser et al. Reference Visser, Giatti, Carvalho and Guerreiro2011). Children have been identified as particularly susceptible to STH infections, probably because their immature immunological system, and inadequate hygiene and sanitation practices, greater contact with the soil, with other children and domestic animals (Ferreira et al. Reference Ferreira, Ferreira and Monteiro2000; Andrade et al. Reference Andrade, Leite, Vieira, Abramo, Tibiriçá and Silva2011). Nutritional impairment caused by STHs, may have a significant impact on growth, and cognitive and physical development in children (Silva et al. Reference Silva, Chan and Bundy1997). Higher mortality among the older people as compared to adults, may reflect severe impairment of nutritional status by STHs, in addition to comorbidities with other common chronic conditions in this age group (Hurtado-Guerrero et al. Reference Hurtado-Guerrero, Alencar and Hurtado-Guerrero2005). Despite a lower number of absolute deaths, the highest proportion of hookworm-related deaths was observed in middle-aged adults and the elderly, with significant difference in mean age at death.
There was no difference in STH-related mortality between genders. Some studies found a higher STH prevalence in males (Ferreira et al. Reference Ferreira, Ferreira and Monteiro2000; Prado et al. Reference Prado, Barreto, Strina, Faria, Nobre and Jesus2001; Hurtado-Guerrero et al. Reference Hurtado-Guerrero, Alencar and Hurtado-Guerrero2005; Fonseca et al. Reference Fonseca, Teixeira, Barreto, Carmo and Costa2010), others in females (Andrade et al. Reference Andrade, Leite, Vieira, Abramo, Tibiriçá and Silva2011; Silva et al. Reference Silva, Bouth, Costa, Carvalho, Hirai, Prado, Araújo, Pereira and Ribeiro2014). Differences of STH prevalence between genders can be influenced by specific activities and behaviours in the studied population groups, such as those in which there is close contact with the soil and water (Andrade et al. Reference Andrade, Leite, Vieira, Abramo, Tibiriçá and Silva2011).
The high death rate in indigenous populations reflects the vulnerability of these ethnic groups, indicating possible social, economic, health and sanitary disparities, and limited access to health services in the determination of complications caused by STHs (Miranda et al. Reference Miranda, Xavier, Nascimento and Menezes1999; Scolari et al. Reference Scolari, Torti, Beltrame, Matteelli, Castelli, Gulletta, Ribas, Morana and Urbani2000). It is known that indigenous population suffer from higher prevalence and morbidity caused by STHs (Miranda et al. Reference Miranda, Xavier and Menezes1998, Reference Miranda, Xavier, Nascimento and Menezes1999). In an epidemiological survey of STH infections conducted in schoolchildren of an urban area and an indigenous reserve in the municipality of Ortigueira, State of Paraná in the South of Brazil, indigenous children had higher overall prevalence and intensity of STH infections than schoolchildren from the urban area (Scolari et al. Reference Scolari, Torti, Beltrame, Matteelli, Castelli, Gulletta, Ribas, Morana and Urbani2000).
The highest mortality burden due to A. lumbricoides among STH infections reflects the pattern found in Brazil and other countries, where the highest number of STH estimates is usually attributed to A. lumbricoides infection (Fonseca et al. Reference Fonseca, Teixeira, Barreto, Carmo and Costa2010; Chammartin et al. Reference Chammartin, Scholte, Guimarães, Tanner, Utzinger and Vounatsou2013, Reference Chammartin, Guimarães, Scholte, Bavia, Utzinger and Vounatsou2014; Scholte et al. Reference Scholte, Schur, Bavia, Carvalho, Chammartin, Utzinger and Vounatsou2013; Pullan et al. Reference Pullan, Smith, Jasrasaria and Brooker2014). The high frequency of A. lumbricoides can be explained by high viability of eggs with infectivity in the soil for months and up to years (Fonseca et al. Reference Fonseca, Teixeira, Barreto, Carmo and Costa2010; Mandarino-Pereira et al. Reference Mandarino-Pereira, Souza, Lopes and Pereira2010; Andrade et al. Reference Andrade, Leite, Vieira, Abramo, Tibiriçá and Silva2011). Ascariasis is considered an infection with low case fatality, but complications due to aggregation or migration of adult worms, mainly in children, may occur, leading to intestinal obstruction, biliary and pancreatic ascariasis, and appendicitis, possibly leading to death (Silva et al. Reference Silva, Chan and Bundy1997; Scolari et al. Reference Scolari, Torti, Beltrame, Matteelli, Castelli, Gulletta, Ribas, Morana and Urbani2000).
The main conditions or causes of death associated with STH-related deaths as underlying or associated cause partly reflect the clinical description and natural history of severe cases, especially complications arising from A. lumbricoides infection such as intestinal obstruction, malnutrition and peritonitis (Silva et al. Reference Silva, Chan and Bundy1997). The presence of associated causes of death considered terminal conditions, such as sepsis, respiratory failure, respiratory arrest, and pneumonia, may reflect the severity of the STH-related complications in the affected individuals (Bethony et al. Reference Bethony, Brooker, Albonico, Geiger, Loukas, Diemert and Hotez2006). In addition, there is association with other intestinal infectious diseases; polyparasitism may aggravate the conditions of patients with STH infections (Naing et al. Reference Naing, Whittaker, Nyunt-Wai, Reid, Wong, Mak and Tanner2013; Bisanzio et al. Reference Bisanzio, Mutuku, Bustinduy, Mungai, Muchiri, King and Kitron2014).
STH-related mortality rates varied among Brazilian regions, with highest rates in the Northeast. The spatial distribution of STH-related mortality is coherent with patterns of prevalence observed by previous studies, indicating that the geographical distribution of A. lumbricoides infection, leading cause of death among STHs, and T. trichiura is concentrated in the North region and along the eastern coast of Brazil, while high-risk areas of hookworm infection is concentrated in the Amazonas region (Scholte et al. Reference Scholte, Schur, Bavia, Carvalho, Chammartin, Utzinger and Vounatsou2013; Chammartin et al. Reference Chammartin, Guimarães, Scholte, Bavia, Utzinger and Vounatsou2014). The high prevalence of STHs in some areas is related to unfavourable socio-economic, educational and sanitary conditions, agglomeration of people, access to health services, inappropriate use and contamination of soil, water and food, and ecological conditions that are favourable for the development infective stages (Fonseca et al. Reference Fonseca, Teixeira, Barreto, Carmo and Costa2010; Andrade et al. Reference Andrade, Leite, Vieira, Abramo, Tibiriçá and Silva2011; Chammartin et al. Reference Chammartin, Guimarães, Scholte, Bavia, Utzinger and Vounatsou2014; WHO, 2016a ).
Age-adjusted STH-related mortality rates showed a declining trend at national level during the study period. This observed pattern can be explained in part by the expansion of primary healthcare services that have allowed greater access to broad-spectrum anthelmintic drugs, in addition to improved living and sanitary conditions in the last decade (Fonseca et al. Reference Fonseca, Teixeira, Barreto, Carmo and Costa2010). However, there is neither systematic disease surveillance nor a compulsory notification of cases, consequently leading to underestimated numbers of infection rates. There are no systematic epidemiological studies for prevalence estimation on national level. The poorest regions of the country, the North and Northeast, did not present declining trends of mortality during the study period, highlighting the need for specific control programmes, and improved access to health (Fonseca et al. Reference Fonseca, Teixeira, Barreto, Carmo and Costa2010).
Parasitic infections are usually detected passively by the health care centres. In endemic regions for schistosomiasis, STHs are detected during routine stool examinations for Schistosoma mansoni (Brazilian Ministry of Health, 2012). The Ministry of Health of Brazil launched a national integrated campaign to confront STHs in the period 2012–2015 together with other NTDs, such as leprosy and trachoma (Brazilian Ministry of Health, 2012). This campaign aimed to reduce the parasite load of schoolchildren (5–14 years) from public elementary education in priority municipalities (Brazilian Ministry of Health, 2012, 2016). The campaign is based on the administration of broad-spectrum drugs to schoolchildren, with the recommendation of preventive and collective treatment in priority endemic municipalities with prevalence >20%. High-risk communities with a greater number of people in extreme poverty and difficult access to health services and basic sanitation were also included (Brazilian Ministry of Health, 2012, 2016). In 2013, the campaign was conducted in 852 municipalities, with more than 2·8 million treatments for STHs in schoolchildren. In 2014, the activities were expanded to 1227 municipalities, and 4·7 million children treated for STHs (Brazilian Ministry of Health, 2016).
The implementation of appropriate surveillance mechanisms and a mandatory reporting system for STH infections throughout the national territory could provide more accurate epidemiological data on prevalence and would allow geographical mapping of high-risk areas to better target disease surveillance and control.
Despite improved SIM coverage and quality of information about causes of death during the last years, secondary mortality data may present inconsistencies of information over time and between regions (Martins-Melo et al. Reference Martins-Melo, Ramos, Alencar, Lange and Heukelbach2012b , Reference Martins-Melo, Ramos, Alencar and Heukelbach2016a , Reference Martins-Melo, Ramos, Alencar and Heukelbach b ). Coverage and proportion of deaths from ill-defined causes varies among Brazilian regions, with higher proportions of underreporting of deaths and ill-defined causes of death in the North and Northeast regions (Santo, Reference Santo2007; Martins-Melo et al. Reference Martins-Melo, Ramos, Alencar, Lange and Heukelbach2012b , Reference Martins-Melo, Ramos, Alencar and Heukelbach2016a ). Interpretation of data should consider these limitations. The underlying cause of death may have been coded as a complication or aggravation associated with STHs. We collected information based on multiple causes of death, i.e. the mention of some STH in any field rather than only the underlying cause, to reduce this error. In fact, an additional 39% of cases were identified by using multiple causes of death. Some demographic variables such as race/ethnicity included a considerable proportion of missing data. In spatial analysis, crude rates suffer from instability in expressing the risk of a rare event or when the population of the geographic unit of analysis is small. Thus, we used smoothed rates by means of local Bayesian estimation method (Martins-Melo et al. Reference Martins-Melo, Pinheiro, Ramos, Alencar, de Moraes Bezerra and Heukelbach2015). Despite all mentioned limitations, the data analysed are consistent and representative for a country of continental dimensions over a period of 12 years.
Concluding remarks
STHs are neglected causes of death, mainly in the most underprivileged population groups in Brazil. STH-related deaths may be largely underestimated in the country. Specific surveillance and control measures for STHs should be developed in high-risk areas in a multidisciplinary manner, including improvements targeting on early diagnosis and treatment, and to improve socioeconomic conditions, sanitation and health education.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182016002341
ACKNOWLEDGEMENTS
J. H. is class 1 research fellow from the Brazilian Research Council (Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq).
FINANCIAL SUPPORT
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.










