INTRODUCTION AND HISTORY
The parasite Toxoplasma gondii and the disease it causes, toxoplasmosis, were first noted in 1908 in the rodent Ctenodactylus gundi in Tunisia by Nicolle and Mancaeux (Reference Nicolle and Manceaux1908, Reference Nicolle and Manceaux1909), and in the domestic rabbit (Oryctolagus cuniculus) in Brazil by Splendore (Reference Splendore1908). Clinical disease was first recognized in Italy in a domestic animal, a dog, by Mello (Reference Mello1910). The first proven case of congenital toxoplasmosis was described in an infant in the USA by Wolf et al. (Reference Wolf, Cowen and Paige1939).
The discovery of a novel and specific serologic test, the dye test, by Sabin and Feldman (Reference Sabin and Feldman1948) made it possible to conduct population-based surveys for this parasite. Soon it became clear that T. gondii infections are common in humans and animals and clinical disease is relatively uncommon.
The earliest publication on toxoplasmosis in Romania we found is that of Dragomir (Reference Dragomir1956) who isolated viable T. gondii from a human infant. At about the same time, Radacovici and Atanasiu (1959), Lupaşcu et al. (Reference Lupaşcu, Bossie-Agavriloaei, Atanasiu, Dahnovici, Burnuz, Elias and Pucă-Ciudin1963), Elias and Porsche (Reference Elias and Porsche1961), Elias et al. (Reference Elias, Gluhovschi, Pucă-Ciudin and Costin1963a , Reference Elias, Pucă-Ciudin, Costin, Porshe, Borbil and Bogdan b ), Elias (Reference Elias1966) and Elias and Budiu (Reference Elias and Budiu1973) reported on toxoplasmosis in humans and animals in Romania. Since then, there have been many reports, mostly serological surveys in women with gynaecological problems. In the present paper we review prevalence, clinical spectrum and epidemiology of T. gondii in humans and animals in Romania.
METHODS FOR PRESENT REVIEW
Romania has a human population of >19 million, and joined the European Union in 2007. The country is divided into 8 regions (Fig. 1). We have used abbreviated names of these regions in the following review; full names with human populations are shown in Fig. 1. Our initial search of the PUBMED database indicated references to only 25 papers on toxoplasmosis in humans and animals from Romania. Subsequently, we found numerous papers, mostly in Romanian journals. In the present review we attempted to incorporate all published reports available to us on natural T. gondii infections. We consulted original papers when possible. Papers published as abstracts, at symposia and conferences, and reviews, or papers we could not access are listed as supplementary information (in Appendix online – in Online version only). The main objectives of the review are to summarize research accomplished on toxoplasmosis in Romania, suggest areas for future research, and to encourage international collaboration.
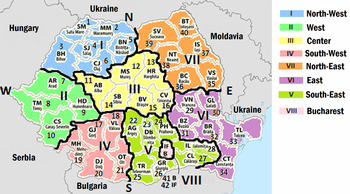
Fig. 1. Map of Romania with 8 regions and distribution of human population. Figures in parentheses are millions of people (in 2011) and % of the total population. Total population of Romania – 19 042 936. I Maramures-Crisana (2·49; 13·1%); II Banat (1·73; 9·08%); III Transilvania (2·25; 11·82%); IV Oltenia (1·98; 10·39%); V Muntenia (2·99; 15·75%); VI Moldova-Tulcea (2·4; 12·6%); VII Bucovina-Moldova (3·15; 16·53%); VIII Bucharest – capital of Romania (2·04; 10·72%).
Detailed historical, serological, parasitological and clinical information on T. gondii infections in humans and other animals are summarized in the tables throughout the review. Different serological techniques used in Romanian studies are listed in Table 1. Cut-off values for serological tests are listed wherever the authors provided the information. Details of in-house tests are not listed in Table 1 or any subsequent tables. Superscripts in the tables and text refer to details of the serological tests provided in Table 1.
Table 1. Details of serological tests used for the detection of antibodies to T. gondii in animals and humans in Romania
a General distributor: SC Aspius SRL, no. 25/21, Aurel Vlaicu street, 310147, Arad, Romania, phone: +40 724574943, E-mail: contact@aspius.ro
The finding of T. gondii antibodies indicates exposure to the parasite. The sensitivity and specificity of different serological tests used to detect T. gondii antibodies varies a lot with the test used, serum dilution and the stage of infection. The skin test (dermal hypersensitivity), one of the first tests used to detect T. gondii exposure, is very insensitive, and is rarely used now. The Sabin–Feldman dye test is the most sensitive and specific test for human toxoplasmosis but it is rarely used now because it requires the use of live parasites and a complement-like factor from human serum; moreover, the test does not work with sera of some animal species. The indirect fluorescent antibody test (IFAT) and the modified agglutination test (MAT) use whole, killed tachyzoites, and the results are comparable with those obtained with the dye test, especially at serum dilution of 1:64 or higher. There are several ELISAs developed to detect T. gondii exposure and some of them are commercially available (Table 1). Some serological tests can distinguish class and type of antibodies (IgM, IgA, IgE, avidity); we have listed them where this information was provided. We would like to emphasize that the detection of antibodies only indicates exposure and the definitive evidence of infection requires demonstration of the parasite.
TOXOPLASMOSIS IN HUMANS
Prevalence of T. gondii infection
There is little information concerning T. gondii prevalence in the general human population in Romania. Most serological surveys are based on convenience samples, except a recent study by Coroiu et al. (Reference Coroiu, Radu, Molnar and Bele2009) who tested 1155 sera based on stratified sampling from the general population in north-west and central Romania with a total population of 4·6 million in 11 counties. Sera were tested for T. gondii IgG antibodies by two commercial tests (ELISA13, LAT2) with similar results. Antibodies to T. gondii were found in 686 (59·4%) of 1155 sera; seropositivity varied from 44·9–70·2% depending on the county sampled; prevalence in different counties were as follows: Alba 59 (70·2%) of 84, Bihor 104 (65·8%) of 158, Bistriţa-Năsăud 46 (64·7%) of 71, Cluj 80 (44·9%) of 178, Covasna 34 (53·1%) of 64, Harghita 26 (36·6%) of 71, Maramureş 77 (64·7%) of 119, Mureş 68 (61·8%) of 110, Satu Mare 75 (63·0%) of 119, Sălaj 63 (67·7%) of 93, and Sibiu 55 (62·5%) of 88. The highest (70·2%) seropositivity was in people from Alba, and the lowest (36·6%) in Harghita county. Prevalence in males, 279 (60·3%) of 462, was similar to that in females, 408 (58·8%) of 693, and slightly lower in the urban population, 264 (55·1%) of 479, than the rural population, 363 (63·6%) of 570. Prevalences by age were: 46 (24·3%) of 189 <14 years, 26 (43·3%) of 60–15 to 19 years, 59 (49·7%) of 120–20 to 29 years, 68 (55·7%) of 122–30 to 39 years, and 191 (71·5%) of 267 at 40 years or older. In this population, prevalence in women of fertile age (defined in this paper as 16–35 years) was high (51·4%). It is of interest that 6 of 68 children 1–9 years old were seropositive. The authors tabulated results for each category in each of 11 counties. Of the total of 687 seropositives of 1155 persons, only 1 had IgM antibodies. To our knowledge this is the only population-based survey for T. gondii in Romania. Unfortunately, risk assessment data were limited. There were several other studies with <100 people (Antoniu et al. Reference Antoniu, Moldoveanu and Ionescu2005; Teodorescu et al. Reference Teodorescu, Steriu, Teodorescu, Miclos and Pop2006; Csep, Reference Csep2010a ).
There are other surveys of T. gondii infection in humans tested for various reasons. Surveys in women of childbearing age or in those who were tested mainly because of gynaecological problems are given in Table 2. In general, seroprevalence was higher in women with gynaecological problems than women in the general population. Data from other patients or the general population are given in Table 3. In general, seroprevalence was higher in persons from rural than urban areas, but data are limited.
Table 2. Reports of T. gondii antibodies in pregnant and childbearing age women tested in hospitals or private clinics in Romania

a Personal communication to J. P. Dubey, April 2013.
Table 3. Occurrence of Toxoplasma gondii antibodies in sera of humans from various sources in Romania and correlates of infection

a Personal communication J.P.D – March 2013.
b Selected from 15 896 births between 2006–2009 because of clinical suspicion of toxoplasmosis in newborn (personal communication to J. P. D March, 2013).
In bold = noteworthy data.
Clinical toxoplasmosis
Congenital
Although T. gondii can sometimes cause abortion in women, there is no evidence that it causes habitual abortion (Dubey and Beattie, Reference Dubey and Beattie1988). Unfortunately, in many eastern European countries and Asia it has been assumed that chronic T. gondii infection is a common cause of infertility and abortion. For this reason, many women in Romania with these problems were tested for T. gondii infection (Table 2). In addition to those listed in Table 2, there are other reports of T. gondii testing of women with pregnancy problems (Neagoe et al. Reference Neagoe, Gherman, Rădulescu, Pop, Bucurenci, Dumitrache and Steriu2007). However, the relationship between T. gondii infection and pregnancy problems cannot be established by serological testing alone.
Little is known of congenital toxoplasmosis in Romania. An estimate of the incidence of clinically manifest prenatal toxoplasmosis may be obtained in two ways (Dubey and Beattie, Reference Dubey and Beattie1988). First from reports of observed cases, and second from calculations based on the infection rates during pregnancy and follow-up of live born infants. Stroia and Ungureanu (Reference Stroia and Ungureanu2007) reported a 4-month-old child with hydrocephalus, cerebral calcification, and IgG and IgM seropositivity to T. gondii but details are scanty. The authors rightly recognized that these symptoms also occur in other diseases. Csep (Reference Csep2010c ) diagnosed 9 cases of congenital toxoplasmosis, but again details are sketchy. Panaitescu et al. (Reference Panaitescu, Căpraru and Bugarin1995b ) reported a very high rate of seroconversion in 96 (19·7%) of 485 women in Bucharest. The seroconverted women were followed clinically until delivery but it is not clear from the results how many of these mothers delivered congenitally infected children.
Crucerescu and Lovin (Reference Crucerescu and Lovin2001) tested cord blood of 1226 newborns for T. gondii antibodies. Antibodies to T. gondii were detected in 546 (44·5%) children. Out of these, 9 children were considered at risk based on differential serology and followed for 1 year. One of these 9 children had persistent T. gondii antibodies after 1 year; whether this child became symptomatic is unknown. Thus, in this select population from eastern Romania, the congenital transmission rate was 1 for 1226 live-born children.
Antibodies to T. gondii were sought by several investigators in children suspected to have congenital toxoplasmosis and their mothers, but the results were not conclusive to establish definitive diagnosis (Elias et al. Reference Elias, Pucă-Ciudin, Costin, Porshe, Borbil and Bogdan1963b ; Elias, Reference Elias1966; Georgescu, Reference Georgescu1976; Proca-Cioban et al. Reference Proca-Cioban, Ştefănoiu, Potcoavă, Panaitescu and Steriu1981; Junie and Coroiu, Reference Junie and Coroiu1995; Panaitescu et al. Reference Panaitescu, Căpraru and Bugarin1995a ; Junie et al. Reference Junie, Coroiu, Mihalache and Costache2002; Costache et al. Reference Costache, Junie and Coroiu2004, 2008a, b, Reference Costache, Colosi and Junie2010; Teodorescu et al. Reference Teodorescu, Steriu, Teodorescu, Miclos and Pop2006; Barabás-Hajdu et al. Reference Barabás-Hajdu, Miklós, Miklós and Simó2007; Lazăr and Barbu, Reference Lazăr and Barbu2007; Neagoe et al. Reference Neagoe, Gherman, Rădulescu, Pop, Bucurenci, Dumitrache and Steriu2007; Oprea et al. 2007; Rugină et al. Reference Rugină, Dumitru and Gorun2007; Stroia and Ungureanu, Reference Stroia and Ungureanu2007; Mării et al. Reference Mării, Iancu, Miu, Zaharie, Samașcă and Idrizi2008; Csep, Reference Csep2010a , Reference Csep b , Reference Csep c ). Three studies from Cluj reported T. gondii antibodies in about one third of malformed children (116 [29·4%] of 394, Junie and Coroiu, Reference Junie and Coroiu1995; 73 [28·9%] of 253, Junie et al. Reference Junie, Coroiu, Costache and Stranţ2000, Reference Junie, Coroiu, Mihalache and Costache2002; 9 [32·1%] of 28). Overall, from the evidence presented in these studies it is difficult to estimate the rate of congenital toxoplasmosis.
Ocular
There are several reports of serological and clinical examinations of patients suspected of ocular toxoplasmosis in Romania (Panaitescu et al. Reference Panaitescu, Steriu, Proca-Cioban, Ştefănoiu, Silard and Potcoavă1978; Proca-Cioban et al. Reference Proca-Cioban, Ştefănoiu, Potcoavă, Panaitescu and Steriu1981; Junie and Coroiu, Reference Junie and Coroiu1995; Crucerescu, Reference Crucerescu1998; Creţu et al. Reference Creţu, Rădulescu, Ristea, Pop de Popa, Tacorian, Voinea, Mihăilescu and Popă2000, Reference Cozma, Şuteu, Titilincu and Osztian2007; Lazăr et al. 2002; Costache et al. Reference Costache, Junie and Coroiu2004; Dogan and Farah, Reference Dogan and Farah2004; Radbea et al. Reference Radbea, Mederle, Barbu and Izvernariu2006; Siloşi et al. Reference Siloşi, Rogoz, Siloşi, Rogoz, Avramescu and Badea2006; Jurja, Reference Jurja2007; Teodorescu et al. Reference Teodorescu, Teodorescu, Raneti, Dumitrica and Ştefan2008). Most of these studies are retrospective. Proca-Cioban et al. (Reference Proca-Cioban, Ştefănoiu, Potcoavă, Panaitescu and Steriu1981) determined IFAT antibodies in 1712 children (3–19 years old) with neurological manifestations and 338 children with ocular diseases; 144 (8·4%) of 1712 with neurological signs and 36 (10·6%) of 338 with ocular disease were seropositive. The authors did not provide any data but stated that seroprevalence in the normal population (presumably children) was 3·7% (Proca-Cioban et al. Reference Proca-Cioban, Ştefănoiu, Potcoavă, Panaitescu and Steriu1981). Dogan and Farah (Reference Dogan and Farah2004) diagnosed ocular toxoplasmosis in 21 (21·9%) of 96 cases of uveitis in children in a hospital in Bucharest from 1993–2002. Similarly, Teodorescu et al. (Reference Teodorescu, Teodorescu, Raneti, Dumitrica and Ştefan2008) reported 90 (66·7%) cases of ocular toxoplasmosis among all 135 hospitalized cases of toxoplasmosis (acquired or congenital) in other hospitals in Bucharest from 2000–2005. Creţu et al. (Reference Creţu, Cilievici, Neagu, Voinea and Corbu2007) retrospectively examined records of 500 patients diagnosed with ocular toxoplasmosis during 1995–2005 in Colentina Teaching Hospital in Bucharest. Concurrent infections (250 toxocariasis, 129 other infections including tuberculosis and syphilis) were associated in two thirds of cases. The diagnosis was based on serology and clinical findings. Patients were 0–76 years old (median 44 months), 20 case-patients (4%) of them were considered to have postnatally acquired toxoplasmosis, based on onset of clinical symptoms and differential serologic (avidity, IgM) testing. Chorioretinitis, present in 410 cases (82%), was the main finding and was unilateral in 298 cases (59·6%), affected both eyes in 112 cases (22·4%), and cataracts were seen in 74 cases (14·8%) of 500 persons. They described onset and progression of lesions, and attempted to determine risk factors. The authors stated that these cases analysed were one-third of cases seen in this hospital. It is not known if more eye patients sought diagnosis at this facility. Overall, toxoplasmic ophthalmitis is considered common in the Romanian population (Lazăr et al. Reference Lazăr, Radu-Niculescu, Ştefănescu and Agârbiceanu2002) but there is no information on prevalence of ocular disease in the general population. Therefore, we are unable to compare these findings with those from other countries in Europe or America.
Lymphadenitis
Cervical lymphadenopathy is the most common sign of acquired toxoplasmosis. Demonstration of T. gondii DNA or live parasites in biopsy is one way to confirm diagnosis. Presumptive diagnosis may be made based on symptoms and serological tests for acute toxoplasmosis. There have been several reports of serological examination of patients with lympadenopathy (Ştefănoiu et al. Reference Ştefănoiu, Panaitescu, Steriu and Proca Ciobanu1983; Crucerescu and Lovin, Reference Crucerescu and Lovin2002; Costache et al. Reference Costache, Junie and Coroiu2004; Codreanu and Rădulescu, Reference Codreanu and Rădulescu2007; Dumitru et al. Reference Dumitru, Rugină and Rugină2007; Ghinea et al. Reference Ghinea, Niculescu and Nicoară2007; Siloşi et al. Reference Siloşi, Ungureanu, Rogoz, Siloşi, Avramescu, Muşetescu, Drackoulogona and Neamţu2007). Crucerescu and Lovin (Reference Crucerescu and Lovin2001) reported that 117 (34·1%) of 343 lymphadenitis patients were seropositive of which 44 (12·8%) had acute acquired infection based on IgG avidity. Ştefănoiu et al. (Reference Ştefănoiu, Panaitescu, Steriu and Proca Ciobanu1983) reported IFAT antibodies in 297 (28·6%) of 1038 patients with lymphadenopathy; histological examination of biopsy of lymph nodes in 19 cases revealed reactive adenopathy but T. gondii was not found.
Human immunodeficiency virus (HIV)
The HIV epidemic in the 1980s brought recognition of cerebral toxoplasmosis in adults but these cases were not reported until 2000 in Romania. Encephalitis is the predominant presentation of clinical toxoplasmosis in HIV-infected patients. Although computer tomography (CT) and serological examination are useful, definitive diagnosis can only be made postmortem or by biopsy examination. Other conditions including lymphomas can mimic toxoplasmosis, and the determination of type of immunoglobulin and the magnitude of T. gondii titre are not helpful in differential diagnosis. In most HIV-infected patients clinical toxoplasmosis is a reactivation of a chronic infection, and most of these patients have T. gondii antibodies.
There are several reports of toxoplasmosis in HIV-infected patients in Romania (Colţan et al. Reference Colţan, Marin and Rebedea2000; Codarcea et al. Reference Codarcea, Gorun, Rugină, Muja, Ilie and Neagu2000; Cambrea et al. Reference Cambrea, Ilie, Cambrea, Ionescu and Rugină2007). Crucerescu and Lovin (Reference Crucerescu and Lovin2001) found T. gondii antibodies in 35 (34·3%) of 102 HIV-infected patients; 11 (2 adults, 9 children) had encephalitis, and 2 patients died despite therapy. A series of cases were reported in 2007 (Cambrea et al. Reference Cambrea, Ilie, Cambrea, Ionescu and Rugină2007; Codarcea et al. Reference Codarcea, Blebea, Basca, Dumea, Cernat and Rugină2007; Erscoiu et al. Reference Erscoiu, Ungureanu, Alecu, Ionescu and Mihăilă2007; Marcaş et al. Reference Marcaş, Cambrea, Ilie and Rugină2007; Oprea et al. 2007). A striking observation is that most of these cases were in young persons. Out of 34 teenage patients with cerebral toxoplasmosis, 16 died despite therapy (Cambrea et al. Reference Cambrea, Ilie, Cambrea, Ionescu and Rugină2007). To our knowledge, none of the cases mentioned above were confirmed by biopsy or postmortem examination.
Toxoplasma gondii isolation from human samples
Dragomir (Reference Dragomir1956) attempted isolation of T. gondii from 3 cases of toxoplasmosis (details of patients not given) by bioassay in mice. For this, the ventricular fluid was centrifuged; the sediment was suspended in 2 mL of saline and inoculated intraperitoneally (i.p.) into 2 white mice. The first mouse was killed 4 days post-inoculation (p.i.) and tachyzoites were found in the peritoneal fluid. The parasite was maintained by serial passage in mice, and the strain became virulent for mice after 4 passages. The photographs provided in this paper leave no doubt about the first isolation of T. gondii in Romania.
Rodacovici and Atanasiu (1959) attempted isolation of T. gondii from 57 congenitally infected children and 6 adults by bioassays in mice. They isolated viable T. gondii from 3 cases of fatal toxoplasmosis in children. They found T. gondii in tissues of another 11 congenitally infected children and 1 adult with acquired toxoplasmosis but were unable to isolate viable T. gondii.
Elias et al. (Reference Elias, Pucă-Ciudin, Costin, Porshe, Borbil and Bogdan1963b ) attempted to isolate T. gondii from tissues of 7 infants with malformations by bioassays in mice. Viable T. gondii was isolated from 1 infant but details are sketchy.
Recently, Costache et al. (Reference Costache, Colosi, Blaga, Györke, Pastiu, Colosi and Ajzenberg2013) isolated viable T. gondii from the cerebrospinal fluid (CSF) of a 32-week gestational age girl born prematurely but naturally to a mother who had serological evidence of recently acquired T. gondii infection during pregnancy (the mother seroconverted between 2 and 6 months of gestation). The girl had gross evidence of hydrocephalus and microphthalmia of the left eye. Ophthalomoscopic examination revealed acute central chorioretinitis of the right eye, retinal detachment and anterior and posterior uveitis of the left eye. The CSF was collected from the girl 4 days after birth, and examined for T. gondii infection. Toxoplasma gondii DNA was demonstrated directly in the CSF, and viable parasite isolated by bioassay in outbred mice. For bioassay, the CSF was centrifuged, the sediment suspended in isotonic saline, and inoculated i.p. into 3 outbred white mice. The inoculated mice remained asymptomatic; T. gondii tissue cysts were demonstrated in the brains of mice killed 4 weeks p.i. This T. gondii strain was designated as ROU-H-001 and cryopreserved. Genotyping with 15 microsatellite markers revealed that it is a Type II strain (Costache et al. Reference Costache, Colosi, Blaga, Györke, Pastiu, Colosi and Ajzenberg2013). These findings are noteworthy because it is the first genotyping of a viable isolate of T. gondii from Romania from any host.
Epidemiology of human toxoplasmosis
To our knowledge there are no statistically well-controlled epidemiological studies in Romania. Epidemiological data were collected mostly retrospectively, without calculation of statistical significance; we have summarized them in Table 3. Most studies revealed an increase of seroprevalence with age and rural living.
TOXOPLASMOSIS IN ANIMALS
Cats
Serological prevalence and risk factors
Toxoplasma gondii antibodies were detected in 30–80% of cats in small surveys involving 20–62 cats (Table 4). Györke et al. (Reference Györke, Opsteegh, Mircean, Iovu and Cozma2011) made an extensive investigation using serum samples from 236 house cats from three regions (Center-III, Southwest-IV and Northwest-I). Several aspects of this study are noteworthy. The sample size was adequate to study risk factors, prevalence was determined using 6 serological tests (MAT, IFAT, 4 ELISAs) and results could be compared directly with a previously published study from the Netherlands (Opsteegh et al. Reference Opsteegh, Haveman, Swart, Mensink-Beerepoot, Hofhuis, Langelaar and van der Giessen2012); the MAT and ELISA-RIVM were performed in the Netherlands by Opsteegh et al. (Reference Opsteegh, Haveman, Swart, Mensink-Beerepoot, Hofhuis, Langelaar and van der Giessen2012).
Table 4. Serological surveys of Toxoplasma gondii in domestic cats in Romania

VC = veterinary clinics.
a = age, b = sex, c = diet, d = habitat.
In the Györke et al. (Reference Györke, Opsteegh, Mircean, Iovu and Cozma2011) study, using a commercial ELISA2, 111 (47%) of 236 cats were seropositive. Of these 236 sera, 203 sera were comparatively tested by 6 tests. Seropositivity varied from 46·7% to 60·5%, depending on the test. It is of interest that results for 147 tests (87 positive, 60 negative) of the 203 sera were the same in all 6 tests. Overall, the IDVet ELISA2 gave most concordant results. Based on the IDVet test age, breed, diet, outdoor access, location and the source of sera affected the T. gondii seropositivity. Seroprevalence increased with age and indoor/outdoor living: outdoors (85 [59%] of 144) vs indoors (19 [26·8%] of 71). Somewhat similar conclusions were obtained in other references listed in Table 4 although the number of cats were too small for a valid comparison. These results were expected because most cats acquire T. gondii infection post-natally, soon after weaning when cats begin to hunt for food (Dubey and Beattie, Reference Dubey and Beattie1988). Most purebred cats are kept indoors and fed processed diets by their economically advantaged owners.
Prevalence of T. gondii-like oocysts in cat feces
Toxoplasma gondii-like oocysts were found in feces of 18 of 300 cats from Bucharest by Pop et al. (Reference Pop, Cerbu, Pop and Andreescu1986), 5 of 414 cats from Transylvania by Mircean et al. (Reference Mircean, Titilincu and Cozma2010) and 1 of 62 cats from Caraş-Severin by Hotea et al. (Reference Hotea, Ilie, Imre, Sorescu, Indre, Brudiu, Colibar and Dărăbuş2012). Additionally, oocysts were not found in feces of 63 cats from Cluj and Dolj (Titilincu et al. Reference Titilincu, Mircean, Blaga, Chiţimia, Cernea, Mirescu and Cozma2008b ) and 36 cats from Arad county (Hotea et al. Reference Hotea, Dărăbuş, Mederle, Ilie, Imre, Balint and Indre2009c ; Dărăbus et al. Reference Dărăbuş, Hotea, Oprescu, Morariu, Brudiu and Olariu2011b – both papers refer to the same data). These results are based on microscopical examination, and not definitive. Toxoplasma gondii-like oocysts in cat feces include T. gondii, Hammondia spp., Neospora caninum and Besnoitia spp., and these oocysts cannot be diagnosed without bioassays or DNA identification (Dubey, Reference Dubey2010).
Clinical toxoplasmosis
Diagnosis of clinical toxoplasmosis in cats is difficult without postmortem examination. At present there is no confirmed report of clinical toxoplasmosis in cats in Romania. Georgescu et al. (Reference Georgescu, Tudor, Tudor, Grosu and Ionescu2009) reported clinical signs of encephalitis and ophthalmitis in a cat (age or type of cat not stated) in Bucharest. The diagnosis was based on finding positive IgM and IgG antibodies to T. gondii (titre or the test performed were not given), and positive response to clindamycin therapy. The diagnosis is at best presumptive because IgM antibodies can persist in asymptomatic cats for months (Dubey, Reference Dubey2010).
Isolation of viable T. gondii
Toxoplasma gondii was isolated from 12 (4%) of 300 tissues of cats from Bucharest by bioassay in mice. The data are only indicative because tissues from 3 cats were pooled and 100 pools were bioassayed (Pop et al. Reference Pop, Cerbu, Pop and Andreescu1986).
Sheep
A significant ovine population in Romania has been exposed to T. gondii infection (Table 5). Seroprevalence varied with the region, age and the serological methods. Elias (Reference Elias1966) found 53% (340 of 635) seropositivity in sheep tested by the dye test, however, most (192) of the sera had only low titres of 4 and 16; significance of these low dye test titres is unknown. Also, sheep sera should be inactivated at 60 °C to inactivate the ovine complement (Dubey and Beattie, Reference Dubey and Beattie1988). Sharma (Reference Sharma1980) found more variability with the IHAT vs IFAT; he found excellent correlation between IFAT and the dye test in 100 sera. Hotea et al. (Reference Hotea, Ilie, Imre, Sorescu and Dărăbuş2011a) found that only 13 (6·5%) of 200, 35–55-day-old lambs slaughtered for Easter were seropositive; it is likely that some of these lambs had colostrally acquired antibodies. Several surveys listed in Table 5 used different ELISAs and there are no data on their specificity and sensitivity based on isolation of T. gondii from asymptomatic sheep. Ştirbu-Teofănescu et al. (Reference Ştirbu-Teofănescu, Amzuţa, Comârzan and Militaru2005) found good correlation between an in-house ELISA and IFAT, and Titilincu et al. (Reference Titilincu, Blaga, Halos, Mircean, Boireau and Cozma2008a , Reference Titilincu, Mircean, Iovu and Cozma2009) found good correlation between MAT and 2 commercial ELISAs and an in-house ELISA.
Table 5. Surveys for T. gondii antibodies in sheep in Romania
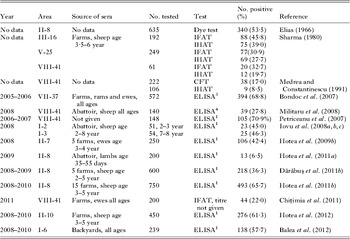
Toxoplasma gondii is an important cause of ovine abortion worldwide (Dubey, Reference Dubey2010), but there is no definitive information on this subject in Romania. Elias et al. (Reference Elias, Gluhovschi, Pucă-Ciudin and Costin1963a ) and Elias (Reference Elias1966) found higher seropositivity in 80 (51·9%) of 154 ewes that aborted vs 61 (44·5%) of 137 healthy sheep from a flock that experienced a storm of abortions (Elias et al. Reference Elias, Gluhovschi, Pucă-Ciudin and Costin1963a , Reference Elias, Pucă-Ciudin, Costin, Porshe, Borbil and Bogdan b ); it is not clear to us if both papers relate to the same farms or different farms. They were unable to isolate viable T. gondii from aborted fetuses but details are sketchy. Pyrimethamine treatment of 20 ewes with high antibody titres prevented abortion but again details are sketchy. Medrea and Constantinescu (Reference Medrea and Constantinescu1991) also reported higher T. gondii seropositivity in sheep with neonatal losses but exact figures are not clearly stated.
Goats
Iovu et al. (Reference Iovu, Györke, Mircean, Gavrea and Cozma2012) studied in-depth epidemiology of toxoplasmosis in dairy goats from Romania. They tested 735 goats from 4 areas of Romania. Goat sera were tested for T. gondii IgG antibodies by ELISA12. Seroprevalence varied from 20–84%, depending on the sampling; antibodies were found in 8 (20·0%) of 40 goats from Muntenia, 144 (39·2%) of 367 goats from Transylvania, 194 (69·8%) of 278 goats from Crişana and 42 (84·0%) of 50 goats from Maramureş (for regions see Fig. 1). As expected, seroprevalence was higher in backyard-raised goats, 58 (79·5%) of 73, than in goats raised on farms, 330 (49·8%) of 662; and in adults, 386 (55·8%) of 692, vs kids, 2 (4·7%) of 43. The goat-kids tested were 2 months old and might still have colostrally acquired antibodies. This paper included results reported by Titilincu et al. (Reference Titilincu, Mircean and Cozma2008c ) and Balea et al. (Reference Balea, Pastiu, Györke, Mircean and Cozma2012) (personal communication to J.P. Dubey, February 2013). Results indicated that most goats acquire infection postnatally by ingesting food or water contaminated with oocysts. This research is significant because the survey was made on dairy goats; T. gondii can be transmitted to humans via goat milk (Dubey, Reference Dubey2010).
Pigs
Seroprevalence varied with the type of pigs surveyed; <3% of fattening pigs (<8 months) were seropositive compared with higher seropositivity in older pigs (Table 6). An extremely high rate of seropositivity, 49 (94%) of 52, was found in wild pigs (Hotea et al. Reference Hotea, Dărăbuş, Păcurar, Rugea, Muntean, Ilie, Imre, Imre, Sorescu, Balint and Indre2010c ). As expected, pigs housed indoors under intensive management were not exposed to T. gondii infection, compared with those housed outdoors, except in the survey reported by Iovu et al. (Reference Iovu, Titilincu, Mircean, Blaga and Cozma2008b ) (Table 6). Iovu et al. (Reference Iovu, Titilincu, Mircean, Blaga and Cozma2008b ) found good correlation between 2 commercial ELISAs2,9. Paştiu et al. (Reference Paștiu, Györke, Blaga, Mircean, Rosenthal and Cozma2013) using IFAT cut-off of 1:32 found antibodies in 24 (16%) of 150 wild boars, 0 of 660 fattening pigs, and 783 (30·5%) of 2564 backyard pigs. The prevalence in backyard pigs varied from 13·3 to 60%. It was remarkable that none of the 200 sows from one establishment were seropositive compared with 46 (26·9%) of 171 sows from another establishment, although both farms used intensive management. The data on pigs reported by Balea et al. (Reference Balea, Pastiu, Györke, Mircean and Cozma2012) were included in Paștiu et al. (Reference Paștiu, Györke, Blaga, Mircean, Rosenthal and Cozma2013) (personal communication to J. P. D.).
Table 6. Surveys of Toxoplasma gondii antibodies in pigs in Romania

Little is known of clinical toxoplasmosis in pigs in Romania. Iovu et al. (Reference Iovu, Titilincu, Voinescu and Cozma2010) examined fetal tissues and fluids from 32 sow abortions and did not find T. gondii DNA in abortus.
Miscellaneous animals
Little is known of T. gondii infection in cattle in Romania. Elias (Reference Elias1966) found dye test antibodies in 31 (18·9%) of 164 cattle. Medrea and Constantinescu (Reference Medrea and Constantinescu1991) reported seropositivity in 84 (12·6%) of 667 cattle by the complement fixation test.
Antibodies to T. gondii were found in 104 (51·4%) of 202 domestic dogs by Elias (Reference Elias1966), and in 14 (25·0%) of 56 of stray dogs from Cluj-Napoca by Cozma et al. (Reference Cozma, Şuteu, Titilincu and Osztian2007) using 1:100 serum dilution in the IFAT.
Elias (Reference Elias1966) found dye test antibodies in 834 (45·3%) of 1840 rabbits, 21 (26·2%) of 80 hamsters and 3 (10·3%) of 29 rats. Dãrãbuș et al. (Reference Dărăbuş, Afrenie, Olariu, Ilie, Balint and Hotea2011b ) detected T. gondii antibodies by ELISA4 in 19 (73·1%) of 26 animals in a zoo, including 1 of 1 Felis catus, 2 of 2 Felis sylvestris, 3 of 3 Panthera leo, 2 of 5 Capra aegagrus, 2 of 3 Capreolus capreolus, 1 of 1 Lama guanicoe, 2 of 2 Rangifer tarandus, 1 of 3 Equus caballus, 4 of 4 Procyon lotor and 1 of 1 Ursus arctos.
Păstârnac (Reference Păstârnac2009) discussed a large outbreak of toxoplasmosis in minks – these results need confirmation using verifiable methods.
Gheoca et al. (Reference Gheoca, Hărânglăvean and Gheoca2009) reported unusual findings that need verification. They found T. gondii-like oocysts in feces of 4 of 6 rodents and cysts in tissues of 3 rodents. They discuss possible spread of T. gondii by rodent feces. In our opinion the cysts illustrated appear to be pollen grains and there is no evidence that T. gondii is transmitted via rodents other than by carnivorism.
Isolation of viable T. gondii from food animals
Pop et al. (Reference Pop, Oprişan, Pop, Cerbu, Stavarache and Niţu1989) bioassayed diaphragms of 740 pigs, 910 cattle and 1340 sheep from slaughterhouses. Five grams of muscle from each of 10 animals were pooled by species, digested in acidic pepsin, and inoculated intraperitoneally into 6 mice. Viable T. gondii was isolated from 7 (9·5%) of 74 swine pools, 9 (9·9%) of 91 beef pools, and 11 (8·2%) of 134 mutton pools. In 19 (70·4%) of 27 positive samples tachyzoites were found in the peritoneal exudates, and tissue cysts were found in 24 groups of positive mice. One isolate from beef and 1 isolate from pork were virulent for mice. The isolation of T. gondii from approximately 10% of beef samples is unusual. Whether beef samples were contaminated with pork or lamb will never be known. It is also unfortunate that the samples were pooled, and there is no archived material for verification. Toxoplasma gondii has been rarely isolated from beef in other attempts worldwide and the role of beef in the epidemiology of toxoplasmosis needs investigation (Dubey and Beattie, Reference Dubey and Beattie1988; Dubey, Reference Dubey2010).
Sharma (Reference Sharma1980) isolated viable T. gondii tissues from 2 (15·4%) of 13 serologically positive sheep and 1 sheep not serologically examined. All 3 isolates were non-pathogenic for mice; these isolates were not cryopreserved. Recently, Turcitu et al. (Reference Turcitu, Pricop, Cioranu, Bărbuceanu, Diaconu, Banu, Chiţimia, Codreanu, Codreanu and Antoniu2012) detected T. gondii genomic DNA in 17 (18·4%) of brain homogenate samples from 92 sheep as part of an investigation on scrapie.
PERSPECTIVE
During the preparation of this review it became clear that most of the research on toxoplasmosis conducted in Romania was probably not read/known to scientists in other countries. As stated earlier our initial search of the PUBMED database indicated references to only 25 papers on toxoplasmosis in humans and animals from Romania. Here, we have listed all >150 papers that we could find and summarized the current status of research on toxoplasmosis. There is a great need to establish a central facility for toxoplasmosis research and to conduct a national surveillance study using a statistically valid survey for prevalence. There are adequately trained scientists in Romania to conduct this research but there is a need for financial support from European funding agency/agencies. The present research was conducted mostly using commercial kits, which are quite expensive for survey research. Little is known regarding the mortality and morbidity of toxoplasmosis in humans and animals in Romania. Until now, nothing was known of the genetic diversity of T. gondii in Romania, although some progress has been made recently in this direction and the first viable isolate of T. gondii from a congenitally infected child has been genotyped (Type II, as in children in France) and deposited in an international reference centre in France (Costache et al. Reference Costache, Colosi, Blaga, Györke, Pastiu, Colosi and Ajzenberg2013). Toxoplasmosis is an important cause of abortion in sheep and goats in many countries but nothing is known of this in Romania. Romania is a major country exporting mutton and other sheep products to Europe and Arab countries. Until the 1990s Romania was a socialist country with little contact with non-Soviet-block countries. International research collaborations are needed for total assimilation of Romania in the western world. There is little information concerning the presence of viable T. gondii in food animals in Romania. For this, bioassays for viable T. gondii are needed because determination of parasite DNA and antibodies only indicate exposure and not the live parasite presence.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/S0031182013001509.
ACKNOWLEDGEMENTS
We would like to thank Drs Adriana Györke (Titilincu), University of Agricultural Science and Veterinary Medicine, and Carmen Costache, University of Medicine and Pharmacy Cluj-Napoca, Romania, the personnel of Banat's University of Agricultural Sciences and Veterinary Medicine Timisoara Library and of Carol Davila University of Medicine and Pharmacy Bucharest Library and all researchers (not mentioned) for providing assistance with the literature. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Department of Health and Human Services, the Centers for Disease Control and Prevention or the US Department of Agriculture.









