INTRODUCTION
Among the numerous parasite species known to alter the phenotype of their intermediate hosts, acanthocephalan parasites have been shown to manipulate the behaviour of their intermediate arthropod host to make it more prone to predation by their final vertebrate host (Poulin, Reference Poulin1995; Lafferty, Reference Lafferty1999; Kennedy, Reference Kennedy2006). The behavioural changes induced by acanthocephalans can vary and include reaction to light (Bauer et al. Reference Bauer, Trouvé, Grégoire, Bollache and Cézilly2000; Cézilly et al. Reference Cézilly, Gregoire and Bertin2000; Perrot-Minnot, Reference Perrot-Minnot2004), vertical distribution (Cézilly et al. Reference Cézilly, Gregoire and Bertin2000; Bauer et al. Reference Bauer, Haine, Perrot-Minnot and Rigaud2005; Médoc et al. Reference Médoc, Bollache and Beisel2006), drift behaviour (McCahon et al. Reference McCahon, Maund and Poulton1991; Maynard et al. Reference Maynard, Wellnitz, Zanini, Wright and Dezfuli1998), activity level (Dezfuli et al. Reference Dezfuli, Giari, Arrighi, Domeneghini and Bosi2003) or the refuge use and the escape performance faced with non-host predators (Baldauf et al. Reference Baldauf, Thünken, Frommen, Bakker, Heupel and Kullmann2007; Perrot-Minnot et al. Reference Perrot-Minnot, Kaldonski and Cézilly2007; Médoc and Beisel, Reference Médoc and Beisel2009; Médoc et al. Reference Médoc, Rigaud, Bollache and Beisel2009; Beisel and Médoc, Reference Beisel and Médoc2010). Behavioural changes make gammarids more likely to be preyed upon by the parasite's final host (Lagrue et al. Reference Lagrue, Kaldonski, Perrot-Minnot, Motreuil and Bollache2007; Perrot-Minnot et al. Reference Perrot-Minnot, Kaldonski and Cézilly2007; Cézilly et al. Reference Cézilly, Thomas, Médoc and Perrot-Minnot2010). Although much attention has been focused on behavioural changes, few studies have been devoted to the physiological consequences of acanthocephalan infection in the intermediate host. For example, Cornet et al. (Reference Cornet, Franceschi, Bauer, Rigaud and Moret2009) described that acanthocephalans reduced the immune capacity of Gammarus pulex. In addition, parasites need their host to survive both in terms of energy supply for their own development and of their transmission to a final host (Plaistow et al. Reference Plaistow, Troussard and Cézilly2001). In disturbed environments, antitoxic defence capacities may play a key-role to allow the survival of the intermediate host faced with biotic (parasites) and abiotic (pollutants) stresses. A conflict between these two factors may occur and compromise the future of infected individuals. The parasite could also protect the host from a pollutant, as sometimes demonstrated for adult acanthocephalans in their fish hosts (Sures and Siddall, Reference Sures and Siddall1999; Sures et al. Reference Sures, Dezfuli and Krug2003).
Gammarus roeseli is a widespread amphipod crustacean of Balkan-European origin (Jazdzewski, Reference Jazdzewski1980; Barnard and Barnard, Reference Barnard and Barnard1983), often used as a biological model in ecotoxicological studies that aim at developing biomarkers, especially antitoxic defence system biomarkers (Sroda and Cossu-Leguille, Reference Sroda and Cossu-Leguille2011a, Reference Sroda and Cossu-Leguilleb). In natural populations, G. roeseli commonly serves as an intermediate host for numerous acanthocephalan parasites, including the water bird acanthocephalan Polymorphus minutus (Médoc and Beisel, Reference Médoc and Beisel2009). G. roeseli get infected as a result of eating P. minutus eggs released in the final host's feces. They hatch in the intestine and release acanthor which move into the haemocoel, where they develop into cystacanths (infective larvae). This larval stage is characterized by an orange carotenoid-based colour which makes it visible through the translucent cuticle of infested gammarids (Kennedy, Reference Kennedy2006).
The aim of our study was to investigate the influence of P. minutus on the energy reserves and antitoxic defence capacities of its intermediate host G. roeseli in May, June and August, which correspond to the period of high prevalence in our study site (Médoc and Beisel, Reference Médoc and Beisel2009). As parasite survival and transmission depend on host survival, we hypothesized that P. minutus could protect its host. We therefore assumed that P. minutus-infected gammarids could have higher defence capacities (higher GSH concentrations) as well as lower cell damage (lower MDA levels) as compared to uninfected ones. Energy reserves were assessed by assaying protein concentrations as well as total lipid and glycogen contents. Glycogen levels are representative of the energy available for current activities (Sparkes et al. Reference Sparkes, Keogh and Pary1996) whereas lipids are stored in fat bodies and serve as nutrients used during starvation or reproduction periods (Cargill et al. Reference Cargill, Cummin, Hanson and Lowry1985). Antitoxic defences were estimated by measuring reduced glutathione (GSH) concentrations. GSH is a tripeptide whose role is essential in the detoxification system thanks to its thiol function, and its action as a scavenger of organic or metal xenobiotics (Griffith, Reference Griffith1999; Vasseur and Leguille, Reference Vasseur and Leguille2004). GSH is commonly used in ecotoxicology studies on invertebrates; its concentration may be reduced in organisms exposed to copper (Doyotte et al. Reference Doyotte, Cossu, Jacquin, Babut and Vasseur1997; Canesi et al. Reference Canesi, Viarengo, Leonzio, Filippelli and Gallo1999) or lead (Yan et al. Reference Yan, Teo and Sin1997). It also plays an important role as a substrate for several antioxidant enzymes like selenium-dependent glutathione peroxidase (SeGPx, EC 1.11.1.9) or glutathione-S-tranferases. The activity of γ-glutamylcysteine ligase (GCL, EC 6.3.2.2), the enzyme that limits de novo synthesis of glutathione, was measured in parallel to glutathione concentrations. Finally, we measured the level of malondialdehyde (MDA), which is a product of lipid peroxidation reflecting cellular damage (Correia et al. Reference Correia, Livingstone and Costa2002; Neuparth et al. Reference Neuparth, Correia, Costa, Lima and Costa2005). This toxicity biomarker enables us to assess the probable protective effect of the parasite on its host: lower MDA levels reflect lower cell damage. In this study, we also hypothesized that gender could have an influence on biomarker variation, as shown by Sroda and Cossu-Leguille (Reference Sroda and Cossu-Leguille2011a, Reference Sroda and Cossu-Leguilleb), on antioxidant enzyme activities; therefore measurements were performed separately on males and females.
MATERIALS AND METHODS
Gammarus roeseli sampling
Male and female G. roeseli were collected using hand nets and artificial traps in the Nied River (Laquenexy, North-eastern France, 49°05′N and 6°19′E) in May, June and August 2009. The low number of infected gammarids in July did not allow us to perform any comparisons. Infected G. roeseli were easily identified as the parasite appears as an intense orange dot through the cuticle. Dissection was performed to confirm infection by P. minutus. According to a previous study, the overall prevalence of P. minutus varied over the sampling period, ranging from 0·08 in May and October to 0·13 in August (Médoc and Beisel, Reference Médoc and Beisel2009). Male and female gammarids were sorted on the spot according to gnathopod size, a sexual dimorphism character. The animals were immediately transported to the laboratory in river water, where they were frozen in liquid nitrogen and stored at −80°C. Four pools of 7 males and 4 pools of 10 non-gravid females were prepared for each analysis. Prior to analyses, G. roeseli gender was checked by observing genital papillae (found in males only) on the 7th ventral segment. Two conditions were studied: (i) uninfected gammarids corresponding to controls, and (ii) infected gammarids out of which the parasite was removed by dissection.
Sample preparation
Each pool was homogenized with a manual Potter Elvejhem tissue grinder in a 50 mM phosphate buffer KH2PO4/K2HPO4 (pH 7·6) supplemented with 1 mM phenylmethylsulphonylfluoride (PMSF) and 1 mM L-serine-borate mixture as protease inhibitors and 5 mM phenylglyoxal as a γ-glutamyl transpeptidase inhibitor. The homogenization buffer was adjusted at a volume 2-fold the wet weight of the sample pool (e.g. 400 μl of homogenization buffer for 200 mg of wet weight tissue). All homogenates were used for the assays immediately after being prepared. The homogenate was divided into 5 parts to measure the different parameters. For each replicate, 2 independent measures were performed for each biomarker. Then, the average of the 2 independent measures was estimated.
Lipid and glycogen assays
The measurement of total lipid and glycogen contents was adapted from Plaistow et al. (Reference Plaistow, Troussard and Cézilly2001). Twenty microlitres of 2% sodium sulphate (w/v) and 540 μl of chloroform/methanol 1:2 (v/v) were added to 40 μl of total homogenate. After 1 h on ice, the samples were centrifuged at 3000 g for 5 min at 4°C. The resulting supernatant and the pellet were used to determine the total lipid and glycogen contents, respectively.
Samples of 100 μl of the supernatant were transferred to culture tubes and placed in a dry bath at 95°C to evaporate the solvent. Then, 200 μl of 95% sulphuric acid were added in each tube and left for 10 min. The culture tubes were cooled on ice and 4·8 ml of a vanillin-phosphoric acid reagent, composed of 120 mg of vanillin, 20 ml of ultrapure ethanol (95%) and 80 ml of phosphoric acid (85%), were added. After a 10-min reaction time, optical density was measured at 535 nm. Commercial cholesterol was used as a standard and lipid contents were expressed in mg.ml−1.
Total dissolution of the pellet was performed in 400 μl of deionized (milliQ) water for 10 min in an ultrasonic bath, and 100 μl of sample were placed in culture tubes and 4·9 ml of anthrone reagent were added. The anthrone reagent is a mixture of 1·13 g of anthrone, 170 ml of ultrapure water and 630 ml of sulphuric acid (95%). The mixture was placed in a dry bath at 95°C for 17 min and then cooled on ice. Optical density was measured at 625 nm. Glucose was used as a standard and concentrations were expressed in μg.mg−1 tissue.
Total protein assay
The total protein content of each sample was quantified according to the method of Bradford (Reference Bradford1976) with bovine serum albumin (BSA) as a standard. The results were expressed in mg.ml−1.
Reduced glutathione assay
Reduced glutathione (GSH) concentration measurement was adapted from Leroy et al. (Reference Leroy, Nicolas, Thioudellet, Oster, Wellman and Siest1993) using High-Pressure Liquid Chromatography (HPLC) separation, which consisted in a post-column derivatization with ortho-phtaldialdehyde solution and fluorimetric detection at 340 nm excitation and 440 nm emission wavelengths. The proteins from 40 μl of the total homogenate were precipitated with 10% perchloric acid (v/v). After centrifugation for 10 min at 20 000 g at 4°C, the resulting supernatant was diluted 40-fold in 0·1 M hydrochloric acid (HCl). Then 20 μl of the diluted supernatant were injected in a reverse-phase LiChrospher 100 RP18-encapped column (125 mm×4 mm, 5 μm) and separation was carried out at 25°C. Elution was performed with 7% acetonitrile (Chromanorm, 95%) in a 0·01 M phosphate buffer KH2PO4/Na2EDTA (pH 2·50) containing 0·5 mM n-decylsodiumsulfate as an ion-pairing reagent. Commercial GSH diluted in 0·1 M HCl was used as a standard and GSH concentrations were expressed in nmol GSH.mg−1 protein.
Enzymatic assay
The activity of γ-glutamylcysteine ligase (GCL) was assayed using an HPLC method adapted from Parmentier et al. (Reference Parmentier, Leroy, Wellman and Nicolas1998). Measurements were carried out on the S12 000 fraction obtained after centrifuging 40 μl of the total homogenate for 15 min at 500 g and then centrifuging the resulting supernatant at 12 000 g and 4°C for 30 min. The resulting S12 000 fraction was diluted 20-fold in the homogenization buffer and 40 μl of this diluted solution were added to 112 μl of incubation cocktail (0·5 M Tris-HCl, 200 mM MgCl2 6H2O, 500 mM KCl, 45 mM glutamic acid, 90 mM cystein, 1 mM DTT, 90 mM ATP, 0·5 mM phénylglyoxal, pH 8·25) in a 1·5 ml tube to initiate the reaction. After a 20-min incubation period at 25°C, the reaction was stopped by a 4-fold dilution with 0·1 M HCl and 20 μl of the resulting solution were injected into a LiChrospher 100 RP18-encapped HPLC column (125 mm×4 mm, 5 μm). Commercial glutamylcysteine (GC) solution was used as a standard and GCL activity was expressed in nmol GC.min−1.mg−1 protein.
Lipoperoxidation
Malondialdehyde (MDA) levels were measured with an HPLC method adapted from Behrens and Madère (Reference Behrens and Madère1991) with UV detection at 267 nm. Seventy microlitres of the total homogenate were diluted 4-fold in 95% ethanol (HPLC grade) and cooled on ice for 1·5 h to de-proteinize them. The mixture was then centrifuged at 18 000 g for 30 min at 4 °C and 100 μl of the resulting supernatant were injected directly into a reserved-phase LiChrospher 100RP18-encapped HPLC column. Separation was performed at 25°C and elution was carried out with sodium phosphate buffer (pH 6·5) containing 25% ethanol and 0·5 mM tetradecylmethylammoniun bromide as an ion-paring reagent. MDA levels were expressed in ng MDA.mg−1 lipid.
Statistical analyses
Data analysis was performed using a multivariate analysis of variance (MANOVA, Pillai's trace) with respect to ‘gender’, ‘infection status’ and ‘sampling month’ as fixed factors. All data met normality and homogeneity of variance assumptions. MDA levels were not included in this analysis because the low number of infected gammarids in August did not allow us to measure them. As the MANOVA test was significant, each biomarker was then analysed using the ANOVA test, followed by the TukeyHSD post-hoc test. All tests were performed with a 5% type I error risk, using R 2.9.0 Software.
RESULTS
Acanthocephalan effect on G. roeseli biomarker
Global MANOVA and ANOVA analysis revealed an effect of the sampling month, of individual gender, of parasite infection and of their interactions on the variations of biomarker levels (Tables 1 and 2). The results are detailed below for each biomarker category.
Table 1. Multivariate analyses of variance (Pillai's trace) investigating variations in energy reserves (protein, lipid, glycogen) and defence capacity (GSH, GCL) of Gammarus roeseli, as a function of infection by acanthocephalan parasites, month sampling and individual gender

| Source of variation | num d.fa, den d.f.b | F | P value |
| Whole model | 55, 180 | 18·94 | <0·0001 |
| Month | 5, 36 | 41·75 | <0·0001 |
| Gender | 5, 36 | 30·10 | <0·0001 |
| Infection status | 5, 36 | 102·27 | <0·0001 |
| Month×Gender | 5, 36 | 10·57 | <0·0001 |
| Month×Infection status | 5, 36 | 10·33 | <0·0001 |
| Gender×Infection status | 5, 36 | 6·37 | 0·0002 |
| Month×Gender×Infection status | 5, 36 | 5·83 | 0·0004 |
a Numerator degrees of freedom.
b Denominator degrees of freedom.
Table 2. Univariate analyses of variance (ANOVA) investigating variations in energy reserves (protein, lipid, and glycogen), in defence capacity (GSH, GCL) and the variation of a toxicity biomarker (MDA), in Gammarus roeseli, according to sampling month, gender and infection by Polymorphus minutus

| d.f. | Mean square | F | P value | ||
| Protein | Month | 2 | 4·96 | 6·52 | 0·004 |
| Gender | 1 | 59·05 | 77·61 | <0·001 | |
| Parasite | 1 | 117·09 | 156·49 | <0·001 | |
| Month:Gender | 2 | 4·8 | 6·30 | 0·004 | |
| Month:Parasite | 2 | 1·46 | 1·91 | 0·162 | |
| Gender:Parasite | 1 | 0·28 | 0·37 | 0·548 | |
| Gender:Parasite:Month | 2 | 12·76 | 16·76 | <0·001 | |
| Lipid | Month | 2 | 9·31 | 64·76 | <0·001 |
| Gender | 1 | 33·99 | 236·4 | <0·001 | |
| Parasite | 1 | 14·98 | 104·16 | <0·001 | |
| Month:Gender | 2 | 1·99 | 13·81 | <0·001 | |
| Month:Parasite | 2 | 1·1 | 7·67 | 0·001 | |
| Gender:Parasite | 1 | 7·8 | 54·26 | <0·001 | |
| Gender:Parasite:Month | 2 | 0·06 | 0·40 | 0·671 | |
| Glycogen | Month | 2 | 14·73 | 25·42 | <0·001 |
| Gender | 1 | 10·38 | 17·91 | <0·001 | |
| Parasite | 1 | 28·52 | 49·23 | <0·001 | |
| Month:Gender | 2 | 5·12 | 8·84 | <0·001 | |
| Month:Parasite | 2 | 23·89 | 41·23 | <0·001 | |
| Gender:Parasite | 1 | 6·87 | 11·86 | 0·001 | |
| Gender:Parasite:Month | 2 | 0·52 | 0·9 | 0·417 | |
| GSH | Month | 2 | 4·06 | 93·26 | <0·001 |
| Gender | 1 | 0·51 | 11·64 | 0·001 | |
| Parasite | 1 | 4·81 | 110·46 | <0·001 | |
| Month:Gender | 2 | 1·47 | 33·82 | <0·001 | |
| Month:Parasite | 2 | 0·11 | 2·63 | 0·086 | |
| Gender:Parasite | 1 | 0·04 | 0·82 | 0·371 | |
| Gender:Parasite:Month | 2 | 0·13 | 2·91 | 0·067 | |
| GCL | Month | 2 | 0·2440 | 67·61 | <0·001 |
| Gender | 1 | 0·0010 | 0·19 | 0·668 | |
| Parasite | 1 | 0·2000 | 55·44 | <0·001 | |
| Month:Gender | 2 | 0·0260 | 7·12 | 0·002 | |
| Month:Parasite | 2 | 0·0240 | 6·75 | 0·003 | |
| Gender:Parasite | 1 | 0·0001 | 0·04 | 0·849 | |
| Gender:Parasite:Month | 2 | 0·0060 | 1·62 | 0·211 | |
| MDA | Month | 1 | 87·09 | 244·33 | <0·001 |
| Gender | 1 | 12·97 | 36·38 | <0·001 | |
| Parasite | 1 | 38·70 | 108·57 | <0·001 | |
| Month:Gender | 1 | 0·18 | 0·49 | 0·489 | |
| Month:Parasite | 1 | 0·51 | 1·44 | 0·242 | |
| Gender:Parasite | 1 | 0·01 | 0·01 | 0·904 | |
| Gender:Parasite:Month | 1 | 1·58 | 4·43 | 0·046 |
Parasitism and energy reserves
Energy reserves (protein, lipid and glycogen) were influenced by P. minutus infection, gender and sampling month (Table 2). Gammarid protein concentrations were lower in the presence of P. minutus in the two genders whatever the sampling month (Fig. 1A). Moreover, no significant monthly variations were observed in uninfected and infected males and females. The same trend was observed for total lipid contents, which were lower in the presence of P. minutus in the two genders at each sampling month, except for males in August (Fig. 1B). Total lipid contents in infected females were on average 1·5-fold lower as compared to uninfected ones; while in infected males, they were on average 1·2-fold lower than in uninfected ones. Comparison of males and females showed that lipid contents were higher in uninfected females than in uninfected males whatever the sampling month, whereas there was no significant difference between infected males and females. In males, monthly variations of total lipid contents were observed only in uninfected individuals, whereas in females, these variations were marked whatever the infection status (Fig. 1B).

Fig. 1. Protein concentrations (A), total lipid (B) and glycogen contents (C) depending on sampling period, Gammarus roeseli gender and infection status. Different letters above the bars indicate significantly different values (Tukey's HSD test, P values <0·05). White bars represent uninfected G. roeseli and black bars represent infected G. roeseli.
Conversely, the presence of the acanthocephalan P. minutus increased glycogen contents whatever the gender and the sampling month, except for females in May (Fig. 1C). In infected males, the glycogen contents were 1·7-fold higher as compared to uninfected ones, whereas in infected females they were 2·4-fold higher as compared to uninfected ones. The differences in glycogen contents were highest in August in both genders. Indeed during that month, glycogen contents in infected males were 1·5-fold higher than in uninfected ones and were 3-fold higher in infected females as compared to uninfected ones. Unlike in uninfected males and females, no significant difference in glycogen contents was observed between infected males and females depending on the sampling month, except in June.
Parasitism and antitoxic defences
P. minutus has an influence on the defence capacities of G. roeseli by decreasing GSH concentrations in both genders whatever the sampling month (Fig. 2A). Indeed, infected males and females displayed on average 1·5- to 2·5-fold less GSH than uninfected ones, whatever the sampling month. The same variation in GSH concentration was observed in uninfected and infected individuals, whatever the gender and the sampling month. The decrease in GSH concentrations could be linked with the decrease in GCL activity, which was also marked in infected males and females (Fig. 2B). GCL activity was on average 2-fold lower in infected gammarids, whatever their gender and the sampling month. No significant difference was observed between males and females each month, whatever the infection status.

Fig. 2. GSH concentration (A) and GCL activity (B) depending on sampling month, Gammarus roeseli gender and infection status. Different letters above the bars indicate significantly different values (Tukey's HSD test, P values <0·05). White bars represent uninfected G. roeseli and black bars represent infected G. roeseli.
Parasitism and toxic effect biomarker
MDA levels were not measured in August due to the lack of infected G. roeseli, which led to a low total homogenate quantity. Univariate analysis of MDA levels revealed an effect of the presence of P. minutus (Table 2). MDA levels were lower in infected gammarids as compared to uninfected ones, whatever their gender and the sampling month (Table 3). Indeed, MDA levels were 1·5-fold lower in infected males and females than in uninfected ones. In addition, MDA levels were 1·5-fold higher in males than in females, whatever the infection status. Monthly variations were observed in uninfected and infected gammarids whatever the gender.
Table 3. MDA levels depending on sampling period, Gammarus roeseli gender and infection status
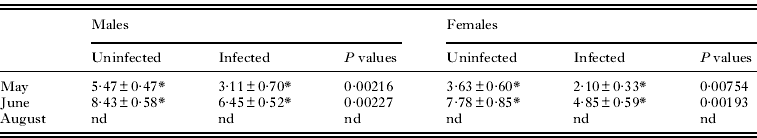
| Males | Females | |||||
| Uninfected | Infected | P values | Uninfected | Infected | P values | |
| May | 5·47±0·47* | 3·11±0·70* | 0·00216 | 3·63±0·60* | 2·10±0·33* | 0·00754 |
| June | 8·43±0·58* | 6·45±0·52* | 0·00227 | 7·78±0·85* | 4·85±0·59* | 0·00193 |
| August | nd | nd | nd | nd | nd | nd |
nd, not determined.
DISCUSSION
This study was carried out (i) to improve knowledge about the physiological effects of the acanthocephalan parasite Polymorphus minutus on its intermediate host Gammarus roeseli, especially on its energy reserves, and (ii) to assess the potential of infected individuals to deal with chemical stress by measuring defence capacities.
The presence of P. minutus in G. roeseli clearly influenced the energy reserves of its host by decreasing protein and total lipid concentrations and increasing glycogen contents in both genders. Plaistow et al. (Reference Plaistow, Troussard and Cézilly2001) demonstrated that the acanthocephalan parasite P. laevis decreased total lipid content in infected G. pulex gravid females, but did not observe any difference in males depending on the infection status. Additionally, they also observed an increase in glycogen content in P. laevis-infected G. pulex whatever the gender. However, Médoc et al. (Reference Médoc, Piscart, Maazouzi, Simon and Beisel2011) showed no difference in neutral lipid contents in P. minutus-infected G. roeseli as compared to uninfected ones. While a decrease in total lipid content in infected individuals has already been observed, the results obtained for glycogen content are rather more contrasting. Some studies highlighted an increase (P. laevis in G. pulex – Plaistow et al. Reference Plaistow, Troussard and Cézilly2001) as in our study, or an absence of modification (P. ringueletti – Isopoda ectoparasite – in P. argentinus – Neves et al. Reference Neves, Sampedro, Pastor, Nery and Santos2004); but no one reported a glycogen decrease in an acanthocephalan-infected host although such a decrease was observed in other host-parasite systems as in Norway lobsters Nephrops norvegicus infected by the dinoflagellate Hematodinium sp. (Stentiford et al. Reference Stentiford, Neil and Coombs2001). Our study underlined higher glycogen contents in P. minutus-infected G. roeseli as compared to uninfected ones, whatever the gender. We can hypothesize that the higher glycogen content in infected gammarids could be due to their immobility, since P. minutus-infected gammarids are known to stay at the water surface where they become more vulnerable to final host predation (Bakker et al. Reference Bakker, Mazzi and Zala1997; Bauer et al. Reference Bauer, Trouvé, Grégoire, Bollache and Cézilly2000, Reference Bauer, Haine, Perrot-Minnot and Rigaud2005; Cézilly and Perrot-Minnot, Reference Cézilly and Perrot-Minnot2005; Médoc et al. Reference Médoc, Bollache and Beisel2006). It could also be due to a feeding rate increase as described in G. pulex infected by Echinorhynchus truttae (Dick et al. Reference Dick, Armstrong, Clarke, Farnsworth, Hatcher, Ennis, Kelly and Dunn2010) but another study showed that P. minutus-infected G. roeseli consumed as many dead isopods, but fewer living isopods and less leaf material as compared to uninfected ones (Médoc et al. Reference Médoc, Piscart, Maazouzi, Simon and Beisel2011). We can also hypothesize that glycogen could be stored by gammarids as an alternative energy source because lipids are partly used by P. minutus.
The decrease in total lipid contents measured in our study could be due to the parasite's development. It is well known that parasites need energy for their own development inside their hosts, as demonstrated for P. minutus, which must store up host nutriments to attain the last larval stage (Crompton and Nickol, Reference Crompton and Nickol1985; Taraschewski, Reference Taraschewski2000). So, the reduction of total lipid contents in infected G. roeseli could be explained by their consumption by P. minutus by osmotrophy. This hypothesis is supported by the study of Barrett and Butterworth (Reference Barrett and Butterworth1968) who demonstrated that P. minutus gets its carotenoids from its host. Carotenoids, which are lipid constituents, are the main compound of the crustacean vitellus (Mantiri et al. Reference Mantiri, Negre-Sadargues, Charmantier, Trilles, Milicua and Castillo1996). Polymorphus minutus diverts carotenoids for its own development and consequently G. roeseli females become castrated (Bollache et al. Reference Bollache, Rigaud and Cézilly2002).
Polymorphus minutus decreased G. roeseli defence capacities: whatever the gender, a drop in reduced glutathione concentrations linked with a decrease in GCL activity was observed in P. minutus-infected G. roeseli as compared to uninfected ones. Several studies of gammarids infected by an acanthocephalan parasite have shown a decrease in host defence capacities. Cornet et al. (Reference Cornet, Franceschi, Bauer, Rigaud and Moret2009) measured a reduction of the prophenoloxidase system as well as of haemocyte concentration, 2 major parameters of crustacean immunity, in G. pulex infected by 1 of the 3 following acanthocephalan parasites: Pomphorhynchus laevis, Pomphorhynchus tereticollis and P. minutus. Sures and Radszuweit (Reference Sures and Radszuweit2007) also demonstrated that the cystacanth stage of P. minutus prevented the synthesis of heat shock protein 70 in G. roeseli subjected to a thermal disturbance or palladium exposure. Additionally, a decrease in defence capacities was also observed in other host-parasite relationships. For example, digenean-infected cockles exposed to cadmium displayed lower metallothionein concentrations than uninfected ones (Baudrimont et al. Reference Baudrimont, De Montaudouin and Palvadeau2006). According to our results, malondialdehyde (MDA), a product of lipid peroxidation reflecting cellular damage, was weaker in infected individuals. Thus, on the one hand the drop in antitoxic defences in infected gammarids suggests a higher sensitivity to stress conditions but, on the other hand, a decrease in MDA levels suggests a protective effect of the parasite on its host. Inside the host, the parasite has to escape from/survive the host's defence system and consequently weaken it, but if the host's antitoxic defence capacities are too low, the survival of the host-parasite pair can be compromised in stressful conditions. A compensation system may occur to counterbalance the weakening of the host's defence system.
Sures and Radszuweit (Reference Sures and Radszuweit2007) demonstrated that P. minutus cystacanths of G. roeseli exposed to palladium had accumulated 10 times as much metal as their hosts. In a previous study, we demonstrated that P. minutus cystacanths could accumulate cadmium (Gismondi et al. unpublished data). So, we can hypothesize that if the parasite can accumulate toxic contaminants, toxicity to the host may be reduced. Consequently, the host may need lower antitoxic defences, and the parasite may protect it during environmental stress. However, this hypothesis remains to be tested.
The present study confirms that an acanthocephalan parasite reduces the energy reserves of its host. We also observed lower defence capacities in infected individuals as compared to uninfected ones, in the absence of stressors. We cannot rule out that parasites may infect organisms with a low defence system but the information provided by glutathione concentrations and MDA levels altogether suggests that the physiological modifications we observed resulted from infection, but did not cause it. To go further, the consequences of the modifications we observed in P. minutus-infected gammarids on fitness will have to be assessed in a contamination context.
ACKNOWLEDGMENTS
This study was supported by the French Ministry of Education and Research (Ministère de l'Enseignement Supérieur et de la Recherche), which we sincerely thank here. The present work is part of the research program EC2CO (Ecosphère Continentale et Côtière). We are grateful to Annie Buchwalter for improving the English text and we wish to thank the two anonymous reviewers for their helpful comments on a previous draft of this paper.