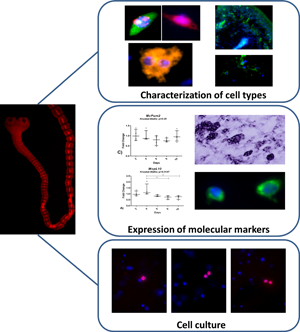
Introduction
The phylum Platyhelminthes includes diverse animals in terms of development, morphology and life cycles, with both parasitic and free-living species (Garcia et al., Reference Garcia, Moro and Schantz2007; Egger et al., Reference Egger, Lapraz, Tomiczek, Müller, Dessimoz, Girstmair, Škunca, Rawlinson, Cameron, Beli, Todaro, Gammoudi, Noreña and Telford2015). These organisms share a unique population of undifferentiated cells with proliferation capacity that is responsible for cell renewal, growth, tissue repair and regeneration (Gustafsson, Reference Gustafsson1990; Peter et al., Reference Peter, Gschwentner, Schürmann, Rieger and Ladurner2004; Reuter and Kreshchenko, Reference Reuter and Kreshchenko2004; Koziol and Castillo, Reference Koziol, Castillo and Esteves2011).
The mechanism of cell renewal, morphological characterization and distribution of neoblasts is well studied in free-living Platyhelminthes, particularly in planarias (Morita et al., Reference Morita, Best and Noel1969; Hay and Coward, Reference Hay and Coward1975; Rossi et al., Reference Rossi, Salvetti, Batistoni, Deri and Gremigni2008; Rink, Reference Rink2013). In cestodes, the presence of a set of undifferentiated cells, denominated germinative cells, has also been demonstrated and characterized in classical morphology studies (Gustafsson, Reference Gustafsson1990; Reuter and Kreshchenko, Reference Reuter and Kreshchenko2004; Koziol and Castillo, Reference Koziol, Castillo and Esteves2011). They were described at the ultrastructural level in oncospheres, metacestodes and adult cestodes, showing characteristics similar to that of planarian neoblasts, except for the absence in cestodes of chromatoid bodies (large, cytoplasmic electron-dense ribonucleoprotein particles) (Douglas, Reference Douglas1961; Bolla and Roberts, Reference Bolla and Roberts1971; Wikgren and Gustafsson, Reference Wikgren and Gustafsson1971; Sulgostowska, Reference Sulgostowska1972; Gustafsson, Reference Gustafsson1976; Loehr and Mead, Reference Loehr and Mead1979; Koziol et al., Reference Koziol, Domínguez, Marín, Kun and Castillo2010, Reference Koziol, Rauschendorfer, Rodríguez, Krohne and Brehm2014). However, molecular approaches to characterize these cells in cestodes are still too scarce.
Several genes involved in cell cycle and developmental regulation have been identified as planarian neoblast markers (Aboobaker, Reference Aboobaker2011). RNAi studies revealed the function of these marker genes, relating them with maintenance, proliferation and differentiation of neoblasts (Ogawa et al., Reference Ogawa, Kobayashi, Hayashi, Orii, Watanabe and Agata2002; Reddien, Reference Reddien2005; Juliano et al., Reference Juliano, Swartz and Wessel2010; Rouhana et al., Reference Rouhana, Shibata, Nishimura and Agata2010; Önal et al., Reference Önal, Grün, Adamidi, Rybak, Solana, Mastrobuoni, Wang, Rahn, Chen, Kempa, Ziebold and Rajewsky2012; Wagner et al., Reference Wagner, Ho and Reddien2012; Rink, Reference Rink2013). More recently, several single-cell RNAseq analyses allowed the identification of diverse marker genes characteristic of different neoblast populations and precursors of differentiated cells (Fincher et al., Reference Fincher, Wurtzel, de Hoog, Kravarik and Reddien2018; Plass et al., Reference Plass, Solana, Wolf, Ayoub, Misios, Glažar, Obermayer, Theis, Kocks and Rajewsky2018; Swapna et al., Reference Swapna, Molinaro, Lindsay-Mosher, Pearson and Parkinson2018; Zeng et al., Reference Zeng, Li, Guo, Gao, McKinney, Wang, Yu, Park, Semerad, Ross, Cheng, Davies, Lei, Wang, Perera, Hall, Peak, Box and Alvarado2018).
Gene-expression analyses of cestode germinative cells are more recent and include the study of homologous genes of planarian neoblast markers in Echinococcus multilocularis (Em-nos-1, Em-nos-2, Em-ago-2, Em-h2b, Em-sox2, Em-fgfr3), as well as a transcriptomic study of irradiated Hymenolepis diminuta (Koziol et al., Reference Koziol, Rauschendorfer, Rodríguez, Krohne and Brehm2014, Reference Koziol, Radio, Smircich, Zarowiecki, Fernández and Brehm2015; Cheng et al., Reference Cheng, Liu, Dai, Wu, Li, Guo, Tian, Heng, Lu, Chai and Wang2017; Förster et al., Reference Förster, Koziol, Schäfer, Duvoisin, Cailliau, Vanderstraete, Dissous and Brehm2019; Rozario et al., Reference Rozario, Quinn, Wang, Davis and Newmark2019). Genomic analyses have shown that trematodes and cestodes have lost important germline multipotency programme (GMP) components, such as P-element Induced WImpy testis (PIWI), VASA and group 9 TUDOR proteins (Tsai et al., Reference Tsai, Zarowiecki, Holroyd, Garciarrubio, Sanchez-Flores, Brooks, Tracey, Bobes, Fragoso, Sciutto, Aslett, Beasley, Bennett, Cai, Camicia, Clark, Cucher, De Silva, Day, Deplazes, Estrada, Fernández, Holland, Hou, Hu, Huckvale, Hung, Kamenetzky, Keane, Kiss, Koziol, Lambert, Liu, Luo, Luo, Macchiaroli, Nichol, Paps, Parkinson, Pouchkina-Stantcheva, Riddiford, Rosenzvit, Salinas, Wasmuth, Zamanian, Zheng, Cai, Soberón, Olson, Laclette, Brehm and Berriman2013; Fontenla et al., Reference Fontenla, Rinaldi, Smircich and Tort2017, Reference Fontenla, Rinaldi and Tort2021). The loss of these genes and the absence of chromatoid bodies highlight differences between proliferative cells from parasitic flatworms and the neoblasts of free-living planarias. However, it has been proposed that paralogues to GMP components in the genomes of cestodes and trematodes might have replaced the original functions. This might be the case for pL10, a DEAD-box helicase paralogue of vasa, involved in ATP-ase and helicase activity and their regulation (Gorbalenya and Koonin, Reference Gorbalenya and Koonin1993; Cordin et al., Reference Cordin, Banroques, Tanner and Linder2006; Linder, Reference Linder and Jankowsky2010; Linder and Jankowsky, Reference Linder and Jankowsky2011). Eukaryotic DEAD-box helicases are involved in developmental regulation (Lasko and Ashburner, Reference Lasko and Ashburner1988). In particular, vasa and related genes are expressed in germinal cells (Ikenishi and Tanaka, Reference Ikenishi and Tanaka1997; Kuznicki et al., Reference Kuznicki, Smith, Leung-Chiu, Estevez, Scott and Bennett2000; Johnstone et al., Reference Johnstone, Deuring, Bock, Linder, Fuller and Lasko2005). At least 3 copies of pL10 genes exist in cestode genomes and it is proposed that they might have replaced the original vasa function in neoblasts (Tsai et al., Reference Tsai, Zarowiecki, Holroyd, Garciarrubio, Sanchez-Flores, Brooks, Tracey, Bobes, Fragoso, Sciutto, Aslett, Beasley, Bennett, Cai, Camicia, Clark, Cucher, De Silva, Day, Deplazes, Estrada, Fernández, Holland, Hou, Hu, Huckvale, Hung, Kamenetzky, Keane, Kiss, Koziol, Lambert, Liu, Luo, Luo, Macchiaroli, Nichol, Paps, Parkinson, Pouchkina-Stantcheva, Riddiford, Rosenzvit, Salinas, Wasmuth, Zamanian, Zheng, Cai, Soberón, Olson, Laclette, Brehm and Berriman2013; Fontenla et al., Reference Fontenla, Rinaldi, Smircich and Tort2017, Reference Fontenla, Rinaldi and Tort2021). Pumilio genes are also planarian neoblast markers. These RNA binding proteins repress target gene translation and play a central role in post-transcriptional regulation. Several organisms show specific pumilio gene expression in germinal cells and other stem cell types, and also in the nervous system (Moore et al., Reference Moore, Jaruzelska, Fox, Urano, Firpo, Turek, Dorfman and Pera2003; Guo et al., Reference Guo, Peters and Newmark2006; Kurisaki et al., Reference Kurisaki, Iwai, Yamashita, Kobayashi, Ito and Matsuoka2007). This is consistent with an ancestral function in maintaining proliferation ability and pluripotency of metazoan stem cells (Curtis et al., Reference Curtis, Treiber, Tao, Zamore, Williamson and Lehmann1997; Wickens et al., Reference Wickens, Bernstein, Kimble and Parker2002).
In this work the focus was on the characterization of germinative cells of the cestode Mesocestoides corti (syn vogae) (Etges, Reference Etges1991). This species has been adopted as a model species to study the biology and development of cestodes (Britos et al., Reference Britos, Domínguez, Ehrlich and Marín2000; Costábile et al., Reference Costábile, Marín and Castillo2017, Reference Costábile, Koziol, Tort, Iriarte and Castillo2018; Camargo de Lima et al., Reference Camargo de Lima, Monteiro, Basika Cabrera, Paludo, Moura, Barr, Zaha and Ferreira2018) due to their low risk of infection to humans, the ease of obtaining large amounts of parasites thanks to the asexual amplification in the rodent host and the possibility to induce strobilar development in vitro (Specht and Voge, Reference Specht and Voge1965; Voge and Seidel, Reference Voge and Seidel1968; Barrett et al., Reference Barrett, Smyth and Ong1982; Thompson et al., Reference Thompson, Jue Sue and Buckley1982; Ong and Smyth, Reference Ong and Smyth1986).
It was previously shown that M. corti germinative cells are localized only in the inner parenchyma, particularly in close proximity to the inner muscle layer, but not in the cortical parenchyma nor in the sub-tegumental tissue, with a spatial distribution similar to that observed in free-living platyhelminths. It was also demonstrated that cellular renewal and growth depends on these cells (Hess, Reference Hess1980, Reference Hess1981; Koziol et al., Reference Koziol, Domínguez, Marín, Kun and Castillo2010). Additionally, an optimized protocol to isolate proliferative cells by flow cytometry was developed (Domínguez et al., Reference Domínguez, Koziol, Porro, Costábile, Estrade, Tort, Bollati-Fogolin and Castillo2014). Here we deepen the characterization of M. corti germinative cells, analysing their morphology in cell suspensions, evaluating the ability to proliferate in vitro and the expression of 3 post-transcriptional regulators considered as markers of undifferentiated cells in other flatworms.
Materials and methods
Parasite material
Mice infected with M. corti tetrathyridia were kindly provided by Jenny Saldaña and Laura Dominguez (Laboratorio de Experimentación Animal, Facultad de Química, UdelaR). This is the same strain isolated by Specht and Voge (Reference Specht and Voge1965), with the species originally identified as M. corti, and has been proposed that it might be another species of Mesocestoides by Etges (Reference Etges1991), although there is no consensus in the literature on its nomenclature. Parasite removal, culture and induction of strobilization were performed as previously described (Britos et al., Reference Britos, Domínguez, Ehrlich and Marín2000; Koziol et al., Reference Koziol, Domínguez, Marín, Kun and Castillo2010).
Fixed cell suspensions
Tissue maceration was performed according to David (Reference David1973) with some modifications. Briefly, M. corti tetrathyridia were cultured for 6 days in modified RPMI-1640 media [RPMI 1640 media, HEPES modified (Sigma-Aldrich, Germany) with 4.3 g L−1 glucose (Sigma-Aldrich, Germany), 4.8 g L−1 yeast extract (Sigma-Aldrich, Germany) and 50 μg mL−1 gentamicin (Sigma-Aldric, Germany) added] supplemented with 10% bovine fetal serum (Biochrom, Germany), washed 3 times in phosphate-buffered saline (PBS) and placed in maceration solution (1:1:13 distilled water:glacial acetic acid:glycerol; 15 mL per 200 μL of tetrathyridia). Samples were pipetted up and down, and incubated overnight at 4°C. The next day, they were disaggregated again by pipetting, diluted 1:10 in maceration solution and spotted on Silane-prep slides (Sigma-Aldrich, USA) or SuperFrost slides (Thermo Scientific, Germany). Slides were air dried overnight at room temperature.
EdU labelling
5-Ethynyl-2´-deoxyuridine (EdU, Thermo Scientific, Germany) is a thymidine analogue used as a proliferation marker in cell type characterization and cell culture. M. corti tetrathyridia were incubated with EdU 20 μ m for 4 h in RPMI-1640 modified media without yeast extract and with 10% bovine fetal serum. Detection was performed with the Click-iT® EdU Alexa Fluor® 594 Imaging Kit (Thermo Scientific, Germany) as described by the manufacturer.
Cell type identification using different dyes
Slides with cell suspensions were stained by one of the following procedures (Koziol et al., Reference Koziol, Rauschendorfer, Rodríguez, Krohne and Brehm2014).
1) EdU detection and whole cell stain (WCS): EdU was detected first, followed by Cellomics™ WCS Green (Thermo Scientific) staining as instructed by the manufacturer.
2) Propidium iodide (PI): Slides were treated with 0.05% Triton X-100 (Sigma-Aldrich), and stained with PI (Sigma-Aldrich, 2.5 μg mL−1 in PBS) for 15 min.
3) Nile red (NR): After washing with PBS, slides were stained with NR (Greenspan et al., Reference Greenspan, Mayer and Fowler1985) (Sigma-Aldrich, 100 ng mL−1 in PBS from a 4.2 mg mL−1 stock in acetone).
Finally, for all the preparations, 4′,6-diamidino-2-phenylindole (DAPI) staining (Sigma-Aldrich, 1 μg mL−1 in PBS) was performed. After staining, slides were washed with PBS and mounted with Fluoprep mounting (bioMérieux). Imaging was performed with Zeiss Axio Imager Z1 microscope (Zeiss).
Acetylated tubulin immunohistochemistry
Cell suspension samples were fixed in 4% paraformaldehyde (PFA, Sigma-Aldrich) for 15 min, washed 3 times in PBS and treated twice with 0.05% Triton X-100. Whole specimens were fixed by embedding in Paraplast (Oxford Labware) as described by the manufacturer and cut in 10 μm thick sections. After dewaxing and rehydration, the slides were boiled for 20 min in a solution of 10 mm sodium citrate, pH 6.0 with 0.1% Triton X-100 in a microwave, washed 5 min in PBS and treated twice with 0.05% Triton X-100 for 5 min. Cell suspension or sections were then incubated in blocking solution [3% bovine serum albumin (BSA, Sigma-Aldrich) plus 5% sheep serum (Sigma-Aldrich) in PBS] for 1 h and incubated overnight at 4°C with anti-acetylated tubulin antibody (mouse monoclonal, clone 6-11B-1, Santa Cruz Biotechnology), diluted 1:50 in PBS with 3% BSA. Then, samples were washed 4 times for 10 min with PBS and incubated for 2 h with Fluorescein Isothiocyanate (FITC) conjugated antibody (donkey anti-mouse, Jackson Immunoresearch) diluted in 1:100 in PBS with 3% BSA. Finally, samples were washed 4 times for 10 min with PBS, and co-stained with DAPI (1 μg mL−1 in PBS).
In vitro culture of primary cell preparations
Cell suspensions were obtained starting with 2.5 mL of tetrathyridia following previously described protocols with minor modifications (Domínguez et al., Reference Domínguez, Koziol, Porro, Costábile, Estrade, Tort, Bollati-Fogolin and Castillo2014). After parasite fragmentation, samples were incubated in 0.1% Trypsin (Sigma-Aldrich) solution for 20 min, dissociated by gentle pipetting, and filtered through a 30 μm mesh. Samples were then centrifuged at 1000 rpm for 1 min to eliminate calcareous corpuscles, resuspended at the appropriate cellular density, and filtered through a 40 μm mesh Easy strainer (Greiner).
Primary cell culture experiments followed the protocols developed for E. multilocularis (Spiliotis et al., Reference Spiliotis, Tappe, Sesterhenn and Brehm2004, Reference Spiliotis, Lechner, Tappe, Scheller, Krohne and Brehm2008; Brehm and Spiliotis, Reference Brehm and Spiliotis2008). Culture media tested were DMEM (Life Technologies), E. multilocularis hydatid fluid and hepatocyte conditioned media in non-reductive or reductive (0.01% l-cysteine, 100 μ m β-mercaptoethanol and 10 μ m bathocuproine disulphonic acid) conditions (Spiliotis et al., Reference Spiliotis, Lechner, Tappe, Scheller, Krohne and Brehm2008). Culture dishes were placed in sealed plastic bags, the gas phase was replaced with nitrogen and incubated in 5% CO2 at 37°C. For proliferative cell labelling, EdU was incorporated 4 h before the end of the cell culture, and detected as described above. Cell cultures were monitored by optical microscopy. Imaging was performed with Zeiss Axio Imager Z1 microscope (Zeiss).
Determination of mRNA expression levels during in vitro strobilar development by qPCR
Strobilization was induced as previously described (Britos et al., Reference Britos, Domínguez, Ehrlich and Marín2000), retrieving samples every 2 days during strobilar development. RNA extraction, DNase treatment, retrotranscription and qPCR were performed as described previously (Costábile et al., Reference Costábile, Marín and Castillo2017). Primers used to amplify and quantify McpL10, McPum1 and McPum2 genes from M. corti are shown in Table 1. Amplification efficiency and primer specificity were determined as described previously (Costábile et al., Reference Costábile, Marín and Castillo2017).
Table 1. Primers for qPCR

a WormBase ParaSite v13 accession code.
In situ hybridization
Probe labelling
Digoxigenin-labelled probes were synthesized by in vitro transcription with SP6 polymerase (New England Biolabs), using the DIG RNA labelling mix (Roche) as described by the manufacturer. A fragment of the target genes was amplified using degenerate primers [FwVasa: 5´-ATGGCNTGYGCNCARACNGGN-3´ and RvVasa: 5´-NCCCATRTCNARCATNCKRTC-3´ for McpL10 gene, and primers described in Koziol et al. (Reference Koziol, Marín and Castillo2008) for pumilio genes] and cloned in pGEM-T Easy (Promega). The McpL10 gene was cloned using the primers indicated above, prior to the sequencing and publication of the M. corti genome (Coghlan et al., Reference Coghlan, Tyagi, Cotton, Holroyd, Rosa, Tsai, Laetsch, Beech, Day, Hallsworth-Pepin, Ke, Kuo, Lee, Martin, Maizels, Mutowo, Ozersky, Parkinson, Reid, Rawlings, Ribeiro, Swapna, Stanley, Taylor, Wheeler, Zamanian, Zhang, Allan, Allen, Asano, Babayan, Bah, Beasley, Bennett, Bisset, Castillo, Cook, Cooper, Cruz-Bustos, Cuéllar, Devaney, Doyle, Eberhard, Emery, Eom, Gilleard, Gordon, Harcus, Harsha, Hawdon, Hill, Hodgkinson, Horák, Howe, Huckvale, Kalbe, Kaur, Kikuchi, Koutsovoulos, Kumar, Leach, Lomax, Makepeace, Matthews, Muro, O'Boyle, Olson, Osuna, Partono, Pfarr, Rinaldi, Foronda, Rollinson, Samblas, Sato, Schnyder, Scholz, Shafie, Tanya, Toledo, Tracey, Urban, Wang, Zarlenga, Blaxter, Mitreva and Berriman2019). These plasmids were used as template for probe synthesis. Probes were checked by agarose gel electrophoresis and quantified by comparison of serial dilutions in a dot blot with the DIG-labelled Control RNA (Roche).
In situ hybridization in sections
Worms cultured for 6 days were fixed in 4% PFA in PBS for 90 min at room temperature, washed 5 times with PBS and incubated in 30% sucrose for 48 h at 4–8°C. Worms were embedded in inclusion resin, snap frozen in liquid nitrogen and sliced in 10 μm sections onto SilanePrep (Sigma-Aldrich) slides. Slides were stored at −80°C until use. Sections were thawed for 30 min at room temperature, re-fixed with 4% PFA in PBS for 15 min at room temperature and washed 3 times in PBS (5 min each). Permeabilization was performed for 30 min at room temperature with 0.5% Triton X-100 in PBS, followed by 0.2 N HCl treatment for 10 min. Sections were incubated 10 min at 60°C in pre-hybridization buffer [5× saline sodium citrate buffer (SSC: 750 mm NaCl, 75 mm trisodium citrate, pH7, all components from Sigma-Aldrich), 50% formamide (Sigma-Aldrich), 10% dextran sulphate (Sigma-Aldrich), 1 mg mL−1 Torula yeast RNA (Sigma-Aldrich), 1× Denhardt's solution (Fluka)] and pre-hybridized for 120 min at 53°C in fresh pre-hybridization buffer. Probes were denatured by heating at 80°C for 2 min, chilled immediately on ice and added to the slides at a concentration of 0.1 ng μL−1 in pre-hybridization buffer. Hybridization was performed in a humidity chamber at 53°C overnight with slides covered with Parafilm. After hybridization, samples were incubated with pre-heated 2× SSC buffer at 53°C until the Parafilm detached from the slide, washed with 1× SSC buffer plus 50% formamide and 0.1% Tween-20 (Sigma-Aldrich) for 20 min at 53°C, and with 1× SSC buffer plus 50% formamide for 20 min at 53°C. Samples were then transferred to room temperature, washed twice (30 min each) with maleic acid buffer (MAB: 100 mm maleic acid, 150 mm NaCl, all components from Sigma-Aldrich) and blocked for 2 h at room temperature with blocking buffer [0.5% w/v blocking reagent for nucleic acid hybridization and detection (Roche), 1% BSA and 5% v/v heat-inactivated sheep serum (Sigma-Aldrich) in MAB]. Then they were incubated in a wet chamber for 90 min at 37°C with anti-digoxigenin antibody conjugated to alkaline phosphatase (Roche) diluted 1:500 in blocking buffer without sheep serum. Finally, samples were washed 6 times (15 min each) with MAB plus 0.1% Tween-20 and colour development was performed by equilibrating samples in Alkaline Phosphatase (AP) buffer (100 mm Tris buffer, 25 mm MgCl2, 150 mm NaCl, pH 9.5, all components from Sigma-Aldrich) before adding NBT/BCIP (330 μg mL−1 of NBT, 167 μg mL−1 of BCIP, Roche) in AP buffer. Once colour development was achieved, the reaction was stopped with PBS-Tw (PBS plus 0.1% Tween-20) and fixed with 4% PFA in PBS for 20 min. Samples were washed with PBS and mounted with 80% glycerol (Sigma-Aldrich).
In situ hybridization in cell suspensions
Fixed cell suspensions were re-fixed with 4% PFA in PBS for 15 min and washed 3 times with PBS (5 min each). Samples were incubated for 1 min in solutions with increasing concentration of ethanol diluted in PBS (25, 50 and 75%) up to absolute ethanol. Pre-hybridization was performed for 5 min at 56°C in pre-hybridization solution [100 ng mL−1 heparin (Sigma-Aldrich), 3× SSC, 100 mm Dithiothreitol (DTT) (Amresco)]. Before hybridization, the probe was denatured at 80°C for 5 min and incubated in ice. Hybridization was performed overnight at 50°C in pre-hybridization solution containing 2 ng mL−1 of probe. Samples were washed twice with pre-heated 2× SSC for 20 min at 50°C and twice with MABT (MAB plus 0.1% Tween-20) for 10 min at room temperature. Blocking was performed for 2 h at room temperature in blocking solution [2% w/v blocking reagent for nucleic acid hybridization and detection (Roche) and 5% v/v heat-inactivated sheep serum (Sigma-Aldrich) in MABT]. Then, they were incubated with anti-digoxigenin antibody conjugated to peroxidase (Roche) diluted 1:50 in blocking solution for 1 h. Finally, the slides were washed 3 times with MABT (5 min each) and incubated for 10 min in imidazole buffer [0.1 m imidazole (Sigma-Aldrich), pH 7.6 in PBS] at room temperature. Tyramide solution was used for detection, prepared and used as described by Hopman et al. (Reference Hopman, Ramaekers and Speel1998) (diluted 1/100 in imidazole buffer with 0.001% hydrogen peroxide). Samples were incubated with detection solution for 5 min in the dark and washed 5 times in PBS (5 min each). Nuclei were stained with DAPI. Imaging was performed with Olympus epifluorescence microscope.
Results
Characterization of cell types
To identify different cell types in M. corti, several cell biology and immunohistochemical techniques were applied to M. corti fixed cell suspensions.
Germinative cells were identified by their morphological characteristics (containing a large nucleus with finely granular chromatin, a large prominent nucleolus, rounded undifferentiated shape and cytoplasm strongly stained with PI). These cells are the only ones with proliferative capacity identified by differential incorporation of the thymidine analogue EdU. In addition these cells have a nucleus intensely stained with DAPI, cytosol intensely stained with WCS and PI and they rarely present lipid inclusion (detected with NR) (Fig. 1)

Fig. 1. Mesocestoides corti cell types identified in cell suspensions stained with different dyes. The cell types are identified on top of each panel. The stain methods used are identified in the left part of the image. WCS, whole cell staining; PI, propidium iodide; NR, Nile red. The bar represents 10 μm.
Muscle cells were identified as small cells with a heterochromatin-rich nucleus, and frequent cell projections (Fig. 1). Tegumental cells had an irregular shape, with a cytoplasm brightly stained with PI or WCS and uniformly stained with NR (Fig. 1). Calcareous corpuscle precursor cells had a small nucleus localized in the cellular periphery, a big cytoplasm with little PI, WCS or NR staining (Fig. 1). Excretory system cells (flame cells) were identified based on their characteristic shape (Fig. 1).
As a complementary strategy to identify nervous cells and cells from the excretory system, immunofluorescence analyses were performed using an antibody that recognizes acetylated tubulin (Koziol et al., Reference Koziol, Krohne and Brehm2013). In sections, the main nerve cords could be identified based on their intense reactivity with the antibody (Fig. 2A and B; continuous arrow). Beside the cords, the presence of neurons in the region of the cortical parenchyma (Fig. 2A–C, discontinue arrows) was observed. Similarly, in cell suspensions, nerve cells presented a very small nucleus with extensions and projections of the cell soma that may correspond to neurites (Fig. 2D–F, discontinuous arrows). This antibody can also recognize flame cell cilia, which allows the identification of flame cells corresponding to the excretory system by their characteristic shape (Fig. 2G).

Fig. 2. Acetylated tubulin immunofluorescence of the nervous and excretory system. (A) Mesocestoides corti transversal section; (B) detail of the nerve cord and genital primordium (10 μm width); (C) transversal section detail; (D–G) M. corti cell suspensions; solid arrows: main nerve cords; dashed arrows: nerve cells; dotted arrows: flame cells. Blue: DAPI, green: acetylated tubulin. Bars represent 100 μm (A), 50 μm (B–C) and 20 μm (D–G).
Mesocestoides corti cell culture
To figure out if any of the different M. corti cell types identified could survive and proliferate in vitro, a culture of live cell suspensions based on E. multilocularis primary cell culture protocols (Spiliotis et al., Reference Spiliotis, Tappe, Sesterhenn and Brehm2004, Reference Spiliotis, Lechner, Tappe, Scheller, Krohne and Brehm2008; Brehm and Spiliotis, Reference Brehm and Spiliotis2008) was assayed. The development of a cell culture system is important to advance in the understanding of the cellular biology of M. corti. Different growth conditions were tested for primary cell preparations obtained from tetrathyridia, using diverse media (DMEM, hydatid fluid and hepatocyte conditioned media), under non-reducing and reducing conditions, in a N2 and CO2-containing atmosphere.
No adherence to the substrate or between cells was observed under all culture conditions after 1 day in culture, with the dissociated M. corti cells remaining free in suspension (Supplementary Fig. 1).
To determine if cells can proliferate after 20 h in vitro, the primary cultures were tested for EdU incorporation. In all conditions analysed, a subpopulation of cells in the culture incorporated the nucleotide analogue, indicative of their proliferative status (Fig. 3, continue arrow, red nuclei). Since the primary cultures were started with all the cells dissociated from whole worms, it is expected that most cells would not represent proliferative cells (Fig. 3, discontinuous arrow, blue nuclei).

Fig. 3. Proliferation assays on isolated M. corti cells cultured in vitro for 20 and 72 h in different culture media. Nuclei are stained with DAPI (blue), and proliferation is indicated by the incorporation of EdU (red). EdU-positive nuclei are indicated by solid arrows while negative nuclei are marked by dashed arrows. The bar represents 20 μm
The proliferative capacity is maintained after 72 h in culture in rich media (DMEM with or without reductive conditions) and in hydatid fluid in non-reducing conditions (Fig. 3).
Expression of pumilio and pL10 genes
Two previously isolated M. corti pumilio genes [McPum1 and McPum2 (Koziol et al., Reference Koziol, Marín and Castillo2008)], as well as the vasa homologue McpL10 were evaluated as putative markers of germinative cells in M. corti. First, the levels of mRNA expression for these marker genes were evaluated by quantitative reverse transcription polymerase chain reaction during strobilar development.
McpL10 gene was slightly downregulated (Fig. 4A) with 25% less expression in segmented worms (10 days) compared to the larval stage (2 days). A similar trend was observed for McPum1 (Fig. 4B), which was downregulated at the beginning of development, with expression levels reduced more than 50% in worms at 8 days of culture, but increasing again in segmented worms (10 days). No significant changes were seen in McPum2 gene expression during strobilar development (Fig. 4C).

Fig. 4. Fold change of the mRNA level of McpL10 (A), McPum1 (B) and McPum2 (C) genes during strobilar development (2, 4, 6, 8 and 10 days of in vitro culture). Fold change relative to worms with 2 days of culture. Median value with interquartile range was plotted. Significant differences are shown with asterisks (n = 4, Kruskal–Wallis, Dunn's post test, *P < 0.05). (D) Scheme showing the time points analysed during strobilar development.
Then, the expression location of these putative marker genes was evaluated by in situ hybridization. The expression pattern of both pumilio genes in sections of worms starting the segmentation process was very similar (although a stronger signal is observed for McPum1) (Fig. 5A and B). In tetrathyridia beginning segmentation, their expression is ubiquitous in all tissues but the observed signal is stronger in cells located in the periphery of the medullary parenchyma and some cells in the cortical parenchyma. A strong signal is also detected in testes primordia (Fig. 5C and D) and in cells of the genital primordia (Fig. 5D). The results obtained for pumilio genes suggest that they are higher expressed in cells with morphology and distribution consistent with germinative cells, but their expression is not restricted to this cell type alone.

Fig. 5. Expression localization of M. corti pumilio genes. (A) In situ hybridization with antisense McPum1 probe in a longitudinal section of a worm starting segmentation. The arrows show testes primordia. (B) In situ hybridization with antisense McPum2 probe in a longitudinal section of a worm starting segmentation. McPum2 is expressed in several tissues, particularly in cells near the inner muscle layer (shown with arrows). (C) McPum1 expression in testes primordia. (D) McPum1 expression in late genital primordia. (E) McPum2 expression in testes primordia (arrow). iml, Inner muscle layer; mp, medular parenchyma; st, subtegument; t, tegument. The bar represents 100 μm (A and B), 20 μm (C) and 50 μm (D and E).
The expression of the vasa homologue McpL10 was studied using in situ hybridization on cell suspensions. While this gene was expressed in cells with germinative morphology (Fig. 6, continuous arrows), it was also detected in other cell types with differentiated morphology, indicating that McpL10 is not exclusively expressed in germinative cells.

Fig. 6. In situ hybridization of McpL10 gene on M. corti cell suspensions. (A and B) Mesocestoides corti cell suspensions. The white arrow indicates cells with germinative cell morphology. Scale bars (A) 20 μm, (B) 10 μm.
Discussion
Studies on cestode cell types and their characteristics are sparse, and generally older than 50 years, preceding all modern molecular and cellular biology tools. This paucity might be related to the small size of the cells, which makes it hard to identify different cell types based on their morphology using routine techniques (Sakamoto and Sugimura, Reference Sakamoto and Sugimura1970; Gustafsson, Reference Gustafsson1976). The characterization of M. corti cell suspensions with diverse fluorescent stains allowed to identify different cell types and compare them to those described in classical histological reports of cestodes (Sakamoto and Sugimura, Reference Sakamoto and Sugimura1970; Lascano et al., Reference Lascano, Coltorti and Varela-Díaz1975; Gustafsson, Reference Gustafsson1976; Loehr and Mead, Reference Loehr and Mead1979; Koziol et al., Reference Koziol, Rauschendorfer, Rodríguez, Krohne and Brehm2014).
While differentiated cells show varied morphologies and were negative for the incorporation of the nucleotide analogue EdU, a group of small cells (5–12 μm across the longest axis) were the only ones EdU positive. These pear-shaped to fusiform cells are strongly stained with PI and WCS. They have large and round nuclei, with 1–3 prominent nucleoli and granular chromatin, giving very bright staining with DAPI (Fig. 1). Cytoplasmic lipid droplets were rare. All of these characteristics are consistent to previous descriptions of germinative cells (Douglas, Reference Douglas1961; Bolla and Roberts, Reference Bolla and Roberts1971; Wikgren and Gustafsson, Reference Wikgren and Gustafsson1971; Loehr and Mead, Reference Loehr and Mead1979; Koziol et al., Reference Koziol, Domínguez, Marín, Kun and Castillo2010) and those observed in whole mount larvae (Koziol et al., Reference Koziol, Domínguez, Marín, Kun and Castillo2010) and the ones obtained for E. multilocularis (Koziol et al., Reference Koziol, Rauschendorfer, Rodríguez, Krohne and Brehm2014).
Immunofluorescence using acetylated tubulin was consistent with early descriptions of the M. corti nervous system (Hart, Reference Hart1967), but also allowed to recognize less abundant cell types, such as nervous and flame cells, which complement morphologic analysis and stains.
Cells with proliferation capacity were also observed when culturing primary cell preparations of macerated M. corti for 20 or 72 h. These cells remain free in suspension, without adherence to the substratum or interacting with each other after 1 day in culture. This was an interesting difference to E. multilocularis primary cultures, where cells begin to associate after 24 h in culture (Spiliotis et al., Reference Spiliotis, Lechner, Tappe, Scheller, Krohne and Brehm2008).
The ability to proliferate in microaerobic anoxic conditions is consistent with what would be the natural environment within the host. Reducing conditions are not necessary nor beneficial to these cells, as this condition does not seem to increase proliferation. Supplements reported to improve cell proliferation in culture like insulin (Hemer et al., Reference Hemer, Konrad, Spiliotis, Koziol, Schaack, Förster, Gelmedin, Stadelmann, Dandekar, Hemphill and Brehm2014) also resulted in no major changes in the cell culture conditions tested (not shown). The ability to survive and proliferate in all media tested, including hydatid cyst fluid, showed that requirements for cell maintenance and proliferation might be simple. Future work will be done to obtain a deep culture characterization, like the proportion of proliferating cells at different time points and comparisons among culture conditions.
To further characterize the proliferative cells, the expression localization of pL10 (homologue to vasa) and pumilio genes, characterized as markers of neoblasts in planarians (Shibata et al., Reference Shibata, Umesono, Orii, Sakurai, Watanabe and Agata1999; Salvetti et al., Reference Salvetti, Rossi, Lena, Batistoni, Deri, Rainaldi, Locci, Evangelista and Gremigni2005; Rouhana et al., Reference Rouhana, Shibata, Nishimura and Agata2010), was evaluated. Both McpL10 and McPum1 show slight variations in the expression during M. corti strobilar development, while no significant changes were observed for McPum2 gene expression.
The pattern of expression of these genes, based on distribution and morphology of the stained cells, is consistent with expression in germinative cells, but not restricted to this cell type. This stresses some differences between parasitic and free-living flatworms, since pumilio gene expression in the planaria Dugesia japonicum is restricted to neoblasts, where they play an essential role in stem cell maintenance (Salvetti et al., Reference Salvetti, Rossi, Lena, Batistoni, Deri, Rainaldi, Locci, Evangelista and Gremigni2005). Interestingly, in other organisms pumilio genes show specific expression in germinal cells and other stem cell types but are also expressed in differentiated tissues like the nervous system (Moore et al., Reference Moore, Jaruzelska, Fox, Urano, Firpo, Turek, Dorfman and Pera2003; Guo et al., Reference Guo, Peters and Newmark2006; Kurisaki et al., Reference Kurisaki, Iwai, Yamashita, Kobayashi, Ito and Matsuoka2007).
The expression of vasa gene is related to germ line cells in different organisms, like Drosophila melanogaster, Caenorhabditis elegans and Xenopus where their expression is involved in the formation and maintenance of the cells of the germ line (Lasko and Ashburner, Reference Lasko and Ashburner1988; Ikenishi and Tanaka, Reference Ikenishi and Tanaka1997; Olsen et al., Reference Olsen, Aasland and Fjose1997; Kuznicki et al., Reference Kuznicki, Smith, Leung-Chiu, Estevez, Scott and Bennett2000). Vasa genes are key components of the Piwi silencing pathway, involved in the maintenance of genome stability by silencing transposable elements (Weick and Miska, Reference Weick and Miska2014).
In free-living flatworms like planarians, vasa and vasa-related genes were shown to be expressed in germ line cells (Shibata et al., Reference Shibata, Umesono, Orii, Sakurai, Watanabe and Agata1999; Ohashi et al., Reference Ohashi, Umeda, Hirazawa, Ozaki, Miura and Miura2007; Skinner et al., Reference Skinner, Rinaldi, Suttiprapa, Mann, Smircich, Cogswell, Williams and Brindley2012, Reference Skinner, Rinaldi, Koziol, Brehm and Brindley2014). However, parasitic flatworms like monogeneans, trematodes and cestodes have lost most of the genes from the Piwi pathway including true orthologues to vasa genes (Shibata et al., Reference Shibata, Umesono, Orii, Sakurai, Watanabe and Agata1999; Ohashi et al., Reference Ohashi, Umeda, Hirazawa, Ozaki, Miura and Miura2007; Skinner et al., Reference Skinner, Rinaldi, Suttiprapa, Mann, Smircich, Cogswell, Williams and Brindley2012, Reference Skinner, Rinaldi, Koziol, Brehm and Brindley2014; Fontenla et al., Reference Fontenla, Rinaldi, Smircich and Tort2017, Reference Fontenla, Rinaldi and Tort2021). However, they have at least 3 paralogue vasa-like genes (Belle, pL10), and it is proposed that they may have taken over some of the function of vasa (Tsai et al., Reference Tsai, Zarowiecki, Holroyd, Garciarrubio, Sanchez-Flores, Brooks, Tracey, Bobes, Fragoso, Sciutto, Aslett, Beasley, Bennett, Cai, Camicia, Clark, Cucher, De Silva, Day, Deplazes, Estrada, Fernández, Holland, Hou, Hu, Huckvale, Hung, Kamenetzky, Keane, Kiss, Koziol, Lambert, Liu, Luo, Luo, Macchiaroli, Nichol, Paps, Parkinson, Pouchkina-Stantcheva, Riddiford, Rosenzvit, Salinas, Wasmuth, Zamanian, Zheng, Cai, Soberón, Olson, Laclette, Brehm and Berriman2013; Fontenla et al., Reference Fontenla, Rinaldi, Smircich and Tort2017). While the expression of pl10-related genes in other organisms, such as mouse and zebrafish (Olsen et al., Reference Olsen, Aasland and Fjose1997), was also observed in a variety of tissues (Gururajan et al., Reference Gururajan, Perry-O'Keefet, Melton and Weeks1991; Gee and Conboy, Reference Gee and Conboy1994), 2 of these vasa-related genes were expressed in germinal cells in the monogenean Neobenedenia girellae (Ohashi et al., Reference Ohashi, Umeda, Hirazawa, Ozaki, Miura and Miura2007).
The results show that Mc-pL10 is expressed in germinative cells, but is not restricted to this cell type. This might be indicative that it can partially take the role of vasa as had been proposed, but it can also be playing other roles in other cell types. Alternatively other pL10 genes not tested in this study might be the ones that substitute the vasa function in M. corti germinative cells.
Conclusions
Using different staining techniques and based on morphological descriptions, diverse cell types were identified in M. corti cell suspensions. Consistent with previous observations in other cestodes, differentiated cells (muscle, tegumentary, calcareous corpuscle cell precursors and flame cells) did not incorporate EdU, while a subset of small fusiform cells with high nucleus/cytoplasm ratio were positively stained (Koziol et al., Reference Koziol, Domínguez, Marín, Kun and Castillo2010). These cells share the morphology and histochemical characteristics of neoblasts and proliferative cells in other flatworms (Douglas, Reference Douglas1961; Bolla and Roberts, Reference Bolla and Roberts1971; Wikgren and Gustafsson, Reference Wikgren and Gustafsson1971; Loehr and Mead, Reference Loehr and Mead1979; Koziol et al., Reference Koziol, Domínguez, Marín, Kun and Castillo2010).
Furthermore, pumilio and pL10 (homologue to vasa) genes, which are known markers of planarian neoblasts (Shibata et al., Reference Shibata, Umesono, Orii, Sakurai, Watanabe and Agata1999; Salvetti et al., Reference Salvetti, Rossi, Lena, Batistoni, Deri, Rainaldi, Locci, Evangelista and Gremigni2005; Rouhana et al., Reference Rouhana, Shibata, Nishimura and Agata2010), are indeed expressed in M. corti proliferative cells, although they are not restricted to this cell type. Further candidate genes need to be analysed in order to find an appropriate marker for proliferative cells in M. corti.
Besides this, the conditions for in vitro culture of M. corti cells obtained from total cell suspensions were optimized, showing that some cells can proliferate in culture for at least 72 h. These advances contribute to the ongoing efforts to characterize and understand the role of proliferative cells in cestode life cycles. In vitro cell culture could improve the characterization of these cells and allow the performance of functional studies.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182022000956
Author contribution
M. F. D., A. C. and U. K. performed experimental work; U. K., E. C. and J. T. conceived and designed the study. M. P. performed image processing. M. F. D. wrote the original draft and all co-authors contributed to editing.
Financial support
M. F. D. was recipient of a fellowship from Agencia Nacional de Investigación e Innovación, Uruguay (ANII) and Comisión Académica de Posgrado (CAP). A. C. and M. P. also received fellowships from ANII working in overlapping projects. This work was supported by PEDECIBA and CSIC (Comisión Sectorial de Investigación Científica – Universidad de la República, Uruguay). M. F. D., A. C., U. K., E. C. and J. T. are members of Sistema Nacional de Investigadores, Uruguay.
Conflict of interest
None.
Ethical standards
Not applicable.