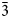Introduction
A new mineral species, hydroxyplumbopyrochlore, ideal formula, (Pb1.5,□0.5)Nb2O6(OH), was discovered in a sample from the Jabal Sayid peralkaline granitic complex in Arabian Shield, Saudi Arabia. The new mineral and its name have been approved by the International Mineralogical Association (IMA) Commission on New Minerals, Nomenclature and Classification (CNMNC) as IMA2018-145 (Li et al., Reference Li, Li, Fan, Fan, Zhong, Jahdali, Qin, Jehani, Wang and Nahdi2020). The holotype specimen is deposited in the Geological Museum of China, Beijing, China (catalogue number: M13239).
There are ten pyrochlore-group minerals approved by IMA–CNMNC (see Table 1). However, no Pb predominant member has been approved. According to the nomenclature of the pyrochlore supergroup (Atencio et al., Reference Atencio, Andrade, Christy, Gieré and Kartashov2010, p. 688), the correspondence between old and new names on the pyrochlore supergroup of minerals is cited as:
Table 1. Current members of the pyrochlore group.

*: Hydroxyplumbopyrochlore, (Pb1.5,□0.5)Nb2O6(OH), (this study)
1: Hydroxynatropyrochlore, (Na,Сa,Ce)2Nb2O6(OH), (Ivanyuk et al., Reference Ivanyuk, Yakovenchuk, Panik orovskii, Konoplyova, Pakhomovsky, Bazai, Bocharov and Krivovichev2017)
2: Fluornatropyrochlore, (Na,Pb,Ca,REE,U)2Nb2O6F, (Yin et al., Reference Yin, Li, Yang, Ge, Xu and Wang2015)
3: Hydroxycalciopyrochlore, (Ca,Na,U,□)2(Nb,Ti)2O6(OH), (Yang et al., Reference Yang, Li, Xiong, Pan and Yan2014)
4: Fluorcalciopyrochlore, (Ca,Na)2(Nb,Ti)2O6F, (Li et al., Reference Li, Yang, Lu, Xiong, Ge, Pan and Fourestier2016)
5: Oxycalciopyrochlore, Ca2Nb2O6O, (Atencio et al., Reference Atencio, Andrade, Christy, Gieré and Kartashov2010)
6: Hydropyrochlore, (H2O,□)2Nb2(O,OH)6(H2O), (Atencio et al., Reference Atencio, Andrade, Christy, Gieré and Kartashov2010)
7: Hydroxykenopyrochlore, (□,Ce,Ba)2(Nb,Ti)2O6(OH,F), (Miyawaki et al., Reference Miyawaki, Momma, Matsubara, Sano, Shigeoka and Horiuchi2017)
8: Hydrokenopyrochlore, (□,Sb3+,Na)2Nb2O6⋅H2O, (Biagioni et al., Reference Biagioni, Gieré, Meisser, Nestola, Pasero, Robyr, Roth and Schnyder2017)
9: Cesiokenopyrochlore, □Nb2(O,OH)6Cs1–x, (Agakhanov et al., Reference Agakhanov, Kasatkin, Britvin, Siidra, Pautov, Pekov and Karpenko2017)
10: Hydroxymanganopyrochlore, (Mn,Th,Na,Ca,REE)2(Nb,Ti)2O6(OH), (Chukanov et al., Reference Chukanov, Blaß, Zubkova, Pekov, Pushcharovskii and Prinz2013)
“plumbopyrochlore of Skorobogatova et al. (Reference Skorobogatova, Sidorenko, Dorofeeva and Stolyarova1966), Kartashov et al. (Reference Kartashov, Voloshin and Pakhomovskiy1992), Voloshin et al. (Reference Voloshin, Pakhomovskiy and Bakhchisaraytsev1993), Kovalenko et al. (Reference Kovalenko, Tsaryeva, Goreglyad, Yarmolyuk, Troitsky, Hervig and Farmer1995), and Xie et al. (Reference Xie, Wang, Wang and Qiu2006) is “plumbopyrochlore”. Plumbopyrochlore of Chakhmouradian & Mitchell (Reference Chakhmouradian and Mitchell2002), Wang et al. (Reference Wang, Fontan, Chen, Hu, Chang, Xu and De Parseval2003), and Beurlen et al. (Reference Beurlen, Soares, Thomas, Prado-Borges and Castro2005) is zero-valent-dominant pyrochlore. Plumbopyrochlore of Voloshin & Pakhomovskiy (Reference Voloshin and Pakhomovskiy1986) corresponds to oxyplumbopyrochlore [their anal. 1, Table 3.1], kenoplumbopyrochlore [their anal. 20, Table 3.1] and “plumbopyrochlore” [several compositions]”.
Instead of an O2– anion and a vacancy, Hydroxyplumbopyrochlore has the dominant OH– anion at the Y site, and therefore differs from ‘oxyplumbopyrochlore’ and ‘kenoplumbopyrochlore’. This paper describes the physical and chemical properties of hydroxyplumbopyrochlore and the characterisation of the structure based on single-crystal X-ray diffraction data.
Occurrence
Hydroxyplumbopyrochlore grains were collected from the pegmatite–aplite mineralised rocks from the Jabal Sayid peralkaline granitic complex in the Arabian Shield, Saudi Arabia (23°49′28.72″N, 40°56′30.93″E). Its associated minerals are quartz, microcline, ‘biotite’, rutile, zircon, calcite, rhodochrosite, columbite-(Fe), goethite, thorite, bastnäsite-(Ce), xenotime-(Y), samarskite-(Y), euxenite-(Y), hydropyrochlore and fluornatropyrochlore (Fig. 1). The hydroxyplumbopyrochlore grains intergrow closely with other rare earth elements (REE) and U–Th–Nb-bearing minerals, which are all hosted within the pegmatite or aplite in this area, signifying their genetic relationship. The U–Th–REE–Nb mineralisation is hosted in the peralkaline granitic pegmatite with hydrothermal superposition in the study areas. The similar geological settings were reported in other areas (Gysi and Williams-Jones, Reference Gysi and Williams-Jones2013; Dostal et al., Reference Dostal, Kontak and Karl2014; Estrade et al., Reference Estrade, Salvi, Beziat, Rakotovao and Rokotondrazafy2014; Harris and Marriner, Reference Harris and Marriner1980). According to the mineral assemblages, the hydroxyplumbopyrochlore is associated originally with the peralkaline granitic pegmatite.

Fig. 1. Back-scattered electron image of hydroxyplumbopyrochlore: 1 – hydroxyplumbopyrochlore; 2 – quartz; 3 – zircon; and 4 – goethite.
Appearance, physical and optical properties
Hydroxyplumbopyrochlore occurs mostly as well-shaped euhedral octahedra, slightly rhombic dodecahedra and cubes or their combination (Fig. 2). The dispersed grain size is generally in the range of 0.01–0.06 mm. Hydroxyplumbopyrochlore is pale yellow to pale brown, transparent with white streak and has adamantine to transparent lustre. It is brittle with conchoidal fracture. No cleavage or parting are observed. The sample is isotropic and non-fluorescent. The microhardness was measured by a Wolpert-401MVD microhardness tester and ranges from 440.6 to 490.4 kg mm–2, with the average of 463.4 kg mm–2, corresponding to a Mohs hardness ≈ 5½. The density is calculated at 6.474 g cm–3 on the basis of empirical formula and unit-cell volume refined from single-crystal X-ray diffraction data. Optically, hydroxyplumbopyrochlore is isotropic, n cal = 2.26(3) (Mandarino, Reference Mandarino1979). The mineral is insoluble in cold HCl and HNO3.

Fig. 2. The crystal forms of hydroxyplumbopyrochlore.
Experimental methods and results
Composition
The composition of hydroxyplumbopyrochlore was determined at Beijing Research Institute of Uranium Geology (BRIUG) with a JEOL JXA-8100 electron microprobe at 20 kV and 10 nA with a 1 μm beam diameter. The standards include bustamite for Ca; celestite for Sr; hematite for Fe; monazite for Ce and Pr; crocoite for Pb; Nb(metal) for Nb; albite for Si; Ta(metal) for Ta; and rutile for Ti.
The 6-point average composition of hydroxyplumbopyrochlore by Electron probe microanalysis (EPMA) is given in Table 2. Due to the paucity of material, direct determination of H2O was not possible. H2O was calculated on the basis of structure refinement, whereas the presence of OH groups and absence of molecular water were confirmed by the Raman spectroscopy.
Table 2. Chemical data (in wt.%) for hydroxyplumbopyrochlore.

b.d.l. – below detection limit; S.D. – standard deviation; Apfu – atoms per formula unit.
*Calculated according to the structure refinement.
Raman spectrum
The Raman spectrum of hydroxyplumbopyrochlore was obtained using a Horiba Jobin-Yvon Lab Raman HR Evolution spectrometer with a laser excitation wavelength of 473 nm and measured in the range of 50–4000 cm–1. This experiment was performed at the lab of Horiba Scientific (Shanghai, China).
The Raman spectrum of hydroxyplumbopyrochlore is shown in Fig. 3. The assignments of absorption bands have been made by analogy with a pyrochlore-like compound (Ivanyuk et al, Reference Ivanyuk, Yakovenchuk, Panik orovskii, Konoplyova, Pakhomovsky, Bazai, Bocharov and Krivovichev2017, Bahfenne and Frost Reference Bahfenne and Frost2010; RRUFF no. R060980 [https://rruff.info/hydropyrochlore/]). The bands at 3408 cm–1 and 3500 cm–1 can be attributed to O–H stretching vibrations. Bands at 727 cm–1 are caused by (Nb,Ti)–O stretching vibrations and those at 560 cm–1 and 511 cm–1 are interpreted as (Nb,Ti)–O6 stretching vibrations. A sharp band at 280 cm–1 is due to the (Nb,Ti)–O6 bending vibrations. There is no characteristic band of the H2O vibrations at 1600 cm–1.

Fig. 3. Raman spectrum of hydroxyplumbopyrochlore.
X-ray crystallography
Both powder and single-crystal X-ray diffraction of hydroxyplumbopyrochlore were collected at BRIUG using a Bruker D8 QUEST diffractometer equipped with a CMOS monochromated MoKα radiation (0.71073 Å) from a sealed microfocus tube.
The powder-diffraction data are reported in Fig. 4 and Table 3, which were integrated using Bruker APEX3 software and refined using TOPAS software. Unit-cell parameters refined from the powder diffraction data are as follows: Fd ![]() $\bar{3}$m (#227), a = 10.5669 (8) Å, V = 1179.8 (9) Å3 and Z = 8, which are in good agreement with the single-crystal data.
$\bar{3}$m (#227), a = 10.5669 (8) Å, V = 1179.8 (9) Å3 and Z = 8, which are in good agreement with the single-crystal data.

Fig. 4. Powder X-ray diffraction pattern for hydroxyplumbopyrochlore.
Table 3. Powder X-ray diffraction data (d in Å) for hydroxyplumbopyrochlore.

The eight strongest lines are in bold.
The single-crystal diffraction data were collected from a single crystal (0.08 mm × 0.10 mm × 0.11 mm). The integration of the data using a cubic unit cell yielded a total of 10,876 reflections to a maximum θ angle of 47.78° (0.48 Å resolution). Data were corrected for absorption effects using the Multi-Scan method (SADABS, Bruker). The final cell constants were a = 10.5456(6) Å and V = 1172.8(2) Å3. The structure was solved and refined using the space group Fd ![]() $\bar{3}$m, with Z = 8 for the formula unit Pb1.34Ca0.06Nb2O6.61. The final anisotropic full-matrix least-squares refinement on F 2 with 10 variables converged at R 1 = 3.17% for the observed data and wR 2 = 13.49% for all data. The goodness-of-fit is 1.136 (Table 4). Final atom coordinates, occupancies, isotropic and anisotropic atomic displacement parameters are given in Table 5. Selected interatomic distances are given in Table 6. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below). The single-crystal structure was solved using direct methods, and the refinement conducted with SHELXTL (Sheldrick, Reference Sheldrick2015).
$\bar{3}$m, with Z = 8 for the formula unit Pb1.34Ca0.06Nb2O6.61. The final anisotropic full-matrix least-squares refinement on F 2 with 10 variables converged at R 1 = 3.17% for the observed data and wR 2 = 13.49% for all data. The goodness-of-fit is 1.136 (Table 4). Final atom coordinates, occupancies, isotropic and anisotropic atomic displacement parameters are given in Table 5. Selected interatomic distances are given in Table 6. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below). The single-crystal structure was solved using direct methods, and the refinement conducted with SHELXTL (Sheldrick, Reference Sheldrick2015).
Table 4. Data collection information and structure-refinement parameters for hydroxyplumbopyrochlore.

Weighting scheme: w = 1/[σ2(F o2)+(0.0609P)2+8.8260P] where P = (F o2+2F c2)/3
Table 5. Coordinates and displacement parameters for hydroxyplumbopyrochlore.

*U eq is defined as one third of the trace of the orthogonalised U ij tensor. The anisotropic atomic displacement factor exponent takes the form: –2π2[ h 2a *2U 11 + … + 2hka*b*U 12]
Wyk. = Wyckoff symbol
Table 6. Selected interatomic distances (Å) for hydroxyplumbopyrochlore.

Discussion
The ideal formula of pyrochlore supergroup is A 2B 2X 6Y 1 (Atencio et al., Reference Atencio, Andrade, Christy, Gieré and Kartashov2010). On the basis of (Nb + Ti + Ta + Si) = 2 atoms per formula unit (B site), the empirical formula of hydroxyplumbopyrochlore is (Pb1.34Ca0.03Fe0.01Sr0.01□0.61)Σ2(Nb1.75Ti0.12Ta0.12Si0.01)Σ2O6 YO*0.345 where O* denotes the amount of charge needed to balance the formula recalculated into O atoms. Therefore, the X site has a charge of −12 and the Y site should have a charge of −0.69.
Because the structure data and composition data are collected from the same particle, we fixed the occupancy of the A and B sites on the basis of the empirical formula. The B site is fully occupied by Nb instead of (Nb1.75Ti0.12Ta0.12Si0.01) for the process of refinement. The refinement results of the Y site are a site occupancy factor (s.o.f.) of 0.68 and an associated U eq of 0.09 Å2, if we allow the site occupancy and displacement parameters for the O1 atom to vary at the same time. However, the U eq of O1 is too high for this sample, leading to an inappropriate Y site occupancy. In order to determine the nature of the occupation of the critical Y site, we refined the structure as follows: (1) a refinement with the O1 atom omitted results in an R 1 = 0.039 and the largest peak in the difference Fourier is 4.39 e – at the Y site, which suggests an oxygen occupancy of a little over 50%; (2) using a range of reasonable fixed U iso values, i.e. 0.03 Å2, 0.04 Å2, 0.05 Å2 and 0.06Å2, refinement of the O1 occupancy then gives the s.o.f., R 1 and difference-Fourier values as shown in Table 7; (3) the U iso value of 0.06 is the most appropriate for this partially filled site, given the lowest R 1 value and the flattest difference map, which suggests this modelling appears to be the best; and (4) after the above steps, (Pb1.34Ca0.06□0.6)Σ2Nb2O6 YO#0.61 is therefore indicated, where O# denotes the amount of occupation needed to balance the formula charge recalculated into O atoms.
Table 7. Site occupancy factors (s.o.f), R 1 and difference-Fourier values for the Y site under different U iso values.

The empirical formula from the EPMA indicates that the Y site requires a charge of –0.69, thus 0.53 OH–, 0.08 O2– and 0.39 □ (vacancy) are assigned to the Y site. According to the Raman spectrum H2O is not present in hydroxyplumbopyrochlore. In summary, based on experiments and analyses, the chemical formula is given as (Pb1.34Ca0.03Fe0.01Sr0.01□0.61)Σ2(Nb1.75Ti0.12Ta0.12Si0.01)Σ2O6(OH0.53O0.08□0.39)Σ1. The simplified formula is (Pb1.5,□0.5)Nb2O6(OH), which requires PbO 54.92%, Nb2O5 43.60%, H2O 1.48%, total 100%.
The bond valences were calculated using the parameters of Brown and Altermatt (Reference Brown and Altermatt1985) with the site-occupancy factors given in Table 5. The bond-valance analysis for A and B positions are 1.37 valence units (vu) and 4.95 vu, respectively. The value for O1 is –1.08 vu in site Y (Table 8), which indicates that OH– exists in this site. No direct proof is given for Fe2+or Fe3+ because of its 0.17% low chemical contents. We used both Fe2+ and Fe3+ for structure refinement separately. No matter what element combinations are assigned, the Y site is occupied predominately by OH.
Table 8. Bond-valence analysis for hydroxyplumbopyrochlore.

Multiplicity is indicated by × → and ↓.
Hydroxyplumbopyrochlore possesses the pyrochlore structure. The A site is dominated by Pb, the B site by Nb and the Y site by OH. The crystal structure of hydroxyplumbopyrochlore is shown in Fig. 5. The structure can be described as a three-dimensional octahedral framework of corner-sharing NbO6 octahedra with Pb cations and OH groups in the interstices.

Fig. 5. The crystal structure of hydroxyplumbopyrochlore along [101].
Acknowledgements
The authors are grateful to Professor Yang Hexiong of Department of Geosciences, University of Arizona, for assistance in the structure refinement. We also thank Professor Ritsuro Miyawaki, the Chairman of the IMA–CNMNC, for his guidance and assistance during the new mineral application. Mr. Tai Zongyao of BRIUG helped with the electron microprobe analysis. The Saudi Geological Survey colleagues are also appreciated for their support during the field work and studies. We also thank reviewer Prof Peter Leverett for structure refinement advice and Dr. Stuart Mills, Dr. Oxana Karimova and an anonymous reviewer for their detailed reviews. This work is supported by the National Natural Science Foundation of China (Grant No. 42002044) and CNNC Science Fund for Talented Young Scholars (Grant No. CEQNYC2020-2).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2020.69