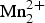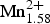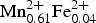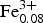Introduction
We present in this paper the complete characterization of a new amphibole of the magnesio-iron-manganese subgroup, clino-suenoite, ideally A□B![]() ${\rm Mn}_{2}^{2 +} $CMg5TSi8O22W(OH)2. The nomenclature of this subgroup has been significantly modified by the amphibole nomenclature scheme in force (Hawthorne et al., Reference Hawthorne, Oberti, Harlow, Maresch, Martin, Schumacher and Welch2012). That report recognizes that dominance of Mn2+ among B cations cannot be dealt with by a prefix – the use of which is hereafter confined to C cations – but requires a new rootname. Indeed, the presence of BCa2+ (ionic radius (hereafter abbreviated i.r.) 1.12 Å in the preferred 8-coordination; Shannon, Reference Shannon1976) or of B(Fe,Mg,Mn)2+ (i.r. 0.78, 0.72 and 0.83 Å, respectively, in 6-coordination) induces strong modifications in the crystal structure. B(Fe,Mg,Mn)2+ occur ~0.4 Å closer than Ca2+ (along the b direction) to the strip of octahedra. Because of the different anion arrangements around the M(4) site, magnesium-iron-manganese amphiboles may have either P21/m or C2/m monoclinic symmetry or Pnmn or Pnma orthorhombic symmetry, depending on their chemical composition and the pressure (P) and temperature (T) conditions of formation (see a short review of the different stereochemistries involved in Hawthorne and Oberti (Reference Hawthorne, Oberti, Hawthorne, Oberti, Della Ventura and Mottana2007) and in the discussion below). In contrast, calcium amphiboles have C2/m symmetry (with the one exception of joesmithite, APb2+ BCa2C(Mg3
${\rm Mn}_{2}^{2 +} $CMg5TSi8O22W(OH)2. The nomenclature of this subgroup has been significantly modified by the amphibole nomenclature scheme in force (Hawthorne et al., Reference Hawthorne, Oberti, Harlow, Maresch, Martin, Schumacher and Welch2012). That report recognizes that dominance of Mn2+ among B cations cannot be dealt with by a prefix – the use of which is hereafter confined to C cations – but requires a new rootname. Indeed, the presence of BCa2+ (ionic radius (hereafter abbreviated i.r.) 1.12 Å in the preferred 8-coordination; Shannon, Reference Shannon1976) or of B(Fe,Mg,Mn)2+ (i.r. 0.78, 0.72 and 0.83 Å, respectively, in 6-coordination) induces strong modifications in the crystal structure. B(Fe,Mg,Mn)2+ occur ~0.4 Å closer than Ca2+ (along the b direction) to the strip of octahedra. Because of the different anion arrangements around the M(4) site, magnesium-iron-manganese amphiboles may have either P21/m or C2/m monoclinic symmetry or Pnmn or Pnma orthorhombic symmetry, depending on their chemical composition and the pressure (P) and temperature (T) conditions of formation (see a short review of the different stereochemistries involved in Hawthorne and Oberti (Reference Hawthorne, Oberti, Hawthorne, Oberti, Della Ventura and Mottana2007) and in the discussion below). In contrast, calcium amphiboles have C2/m symmetry (with the one exception of joesmithite, APb2+ BCa2C(Mg3![]() ${\rm Fe}_{2}^{3 +} $)T(Si6Be2)O22W(OH)2, which has P2/a symmetry).
${\rm Fe}_{2}^{3 +} $)T(Si6Be2)O22W(OH)2, which has P2/a symmetry).
Hawthorne et al. (Reference Hawthorne, Oberti, Harlow, Maresch, Martin, Schumacher and Welch2012) proposed that a new rootname should be assigned to the composition A□B![]() ${\rm Mn}_{2}^{2 +} $CMg5TSi8O22W(OH)2. A subsequent Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA) decision to use the rootname suenoite for that composition was taken considering the data available in the literature for its orthorhombic Pnmn ferro-dominant counterpart, with ideal chemical formula: A□B
${\rm Mn}_{2}^{2 +} $CMg5TSi8O22W(OH)2. A subsequent Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA) decision to use the rootname suenoite for that composition was taken considering the data available in the literature for its orthorhombic Pnmn ferro-dominant counterpart, with ideal chemical formula: A□B![]() ${\rm Mn}_{2}^{2 + {\rm C}}{\rm Fe}_{5}^{2 +} $TSi8O22W(OH)2; this is now called proto-ferro-suenoite (vote 13-A, Williams et al., 2013; proponents: Oberti, Hawthorne and Kurosawa). This composition was first reported by Matsuura (Reference Matsuura1984), and later by Sueno et al. (Reference Sueno, Matsuura, Gibbs and Boisen1998), and its complete mineral description (under the name protomangano-ferro-anthophyllite, PMFA) was reported by Sueno et al. (Reference Sueno, Matsuura, Bunno and Kurosawa2002). The mineral occurs in pegmatites in Fukushima Prefecture and in a Mn mine in the Tochigi Prefecture, Japan, and has the empirical formula B(Mn1.40Fe0.60) C(Fe4.10Mg0.90)TSi8O22W(OH)2. In 2002, Mn was assumed to order as a B cation during the structure refinement. The crystal-chemical formula of PMFA was later confirmed by Zanazzi et al. (Reference Zanazzi, Nestola and Pasqual2010) on a sample provided by M. Kurosawa, for which the authors proposed the formula B(Mn1.39Fe0.59)C(Fe3.98Mg1.02)TSi8O22W(OH)2.
${\rm Mn}_{2}^{2 + {\rm C}}{\rm Fe}_{5}^{2 +} $TSi8O22W(OH)2; this is now called proto-ferro-suenoite (vote 13-A, Williams et al., 2013; proponents: Oberti, Hawthorne and Kurosawa). This composition was first reported by Matsuura (Reference Matsuura1984), and later by Sueno et al. (Reference Sueno, Matsuura, Gibbs and Boisen1998), and its complete mineral description (under the name protomangano-ferro-anthophyllite, PMFA) was reported by Sueno et al. (Reference Sueno, Matsuura, Bunno and Kurosawa2002). The mineral occurs in pegmatites in Fukushima Prefecture and in a Mn mine in the Tochigi Prefecture, Japan, and has the empirical formula B(Mn1.40Fe0.60) C(Fe4.10Mg0.90)TSi8O22W(OH)2. In 2002, Mn was assumed to order as a B cation during the structure refinement. The crystal-chemical formula of PMFA was later confirmed by Zanazzi et al. (Reference Zanazzi, Nestola and Pasqual2010) on a sample provided by M. Kurosawa, for which the authors proposed the formula B(Mn1.39Fe0.59)C(Fe3.98Mg1.02)TSi8O22W(OH)2.
The crystal structure of a monoclinic amphibole with a composition fairly compatible with the name clino-suenoite had been described by Hawthorne and Grundy (Reference Hawthorne and Grundy1977) under the name ‘zincian tirodite’, but a complete mineral characterization was not provided and the sample is no longer available. Therefore, a new search was made with the help of mineral collectors. The mineral clino-suenoite and its name were approved by the IMA-CNMNC (IMA2016-111) The holotype material is deposited in the mineralogical collections of the Museo di Mineralogia of the Università di Pavia, under the catalogue number 2016-01.
Occurrence and optical properties
The specimen studied in this work was found in the early 2000s at the Vedretta Inferiore di Scerscen (Lower Scerscen Glacier in Stalder et al., Reference Stalder, Wagner, Graeser and Stuker1998), Lanzada (46°16′9″N, 9°54′8″E), Valmalenco, Sondrio, Italy (Fig. 1). At this locality, clino-suenoite occurs in Mn-rich quartzite erratics in which the main mineralization consists of rhodonite, braunite, pyroxmangite, rhodochrosite, Mn-bearing calcite, kutnohorite, pyrophanite, tiragalloite, magnesio-riebeckite, manganberzeliite, aegirine–augite, tephroite, albite, Mn-rich muscovite, hausmannite, bixbyite, friedelite, hematite, clinochlore, romanèchite, ranciéite, Mn-bearing tremolite, jacobsite, anatase and johannsenite. Clino-suenoite is yellow or yellow-brown to brown, and may be found both as fibrous aggregates and as flattened elongated crystals, often in tufts up to 2 cm long (Figs 2 and 3), usually included in rhodonite or, more rarely, in small carbonate veins associated with tiragalloite and pyrophanite (Bedognè et al., Reference Bedognè, Montrasio and Sciesa1993; Reference Bedognè, Montrasio and Sciesa2006). Anatase occurs as yellow tabular crystals in fissures inside quartz schist, at the contact with the manganese mineralization. Pyrophanite and tiragalloite may also occur, the first as yellow-brown plates and cherry-red to blood-red hexagonal tabular crystals, often grouped in rosettes up to 1 cm in diameter, the second as orange to orange-red, transparent, wedge-like crystals up to several mm long (Bedognè et al., Reference Bedognè, Montrasio and Sciesa1993; 2006).

Fig. 1. View of the Vedretta inferiore (lower glacier) of Scerscen. The numbers indicate: 1 = rutile-rich dolomitic marble; 2 = manganese mineralization (picture taken by A. Montrasio in Bedognè et al., Reference Bedognè, Montrasio and Sciesa1993, and modified by M.E. Ciriotti)

Fig. 2. Orange-brown clino-suenoite crystal aggregate in rhodonite matrix from Vedretta inferiore di Scerscen. Field of view = 3.5 mm. (photo: R. Bracco)

Fig. 3. Fascicular yellow-orange crystals of clino-suenoite from Vedretta inferiore di Scerscen. Field of view = 6 mm. (photo: R. Bracco)
The geological setting of the Scerscen manganese orebody, like most Alpine manganese occurrences, belongs to the typical seafloor sequences of the Tethys Ocean and is dated mid to late Jurassic. The mineralization formed in shallow radiolaritic sediments of the Margna nappe sedimentary sequence, which were converted into quartzites by Alpine metamorphism.
In the type specimen, clino-suenoite occurs as pale yellow, honey-yellow, yellow-brown to light brown acicular to lamellar crystals embedded in a rhodonite matrix (Fig. 4). Its colour depends strongly on grain size, it does not fluoresce, and has a vitreous lustre. The calculated density of the clino-suenoite sample of this work is 3.175 g/cm3.

Fig. 4. The holotype rock specimen studied in this work.
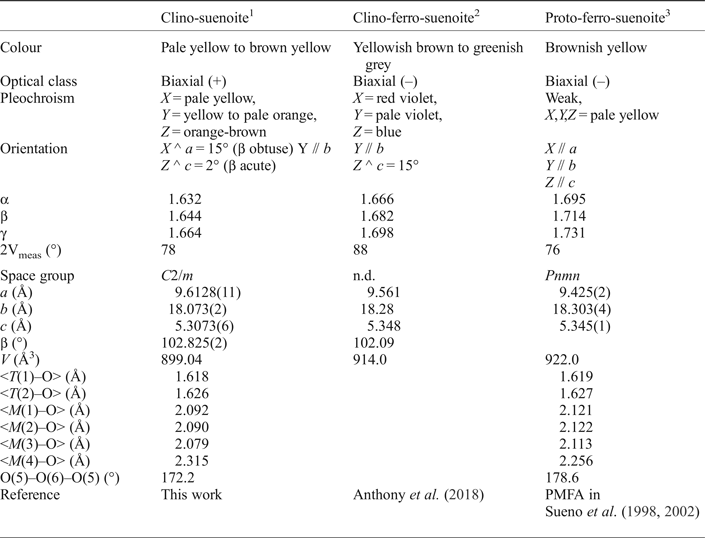
A spindle stage was used to orient a crystal for measurement of refractive indices and 2V by extinction curves (Bartelmehs et al., Reference Bartelmehs, Bloss, Downs and Birch1992). The optical orientation was determined by transferring the crystal from the spindle stage to a single-crystal diffractometer and measuring the relative axial relations by X-ray diffraction. In transmitted plane-polarized light, clino-suenoite is pleochroic (X = pale yellow to yellow, Y = yellow to pale orange and Z = orange brown). It is biaxial (+), α = 1.632(2), β = 1.644(2), γ = 1.664(2), 2Vmeas. = 78(2) and 2Vcalc = 76.3°. The dispersion is weak (v > r), and the orientation is: X ˄ a = 15o (in β obtuse), Y || b and Z ˄ c = 2o (in β acute).
The compatibility index (1 – (Kp/Kc); Mandarino, Reference Mandarino1981) is 0.014 (superior).
Single-crystal and powder diffraction analysis
Diffraction data for a lamellar single crystal 350 µm × 100 µm × 40 µm in size (no. 1316 in the amphibole database at CNR-IGG Pavia) were collected in the θ range 2–30° with a Bruker-AXS CCD diffractometer using graphite-monochromatized MoKα X-radiation (λ = 0.7107 Å). Omega rotation frames (scan width 0.3°, scan time 20 s and sample-to-detector distance 50 mm) were processed with the SAINT software (Bruker, Reference Bruker2003) and intensities were corrected for Lorentz and polarization effects; absorption effects were empirically evaluated with the SADABS software (Krause et al., Reference Krause, Herbst-Irmer, Sheldrick and Stalkeand2015) and an absorption correction was applied to the data. A total of 6704 collected reflections was reduced to 1355 unique reflections (mean redundancy = 5 and R int = 4.4%). Following the procedures and terminology described in Hawthorne et al. (Reference Hawthorne, Ungaretti and Oberti1995) and Oberti et al. (Reference Oberti, Ungaretti, Cannillo and Hawthorne1992), scattering curves for fully ionized chemical species were used at sites where chemical substitutions occur; neutral vs. ionized scattering curves were used at the T and anion sites [except O(3)]. Full-matrix unweighted least-squares refinement on the 971 reflections with I > 3 σI was done with a program locally written to handle complex solid-solutions (Cannillo et al., Reference Cannillo, Germani and Mazzi1983) and gave R obs = 4.3% and R all = 6.4%. Crystallographic details are summarized in Table 1, and refined atom coordinates and displacement parameters, as well as selected bond lengths and angles are given in Tables 2 and 3, respectively. Observed structure factors have been deposited with the crystallographic information file as Supplementary materials (see below). The a:b:c ratio calculated from the unit-cell parameters is 0.532:1:0.294
Table 1. Miscellaneous information for holotype clino-suenoite (crystal 1316).

Table 2. Atom coordinates, refined site-scattering values (ss, electrons pfu) and atom-displacement parameters (B eq, Å2;βij x 104) for clino-suenoite crystal 1316.

Table 3. Selected interatomic distances (Å), angles (°), tetrahedral and octahedral angle variances (TAV, OAV, °^2) and quadratic elongations (TQE, OQE) according to Robinson et al. (Reference Robinson, Gibbs and Ribbe1971) in clino-suenoite crystal 1316.

Powder X-ray diffraction data (CuKα, λ = 1.54178 Å) were obtained using the XPREP utility of SAINT (Bruker, Reference Bruker2003), which generates a 2D powder diffractogram (Debye-Scherrer technique) starting from the F obs2 collected on the single crystal and taking into account solely the information concerning the unit-cell dimensions and the Laue symmetry. No Lorentz and polarization corrections were applied. Data are given in Table 4.
Table 4. Powder X-ray diffraction data for clino-suenoite crystal 1316.

Note: the strongest ten reflections are in bold. Only peaks with I rel ≥ 5 are reported.
Chemical analysis
Chemical analysis (10 points) on the crystal used for structure refinement was undertaken using a with a Cameca SX-100 electron microprobe (wavelength dispersive spectroscopy mode, 15 kV, 20 nA, count time 20 s and 5 µm beam diameter). Chromium and V are below their detection limits. The standards and dispersive crystals used are as follows: Si and Ca: diopside (TAP); Ti: titanite (LPET); Al: andalusite (TAP); Fe: fayalite (LLiF); Mn: spessartine (LLiF); Mg: forsterite (LTAP); Zn: gahnite (LLiF); Ni: pentlandite (LLiF); Na: albite (TAP); K: orthoclase (LPET); F: fluoro-riebeckite (TAP); and Cl: tugtupite (LPET). The amount of H2O used in the calculation is that required for F+OH+Cl = 2 anions pfu and 15.04 cations pfu (as required to obtain a non-negative value for Fe2+). The oxide wt.% and the calculated unit-formula are reported in Table 5. The proposed empirical formula for crystal 1316 is: ANa0.04B(![]() ${\bf Mn}^{\bf 2 +}_{\bf 1.58} $ Ca0.26Na0.16)Σ2.00 C(Mg4.21
${\bf Mn}^{\bf 2 +}_{\bf 1.58} $ Ca0.26Na0.16)Σ2.00 C(Mg4.21![]() ${\rm Mn}_{0.61}^{2 +} \; $Zn0.01Ni0.01
${\rm Mn}_{0.61}^{2 +} \; $Zn0.01Ni0.01![]() ${\rm Fe}_{0.08}^{3 +} $ Al0.04)Σ5.00TSi8.00O22 W[(OH1.94F0.06)]Σ2.00 (where the dominant cations/anions are in bold). Note that the Fe3+ content is that required for electroneutrality. The end-member formula of clino-suenoite is A□B
${\rm Fe}_{0.08}^{3 +} $ Al0.04)Σ5.00TSi8.00O22 W[(OH1.94F0.06)]Σ2.00 (where the dominant cations/anions are in bold). Note that the Fe3+ content is that required for electroneutrality. The end-member formula of clino-suenoite is A□B![]() ${\rm Mn}_{2}^{2 +} $CMg5TSi8O22W(OH)2, which requires SiO2 57.08, MgO 23.93, MnO 16.85 H2O 2.14, total 100.00 wt.%.
${\rm Mn}_{2}^{2 +} $CMg5TSi8O22W(OH)2, which requires SiO2 57.08, MgO 23.93, MnO 16.85 H2O 2.14, total 100.00 wt.%.
Table 5. Chemical composition (average of 10 points), unit formula (based on 24 anions and 15.04 cations; cf. text) and a comparison between observed and calculated site scattering values for clino-suenoite (1316).

* calculated based on 15.04 cations and 24 (O, OH, F, Cl) with (OH + F + Cl) = 2 apfu.
The crystal-chemistry of clino-suenoite
The site populations were obtained by distributing the ions of the unit formula under the constraints of the refined site-scattering values (Hawthorne et al., Reference Hawthorne, Ungaretti and Oberti1995) and mean bond lengths (for the C cations occurring at the M(1), M(2) and M(3) sites). The correct distribution of Mn among the M(1), M(2) and M(3) sites could be determined because of the very low amount of Fe2+ in the formula, and was checked via the refined mean bond-lengths. The Mn site preference is M(4) >> M(1) > M(2) >> M(3). The results are reported in Table 6. The agreement between the refined and calculated site-scattering values is close.
Table 6. Site populations for clino-suenoite, crystal 1316. There is close agreement between the refined values of site-scattering (ss, electrons per formula unit) and mean bond-lengths (mbl, Å) and those calculated based on the proposed site-populations*.

*Hawthorne et al. (Reference Hawthorne, Ungaretti and Oberti1995)
The residual electron density at the A site is very low, in agreement with the presence of 0.04 Na atoms per formula unit (apfu) in the chemical analysis; therefore A sites were not inserted in the refined model. The M(4) site is [6 + 2]-coordinated, as expected where small cations are the dominant B cations: the M(4)–O(5) distance of 3.068 Å is even longer than the distance M(4)–T(2), i.e. 2.994 Å. The <T–O> distances are very short, confirming that no TAl is present, and the M(1,3) octahedra are quite distorted, in accord with the presence of Mn2+. Oberti and Ghose (Reference Oberti and Ghose1993; Fig. 3) observed that in monoclinic amphiboles, the value of the β angle is linearly related to the average ionic radius of the B cations; the β angle measured for clino-suenoite is in perfect agreement with this behaviour.
Relation with other species and a review of related compositions
As noted in the Introduction, Sueno et al. (Reference Sueno, Matsuura, Gibbs and Boisen1998, Reference Sueno, Matsuura, Bunno and Kurosawa2002) described an orthorhombic amphibole (Pnmn symmetry) with the empirical formula B(Mn1.40Fe0.60)C(Fe4.10Mg0.90)TSi8O22W(OH) (root composition A□B![]() ${\rm Mn}_{2}^{2 +{\rm C}}{\rm Fe}_{5}^{2 +} $TSi8O22 W(OH)2) and called it ‘protomangano-ferro-anthophyllite’ (PMFA). According to the rules currently in force, this mineral must now be referred to as proto-ferro-suenoite (Williams et al., Reference Williams, Hatert, Pasero and Mills2013). Proto-ferro-suenoite was discovered in a fayalite pegmatite associated with laihunite, magnetite and quartz and in mineralized blocks associated with pyroxmangite, rhodonite, rhodochrosite and spessartine at: (1) Suishoyama pegmatite, Iizaka, Kawamata, Fukushima Prefecture, Tohoku Region; and (2) Nippyo and Yokoneyama mines, Awano, Kanuma City, Tochigi Prefecture, Kanto Region, both in Honshu Island, Japan. It occurs as fibrous yellow-brown crystals, a few mm long, sometimes in aggregates resembling sheaves of wheat (Sueno et al., Reference Sueno, Matsuura, Bunno and Kurosawa2002).
${\rm Mn}_{2}^{2 +{\rm C}}{\rm Fe}_{5}^{2 +} $TSi8O22 W(OH)2) and called it ‘protomangano-ferro-anthophyllite’ (PMFA). According to the rules currently in force, this mineral must now be referred to as proto-ferro-suenoite (Williams et al., Reference Williams, Hatert, Pasero and Mills2013). Proto-ferro-suenoite was discovered in a fayalite pegmatite associated with laihunite, magnetite and quartz and in mineralized blocks associated with pyroxmangite, rhodonite, rhodochrosite and spessartine at: (1) Suishoyama pegmatite, Iizaka, Kawamata, Fukushima Prefecture, Tohoku Region; and (2) Nippyo and Yokoneyama mines, Awano, Kanuma City, Tochigi Prefecture, Kanto Region, both in Honshu Island, Japan. It occurs as fibrous yellow-brown crystals, a few mm long, sometimes in aggregates resembling sheaves of wheat (Sueno et al., Reference Sueno, Matsuura, Bunno and Kurosawa2002).
The different crystallographic symmetries observed in amphiboles containing small B cations (i.e. in the magnesium-iron-manganese subgroup) derive from the different stacking of the I beams along the a direction: a ++++ stacking corresponds to the monoclinic structure (possible space groups C2/m and P21/m), whereas alternating positive and negative stackings correspond to the orthorhombic structure (++ –– to Pnma, implying a doubling of the a edge, and +–+– to Pnmn) (Hawthorne and Oberti, Reference Hawthorne, Oberti, Hawthorne, Oberti, Della Ventura and Mottana2007, fig. 14). The relative stability of the monoclinic and orthorhombic structures is determined mainly by the B cations, the Ca- and Na-rich compositions having monoclinic C2/m symmetry. Evans and Ghiorso (Reference Evans and Ghiorso1995) showed that orthorhombic (Pnma) anthophyllite is more stable than cummingtonite for Fe/(Fe+Mg) > 0.10 at 600–700°C, the opposite of what is observed for the Fe- and Mn-rich species. In monoclinic amphiboles, P21/m symmetry is preferred to C2/m symmetry at high Mg/Mg+Fe values. Displacive phase transitions are observed at different T values, as shown by many studies reviewed in Welch et al. (Reference Welch, Cámara, Della Ventura, Iezzi, Hawthorne, Oberti, Della Ventura and Mottana2007).
The available data for amphiboles related to the rootname suenoite are given in Table 7. Comparison of the geometry observed in clino-suenoite and in proto-ferro-suenoite is quite interesting. The sizes of the T(1–2) tetrahedra are very similar, but the M(1–3) octahedra are larger in proto-ferro-suenoite, in agreement with its higher CFe2+ content. As a result, the double chain of tetrahedra is almost completely extended (as measured by the O(5)–O(6)–O(5) angle) in proto-ferro-suenoite, a situation which is never observed in monoclinic amphiboles. The M(4) site in amphibole acts as an hinge between the double chain of tetrahedra and the strip of octahedra. In proto-ferro-suenoite, it is not only much smaller, (which again is consistent with the low Ca2+ content, 0.03 apfu), but has a different coordination. The individual distances are: M(4)–O(2) = 2.155 Å, M(4)–O(4) = 2.032 Å and M(4)–O(6) = 2.582 Å (Sueno et al., Reference Sueno, Matsuura, Bunno and Kurosawa2002) so that a [4 + 2] coordination could even be considered.
Table 7. A comparison of the optical and crystallographic properties reported for the three amphiboles related to the rootname suenoite.
(1): ANa0.04B(![]() ${\rm Mn}_{1.58}^{2+} $Ca0.26Na0.16)Σ2.00C(Mg4.21
${\rm Mn}_{1.58}^{2+} $Ca0.26Na0.16)Σ2.00C(Mg4.21![]() ${\rm Mn}_{0.61}^{2 +} {\rm Fe}_{0.04}^{2 +} $Zn0.01Ni0.01
${\rm Mn}_{0.61}^{2 +} {\rm Fe}_{0.04}^{2 +} $Zn0.01Ni0.01![]() ${\rm Fe}_{0.08}^{3 +} $Al0.04)Σ5.00 TSi8.00O22W[(OH)1.94F0.06]Σ2.00
${\rm Fe}_{0.08}^{3 +} $Al0.04)Σ5.00 TSi8.00O22W[(OH)1.94F0.06]Σ2.00
(2): (![]() ${\rm Fe}_{3.10}^{2 +} $ Mg2.42Mn0.96Ca0.33
${\rm Fe}_{3.10}^{2 +} $ Mg2.42Mn0.96Ca0.33![]() ${\rm Fe}_{0.21}^{3 +} $ Na0.07Ti0.01K0.01)Σ7.11(Si7.80Al0.16) Σ7.96O22 [(OH)1.99F0.03]Σ2.02
${\rm Fe}_{0.21}^{3 +} $ Na0.07Ti0.01K0.01)Σ7.11(Si7.80Al0.16) Σ7.96O22 [(OH)1.99F0.03]Σ2.02
(3): B(Mn1.38Fe0.59Ca0.03)C(Fe4.10Mg0.9)Si8O22W(OH)2
Monoclinic amphiboles with composition close to A□B![]() ${\rm Mn}_{2}^{2 +} $CMg5TSi8O22W(OH)2 have previously been reported as ‘tirodite’ after the Tirodi locality (Madhya Pradesh, India; Dunn and Roy, Reference Dunn and Roy1938; Segeler, Reference Segeler1961; Hawthorne and Grundy, Reference Hawthorne and Grundy1977) and later as ‘manganocummingtonite’. The name ‘tirodite’ was discredited by Leake et al. (Reference Leake, Woolley, Arps, Birch, Gilbert, Grice, Hawthorne, Kato, Kisch, Krivovichev, Linthout, Laird, Mandarino, Maresch, Nickel, Rock, Schumacher, Smith, Stephenson, Ungaretti, Whittaker and Guo1997), and the name ‘manganocummingtonite’ was discredited by Hawthorne et al. (Reference Hawthorne, Oberti, Harlow, Maresch, Martin, Schumacher and Welch2012), who stated that the prefix mangano- has to be used only when Mn2+ is dominant among C cations. Analyses close to the ideal composition A□B
${\rm Mn}_{2}^{2 +} $CMg5TSi8O22W(OH)2 have previously been reported as ‘tirodite’ after the Tirodi locality (Madhya Pradesh, India; Dunn and Roy, Reference Dunn and Roy1938; Segeler, Reference Segeler1961; Hawthorne and Grundy, Reference Hawthorne and Grundy1977) and later as ‘manganocummingtonite’. The name ‘tirodite’ was discredited by Leake et al. (Reference Leake, Woolley, Arps, Birch, Gilbert, Grice, Hawthorne, Kato, Kisch, Krivovichev, Linthout, Laird, Mandarino, Maresch, Nickel, Rock, Schumacher, Smith, Stephenson, Ungaretti, Whittaker and Guo1997), and the name ‘manganocummingtonite’ was discredited by Hawthorne et al. (Reference Hawthorne, Oberti, Harlow, Maresch, Martin, Schumacher and Welch2012), who stated that the prefix mangano- has to be used only when Mn2+ is dominant among C cations. Analyses close to the ideal composition A□B![]() ${\rm Mn}_{2}^{2 +} $CMg5TSi8 O22W(OH)2 are listed from 55 localities in mindat.org [https:// www.mindat.org/]. They typically occur in metamorphic manganese ores, and a closer examination of papers on the petrology and ore geology of these deposits would undoubtedly increase the number of localities.
${\rm Mn}_{2}^{2 +} $CMg5TSi8 O22W(OH)2 are listed from 55 localities in mindat.org [https:// www.mindat.org/]. They typically occur in metamorphic manganese ores, and a closer examination of papers on the petrology and ore geology of these deposits would undoubtedly increase the number of localities.
Incidentally, the composition Ca0.1Mn1.9Mg1.25 ![]() ${\rm Fe}_{3.56}^{2 +} {\rm Fe}_{0.38}^{3 +} $Si7.81O22(OH)2 described from Dannemora, Sweden, and thus called ‘dannemorite’ (first reported by Kenngott (Reference Kenngott1855) and Dana (Reference Dana1892); see Anthony et al. (Reference Anthony, Bideaux, Bladh and Nichols2018) at http://www.handbookofmineralogy.org/pdfs/dannemorite.pdf) should hereafter be called clino-ferro-suenoite (Table 7). Kenngott (Reference Kenngott1855) referred to the analysis of Erdmann (Reference Erdmann1851): B(Mn1.13Fe0.74Ca0.12)Σ1.99C(Fe2+4.04Mg0.69Fe3+0.27)Σ5.00T(Si7.73Al0.27)Σ8O22W(OH)2.
${\rm Fe}_{3.56}^{2 +} {\rm Fe}_{0.38}^{3 +} $Si7.81O22(OH)2 described from Dannemora, Sweden, and thus called ‘dannemorite’ (first reported by Kenngott (Reference Kenngott1855) and Dana (Reference Dana1892); see Anthony et al. (Reference Anthony, Bideaux, Bladh and Nichols2018) at http://www.handbookofmineralogy.org/pdfs/dannemorite.pdf) should hereafter be called clino-ferro-suenoite (Table 7). Kenngott (Reference Kenngott1855) referred to the analysis of Erdmann (Reference Erdmann1851): B(Mn1.13Fe0.74Ca0.12)Σ1.99C(Fe2+4.04Mg0.69Fe3+0.27)Σ5.00T(Si7.73Al0.27)Σ8O22W(OH)2.
Vassileva and Bonev (Reference Vassileva and Bonev2001) analysed amphiboles occurring in Pb-Zn skarn ore deposits in the Madan district, Central Bulgaria, and found compositions ranging from manganoan ferro-actinolite to clino-suenoite and clino-ferro-suenoite. Among the latter, the TAl and A(Na,K) contents are always zero or very low, whereas the BCa content ranges from 0.14 to 0.28 apfu. These amphiboles were described as “developed by partial replacement of ferroan johansennite”, occur as fibres of fibrous aggregates with rounded voids formed by the dissolution of calcite, and are associated with rhodonite, quartz and Mn-rich ilvaite.
Clino-suenoite can form a continuous series with cummingtonite A□BMg2CMg5TSi8O22W(OH)2 and grunerite A□BFe2+CFe2+TSi8O22W(OH)2, which are commonly associated with metamorphic manganese ores. Many of the analyses reported in the literature (cf. mindat.org) show MnO ≈ 8%, which is more or less equivalent to 1 apfu, thus close to the midpoint between cummingtonite and/or grunerite and clino-suenoite.
Acknowledgements
Andrew Christy, Peter Leverett and Ritsuro Miyawaki are grateful acknowledged for their helpful comments. FCH was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada and Innovation Grants from the Canada Foundation for Innovation.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/minmag.2017.081.034