We may summarize our conclusions as follows. If the rotating doublets have quite different angular velocitiesinitially, then they repel each other with a force (R) given by

where
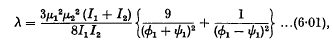
and
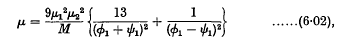
ø1 and ψ1 being the (constant) angular velocities of the two doublets.
If the doublets have the same angular velocities initially, and the same moments of inertia, then over a certain range of r we have

where
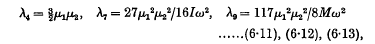
and ω is the common value of the angular velocities of the doublets. When the doublets correspond to hydrogen atoms in their principal quantum orbits, the range of distance becomes 5 Å. to 50 Å. and the formula for R reduces to
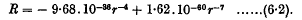
This is a law of force of the type found empirically by Lennard-Jones for helium, neon, and argon. The attractive term in this formula is larger than the attractive terms found by Lennard-Jones. The repulsive term, however, which leads to a “diameter” of 3·31 Å., is in very satisfactory agreement with the repulsive terms found by Lennard-Jones.