Introduction
Lichenization is a symbiotic ecological strategy that is widely distributed among the fungi, and in which fungi are obligately or facultatively associated with a photosynthetic partner as a carbon source. In these morphologically and ecologically diverse symbioses, many of the fungi are both perennial and macroscopically visible, facilitating their study. Although the field of species interactions had long been limited by morphologically enigmatic photosynthetic partners (Kroken & Taylor Reference Kroken and Taylor2000; Škaloud & Peksa Reference Škaloud and Peksa2010) and cryptic fungal lineages (Kroken & Taylor Reference Kroken and Taylor2001; Leavitt et al. Reference Leavitt, Esslinger, Divakar and Lumbsch2012; Lücking et al. Reference Lücking, Dal-Forno, Sikaroodi, Gillevet, Bungartz, Moncada, Yánez-Ayabaca, Chaves, Coca and Lawrey2014), molecular tools have allowed huge advances to be made in the recognition of patterns of association and the description of diversity of these and other symbiotic lineages (Grube & Kroken Reference Grube and Kroken2000; Letsch et al. Reference Letsch, Muller-Parker, Friedl and Lewis2009; Fernández-Martínez et al. Reference Fernández-Martínez, de los Ríos, Sancho and Pérez-Ortega2013). A major theme in recent research on lichens has been the description of diversity encompassed within lichen symbioses and the elucidation of factors promoting and maintaining this diversity (Grube & Kroken Reference Grube and Kroken2000; Piercey-Normore & DePriest Reference Piercey-Normore and DePriest2001; Yahr et al. Reference Yahr, Vilgalys and DePriest2006; Fernández-Mendoza et al. Reference Fernández-Mendoza, Domaschke, García, Jordan, Martín and Printzen2011; Werth Reference Werth2012; del Campo et al. Reference del Campo, Catala, Gimeno, del Hoyo, Martinez-Alberola, Casano, Grube and Barreno2013; O’Brien Reference O’Brien2013a , Reference O’Brien b ).
Photobionts in lichens have been studied in only a very small number of the lichen fungi currently known (Honegger Reference Honegger2008; Voytsekhovich et al. Reference Voytsekhovich, Mikhailyuk and Darienko2011b ), and genetic studies have until recently been mostly limited to those partnerships formed with the conspicuous macrolichen lineages (but see Beck et al. Reference Beck, Friedl and Rambold1998; Beck Reference Beck1999). However, most of the diversity of lichen fungi in any given area comes from the inconspicuous and often less tractable microlichens (e.g. nearly 80% of the well-studied British lichen mycota belong to the microlichens; Yahr et al. Reference Yahr, Coppins and Ellis2011), where it is expected that most of the photobiont diversity remains unexplored.
Recent work has begun to expand the pool of lichens studied to include these much more diverse species, and in less well investigated parts of the world (e.g. Thus et al. 2011; Ruprecht 2012; Muggia et al. 2014). Even in the relatively well-studied macrolichens, the genetic diversity at the strain level is higher than was once expected based on morphology alone, with many undescribed and morphologically cryptic lineages (Škaloud & Peksa Reference Škaloud and Peksa2010; Mansournia et al. Reference Mansournia, Wu, Matsushita and Hogetsu2012; Leavitt et al. Reference Leavitt, Nelsen, Lumbsch, Johnson and St. Clair2013; Sadowska-Des et al. Reference Sadowska-Des, Dal Grande, Lumbsch, Beck, Otte, Hur, Kim and Schmitt2014). In general, it is suspected that the diversity of photobionts is much higher than is currently known.
The genus Micarea s. lat. has been shown using molecular data to be polyphyletic, consistent with suggestions from morphological studies in the monographs by Coppins (Reference Coppins1983) and Czarnota (Reference Czarnota2007). In the former work, Coppins described three groups of species differentiated based on their photobionts: the small and distinctive ‘micareoid’ type found in ‘most species’ including the type, Micarea prasina Fr.; the larger and irregular cells in the M. sylvicola (Flot.) Vězda & Wirth group (including M. bauschiana (Körb) V. Wirth & Vězda, M. lutulata (Nyl.) Coppins, and M. tuberculata (Sommerf.) R. A. Anderson); and the large globose cells from M. intrusa (Th. Fr.) Coppins & Killias (Coppins Reference Coppins1983). He suggested that these latter two groups may require further taxonomic work. Indeed, more recently M. intrusa has been transferred to Scoliciosporum (Hafellner Reference Hafellner2004), with which the similarities in both thallus and photobiont characters had already been noted. Along similar lines, the M. sylvicola group has recently been elevated to the generic level as Brianaria, in recognition of several important characters, including the different photobiont, which are now known to correlate with phylogenetic distinctiveness for this separate lineage in the Psoraceae (Ekman & Svensson Reference Ekman and Svensson2014). Photobionts from Brianaria (Psoraceae) are irregular, larger, and have indistinct haustorial pegs, whereas those in Micarea s. str. (Pilocarpaceae) have so-called ‘micareoid’ algae, with more delicate haustorial connections and regular, smaller cells (Coppins Reference Coppins1983). Until very recently, the identity of the photobionts in any of these groups had not been studied systematically.
A recent study (Voytsekhovich et al. Reference Voytsekhovich, Dymytrova and Nadyeina2011a ) investigated the algae from several species of fungi belonging to Micarea s. str. and found that the photobiont in most samples (8 of 11) belonged morphologically to the genus Elliptochloris, confirming very early work by Brunner (Reference Brunner1985). One specimen contained Pseudococcomyxa sp. and another two accessions associated with several algal species simultaneously (Voytsekhovich et al. Reference Voytsekhovich, Dymytrova and Nadyeina2011a ). Pseudococcomyxa has only rarely been reported as the photobiont of lichen fungi (Muggia et al. Reference Muggia, Baloch, Stabentheiner, Grube and Wedin2010), but this fact certainly reflects problems using morphology to distinguish Pseudococcomyxa from Coccomyxa, and recent work by Pröschold et al.Reference Pröschold, Darienko, Silva, Reisser and Krienitz demonstrated that the authentic strain of P. simplex SAG 216-9a belongs within the genus Coccomyxa in the Elliptochloris clade (2011). On the other hand, some species from the genus Elliptochloris (for example, Elliptochloris bilobata Tsch.-Woess) are widely recognized as symbionts in lichens (Tschermak-Woess Reference Tschermak-Woess1985) and as free-living algae of sub-aerial habitats (Eliáš et al. Reference Eliáš, Neustupa and Škaloud2008; Tsarenko Reference Tsarenko2011). Other species in the genus are recognized as photobionts in marine invertebrates (Letsch et al. Reference Letsch, Muller-Parker, Friedl and Lewis2009) or as free-living terrestrial algae (Ettl & Gärtner Reference Ettl and Gärtner1995). Using molecular data, E. bilobata has also been reported as the symbiont of several other lichens, including Verrucaria sublobulata Eitner ex Servít, using a combined morphological and molecular approach (Thüs et al. Reference Thüs, Muggia, Perez-Ortega, Favero-Longo, Joneson, O’Brien, Nelsen, Duque-Thues, Grube and Friedl2011). However, given the relatively wide variation in cell morphology contrasted with the relative paucity of discrete characters visible with light microscopy, a certain degree of unrecognized genetic variation may be expected in lichen photobionts, particularly based on results of other molecular studies of chlorophyte algae (e.g. Darienko et al. Reference Darienko, Gustavs, Mudimu, Menendez, Schumann, Karsten, Friedl and Pröschold2010; Škaloud & Peksa Reference Škaloud and Peksa2010; Thüs et al. Reference Thüs, Muggia, Perez-Ortega, Favero-Longo, Joneson, O’Brien, Nelsen, Duque-Thues, Grube and Friedl2011; Muggia et al. Reference Muggia, Pérez-Ortega, Kopun, Zellnig and Grube2014).
This study was undertaken to examine the range of photobionts within Micarea s. str., recognizing that several lineages of green algae are likely to be involved in the segregate genera (Coppins Reference Coppins1983), which we do not treat at this time. We used culturing techniques to isolate algae and sequenced cleaned, fresh specimens directly, in addition to obtaining sequences from uni-algal cultures from eight Micarea species in the Pilocarpaceae.
Methods
Sample collection
New samples were collected in 2013 from several locations in Great Britain (Table 1) and were immediately frozen to promote and prolong viability (Honegger Reference Honegger1999). These collections have been deposited in the herbarium of the Royal Botanic Garden Edinburgh (E). Four specimens of Micarea from Ukraine and the cultures arising from these were also examined (from Voytsekhovich et al. Reference Voytsekhovich, Dymytrova and Nadyeina2011a ).
Table 1 Specimens examined with collection details and newly deposited GenBank accession numbers.

Letters in parentheses following GenBank numbers indicate if sequences are direct from fresh material (F) or from cultures (C).
Species identification
Collections were identified using standard morphological characteristics and thin-layer chromatography (TLC) for separation of morphologically similar species in the M. prasina group. TLC was performed using solvent systems A and G, following standard procedures (Orange et al. Reference Orange, James and White2001).
Culturing
All samples were washed thoroughly to remove superficial contamination by placing them in a muslin bag and running a jet of water over them for three hours. Samples for culturing were selected under a dissecting microscope to find a clean section of washed thallus which had no obvious epiphytes or other lichens nearby. For one specimen (AF4), direct inoculations were picked from the medulla of the washed thallus, and for the remaining specimens, a piece of thallus was ground between two microscope slides to form a suspension, from which algal cells with hyphal connections were selected on an inverted microscope (Ahmadjian Reference Ahmadjian1967). All samples were inoculated onto Petri dishes containing agarized Bold’s Basal Medium at 1·5%. Cultures were grown in growth cabinets under 12 h light/dark cycles at 15 °C. After the first inoculations, up to four separate colonies from each plate were maintained separately to test for heterogeneity in the original sample. Representative cultures were deposited in Culture Collection of Algae and Protozoa (CCAP), Oban, Scotland.
Morphology
Established cultures were examined to ensure replicate inoculations were homogeneous, and this examination was carried out using light microscopy during culture conditions, observing both vegetative as well as reproductive cells. Measurements were made using an Olympus BX51 microscope (Olympus Corp., Tokyo, Japan) equipped with a differential interference contrast (DIC). The photographs were taken using an Olympus Z5060 camera.
Molecular methods
Material for extraction was chosen as follows: for the fungal partner, slices of cleaned apothecia were chosen and placed in an extraction tube; for the photobiont from the fresh samples, an apothecial slice was taken and examined under a compound microscope at ×400. There were algal cells present in the thallus material below the apothecia, and these were checked to ensure that they were all morphologically similar and had hyphal connections. Any parts of the material which were seen to contain epiphytic algae that did not have a hyphal connection were cut away and not included in the material for DNA extraction. For cultured algal material, two independent extractions were made from replicates of each established culture. A mixer-mill (Qiagen Tissuelyser II) was used to grind fungal samples in preparation for DNA extraction, for two 30 s cycles at 20 beats per s. For algal cultures, cells were scraped into tubes and ground with a mini-pestle in extraction buffer. Qiagen Plant MiniKits were used for extraction according to the manufacturer’s instructions, but with an extended incubation time at the start (1 h at 65°C), and reduced final elution volumes (50 µl). The DNA was quantified using Nanovue (GE), and DNA concentrations were standardized to c. 2–5 ngµl-1 across samples by vacuum centrifuge or dilution with sterile water.
Algal rbcL and nuclear small subunit ribosomal (nucSSU) DNA were amplified using PRASF1 and PRASR2 (Sherwood et al. Reference Sherwood, Garbary and Sheath2000) and NS1 and NS4 (White et al. Reference White, Bruns, Lee and Taylor1990), respectively, from both fresh collections and from cultures. Fungal amplification reactions each contained 2·5 µl of 10×NH4 reaction buffer (Bioline, London, UK), 2·5 mM MgCl2 (Bioline), 0·2 mM dNTPs, 0·3 µM of each primer, 0·125 units of BIOTAQ DNA polymerase (Bioline), and 1 µl genomic DNA with double-distilled water for a total of 25 µl. The fungal mtSSU amplifications were performed with a programme of 95°C for 2 min, followed by 35 cycles of 95°C for 30 s, 55°C for 90 s, and 72°C for 30 s, with a final 7 min at 72°C. For algal rbcL and nucSSU amplifications, the same PCR reaction recipes were used except that primer concentrations were increased to 0·8 µM, and 5 µl of TBT-PAR (Samarakoon et al. Reference Samarakoon, Wang and Alford2013) was added. Cycling conditions were 95°C for 2 min, followed by 35 cycles of 95°C for 45 s, 47°C (rbcL) or 52°C (nucSSU) for 90 s, and 72°C for 120 s, with a final 7 min at 72°C. Products from PCR amplifications were cleaned using ExoSAP-IT (Affymetrix, Carlsbad, California), according to the manufacturer’s instructions.
Cycle sequencing was conducted with a BigDye Terminator v 3.1 100 Reaction Ready kit (Applied Biosystems, Carlsbad, California, USA) following the manufacturer’s protocol and using the same primers as for the original PCR. Sequences were analyzed by The GenePool (University of Edinburgh, Edinburgh, UK), and electropherograms were visually inspected and edited using Geneious (v 6.1.4, Biomatters). All sequences were subjected to megaBLAST searches (NCBI) to ensure forward and reverse sequences represented the same strains. Alignments were initially generated by adding newly assembled sequences and those with high BLAST matches from GenBank to matrices generated by Thüs et al. (Reference Thüs, Muggia, Perez-Ortega, Favero-Longo, Joneson, O’Brien, Nelsen, Duque-Thues, Grube and Friedl2011). Three separate alignments were created: rbcL, nucSSU and combined matrices. Datasets for both rbcL and nucSSU were analyzed independently and checked for conflicting support values on the branches prior to creation of a combined alignment. Sequences included in all analyses are listed in Appendix 1 (see Supplementary Material Appendix S1, available on-line). Alignments were exported to Mesquite (Maddison & Maddison Reference Maddison and Maddison2011), where they were examined by eye and ambiguous sites and introns (in nucSSU) were delimited and removed. Phylogenetic analysis was completed using both maximum likelihood (ML) using partitioned models for rbcL (codon positions 1, 2 and 3) and combined matrices (nucSSU, codon position 1, 2, and 3), and non-partitioned for nucSSU data alone. ML analysis was performed using RaxML HPC Black Box on the Cipres Web Portal (v 7.2+), which uses the GTRGAMMA model for both bootstrapping and inference of the most-likely tree. The combined dataset was also analyzed using MrBayes v.3.1.2 (BI; Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2001) using 4 partitions (SSU plus three codon positions) and best-fit models as determined by jModeltest v.2.1.3 under the AICc criterion (Darriba et al. Reference Darriba, Taboada, Doallo and Posada2012). Four chains were used in each of two runs of 10 million generations each, sampling every 1000 generations. Convergence and stationarity were assessed using diagnostics in Tracer v1.6 (Rambaut et al. Reference Rambaut, Suchard, Xie and Drummond2014), including examination of the potential scale reduction factor and average standard deviation of split frequencies.
Several algal sequences generated from freshly extracted tissues produced only short sequences with high background (AF11F) or were not matched by the sequence from photobiont in culture (AF4F) and were excluded from the final analysis, although the data generated for these was used in preliminary analyses to check likely group membership.
Results
We examined algal photobionts from 14 specimens of Micarea s. str. using both sequence-based and morphological identification. All the British photobionts matched Coccomyxa, or what has been referred to previously as Pseudococcomyxa (AF17, AF25, AV12) in morphology (Fig. 1). The Ukrainian material was identified by Voytsekhovich et al. (Reference Voytsekhovich, Dymytrova and Nadyeina2011a ). The cultures from both AV12 and AV14 varied in morphology, with those represented in this study corresponding to Coccomyxa when young, but fitting Elliptochloris when more mature. A single Ukrainian photobiont matched Elliptochloris bilobata (AV s. n.).
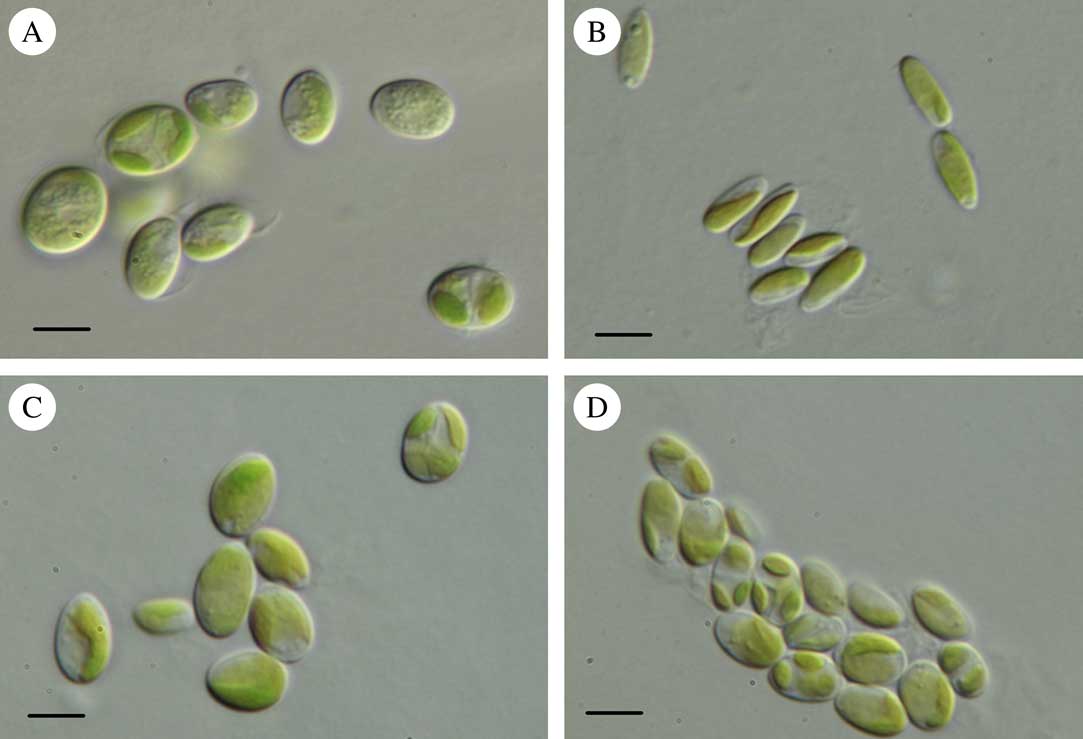
Fig. 1 Light micrographs of photobionts belonging to the Coccomyxa clade. A, Coccomyxa cf. olivacea 078 (AF10); B, Coccomyxa simplex 085 (AF17); C, Coccomyxa simplex 093 (AF23); D, Coccomyxa sp. 208 (AF4). Scales:=10 µm.
Twelve new rbcL sequences and 17 new nucSSU sequences were generated for this study (Table 1). The rbcL alignment was 39 taxa and 1299 positions, with 467 parsimony-informative and 762 constant positions. The nucSSU alignment included 55 taxa and 3987 aligned positions, of which 2331 were excluded, resulting in 216 parsimony-informative and 1306 constant sites. BLAST searches and preliminary analyses showed that all photobiont sequences recovered belong in the Trebouxiophyceae, with most closely matching sequences of Coccomyxa in rbcL and nucSSU. In phylogenetic reconstructions of relationships among photobiont sequences using ML analyses, no conflicts were found between topologies comparing both gene regions individually; therefore, a combined analysis with 64 taxa, 5286 positions (2331 excluded), and 4 partitions was undertaken. In BI analysis, convergence and stationarity were confirmed with all sample sizes exceeding 1000 in Tracer v1.6 (Rambaut et al. Reference Rambaut, Suchard, Xie and Drummond2014), potential scale reduction factor not greater than 1·005, and average standard deviation of split frequencies not greater than 0·005.
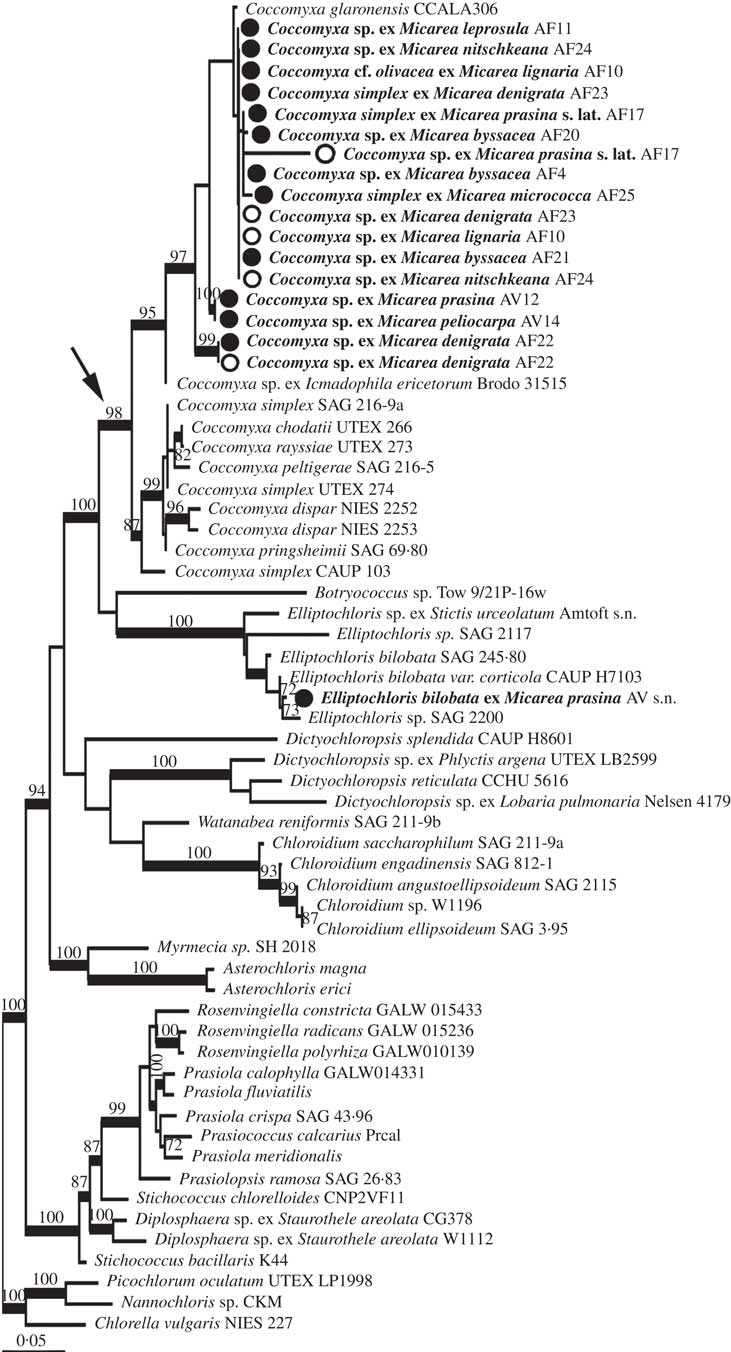
The combined analysis of Micarea photobionts shows that a single photobiont from Micarea prasina collected in the Ukraine belongs to Elliptochloris, whereas all other studied photobionts from Micarea belong to a well-supported clade including Coccomyxa, hereafter referred to as the Coccomyxa clade (arrow in Fig. 2), with 98% bootstrap support and 100% posterior probability. Two well-supported groups were resolved in ML within this clade, with one group predominantly comprised of symbiotic strains from this study and symbionts from Ginkgo biloba L. (C. glaronensis CCALA306, Tremouillaux-Guiller & Huss Reference Tremouillaux-Guiller and Huss2007) and Icmadophila ericetorum (L.) Zahlbr. (supported with 97% bootstrap in ML, and with PP at 100% in BI). The other group predominantly contained free-living strains, including sequences from strains representing the types of both Coccomyxa (C. dispar Schmidle) and Pseudococcomyxa (P. simplex (Mainx) Fott), C. peltigerae Warèn (symbiont of Peltigera) and C. rayssiae Chodat & Jaag (free-living) (supported with 87% bootstrap and 100% in BI). In both the predominantly symbiotic and free-living clades, strains with Coccomyxa and Pseudococcomyxa morphology were mixed. Topologies in BI and ML were congruent.
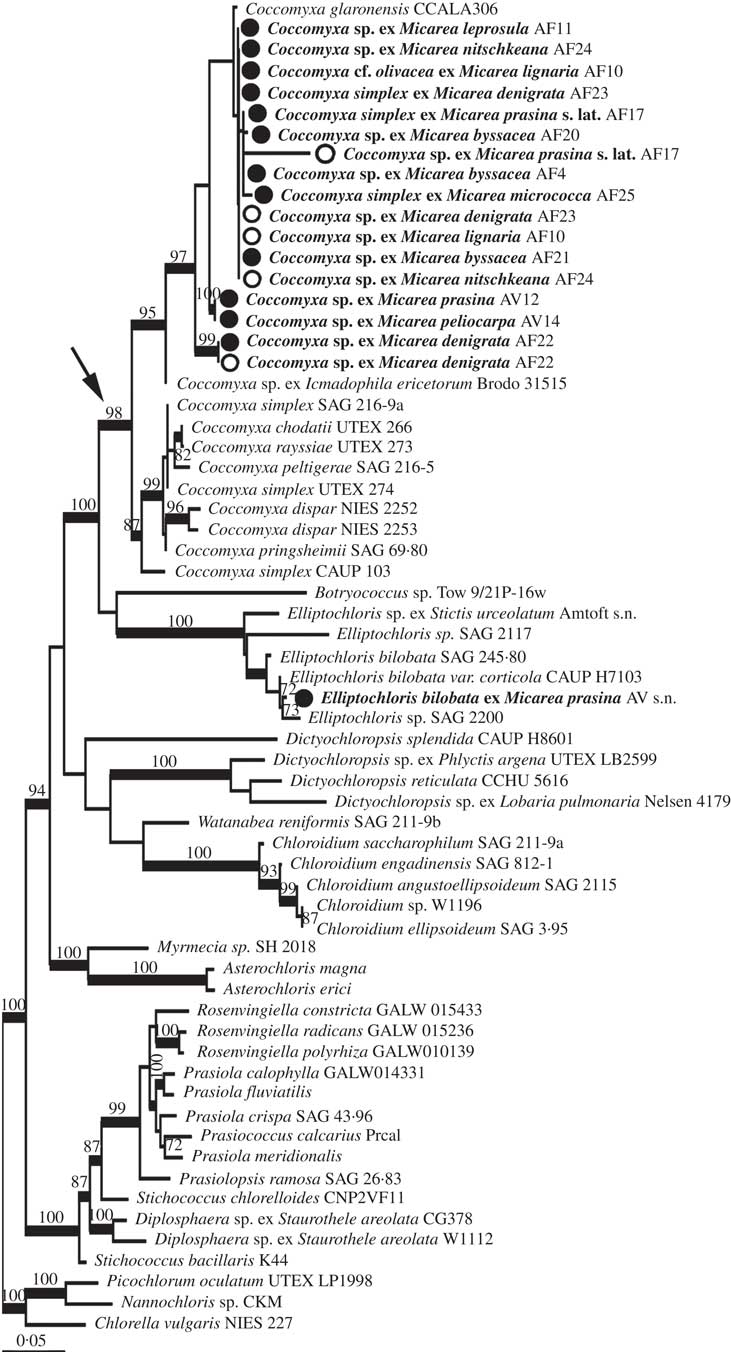
Fig. 2 Maximum likelihood phylogeny from combined rbcL and nucSSU of Micarea photobionts. Morphological determinations of studied strains are labelled on the terminals according to Micarea species with which they were associated, and the collection code for the specimen. Bootstrap support is shown above nodes for values above 70%. Support for nodes from ML analysis but without support from BI is shown to the right of nodes. Thickened branches indicate support over 0·97 in BI. Terminal names in bold show strains studied here. Open circles depict sequences derived from direct amplification of algae from fresh material, closed circles for photobionts sequenced from cultures and arrow indicates the Coccomyxa clade.
The phylogenetic analysis strongly supports the conclusion that the three strains with morphology corresponding to Pseudococcomyxa (AF17, AF25 and AV12) are not distinct from Coccomyxa, although no sequences could be obtained from cultures of AF17. One specimen (AV14) reported to have a photobiont corresponding to Elliptochloris reniformis (S.Watanabe) Ettl & Gärtner (No. 14, Voytsekhovich et al. Reference Voytsekhovich, Dymytrova and Nadyeina2011a ) also groups with these Coccomyxa sequences. No sequences could be obtained from the specimen (AV16; No. 16, Voytsekhovich et al. Reference Voytsekhovich, Dymytrova and Nadyeina2011a ) reported with E. subsphaerica (Reisigl) Ettl & Gärtner as photobiont.
In all but one case from the five specimens (AF4, AF10, AF22, AF23, AF24) where sequences were obtained from both fresh and cultured material (shown as open vs closed circles, respectively, in Fig. 2), the sequences obtained were identical or very closely related, strengthening the case for the algae identified being the dominant photobionts. In the case of AF4, the sequence from fresh material only poorly matched with Stichococcus / Diplosphaera according to BLAST and was not included in further analysis, whereas the cultured photobiont belonged to Coccomyxa. We interpret the partial sequence from the fresh material as an epiphytic alga.
Representative cultures of both the Coccomyxa and the Elliptochloris clades have been deposited in CCAP with the following numbers: CCAP 216/27 Coccomyxa sp. AF23, and CCAP 240/2 Elliptochloris bilobata AV s. n., respectively.
Discussion
Until now, the evolutionary position of ‘micareoid algae’ has been unknown. With the combined perspectives of morphological and molecular data (Voytsekhovich et al. Reference Voytsekhovich, Dymytrova and Nadyeina2011a and this study), it is clear that the typical micareoid algae belong to the ‘Choricystis clade’ of the Trebouxiophyceae (Eliáš et al. Reference Eliáš, Neustupa and Škaloud2008; Leliaert et al. Reference Leliaert, Smith, Moreau, Herron, Verbruggen, Delwiche and De Clerck2012), which includes the genera Choricystis, Botryococcus, and Coccomyxa, among others. The majority of photobionts from eight species of Micarea belong to Coccomyxa (including Pseudococcomyxa), with Elliptochloris also occasionally present (Voytsekhovich et al. Reference Voytsekhovich, Dymytrova and Nadyeina2011a ; this study). Our data support previous findings that symbiotic and free-living strains of Coccomyxa are largely well separated phylogenetically (Zoller & Lutzoni Reference Zoller and Lutzoni2003; Pröschold et al. Reference Pröschold, Darienko, Silva, Reisser and Krienitz2011). Further studies of these strains are required to identify the strains to species, for example using ITS-2, recently proposed as a barcode marker for the genus (Darienko et al. Reference Darienko, Gustavs, Eggert, Wolf and Pröschold2015).
Both fresh and cultured algae were targeted for sequencing, but obtaining clean algal sequence reads from fresh material proved challenging, typically with only a few hundred bases of high quality for both rbcL and nucSSU. In contrast, algal cultures proved easier to sequence. As great care was taken to clean specimens and begin algal cultures from homogeneous algal populations liberated from within thallus granules, we are confident that the algae studied are the dominant ones in the thallus. Identical sequences were also obtained from each of the original multiple isolates per culture. Observations of algal populations obtained by squashing carefully-cleaned and selected granules showed that cells were apparently homogenous and some, though few, maintained hyphal connections.
However, from the combined morphological and molecular perspective offered here, it is clear that Coccomyxa and strains previously referred to as Pseudococcomyxa are intermixed in a single clade using combined rbcL and nucSSU data; they are also very similar morphologically, and both Coccomyxa and Pseudococcomyxa are characterized as having a mucilaginous envelope or mucilage on the tip of the cell (Tsarenko Reference Tsarenko2011). In Coccomyxa, this feature varies with environmental and culture conditions (e.g. Darienko et al. Reference Darienko, Gustavs, Eggert, Wolf and Pröschold2015), and must be interpreted with care. For example, the mucilaginous envelope is not apparent surrounding individual cells on solid media, but is consistently produced in liquid media for most strains of Coccomyxa in culture (Fig. 1B). The mucilage cap on the tip (one pole) of the cell is very fragile, but can be clearly observed after staining with methylene blue and black ink. On the other hand, the type species C. dispar is known to produce a clear mucilaginous layer only in the free-living or lichenized state and not in culture (AV, pers. obs.).
The close relationship found here between Elliptochloris and Coccomyxa is supported both by their sharing fungal symbionts (Micarea) and by molecular studies (e.g. Eliáš et al. Reference Eliáš, Neustupa and Škaloud2008; Letsch & Lewis Reference Letsch and Lewis2012). Morphologically, these two algal genera are similar, but are usually clearly differentiated morphologically since Elliptochloris has generally spherical vegetative cells, two types of autospores, and consistently lacks mucilage layers, whereas cells are oval/kidney-shaped in Coccomyxa, have a single type of autospore, and have mucilage production (Ettl & Gärtner Reference Ettl and Gärtner1995). However, it can be difficult to assign strains to genera even with cultured material due to wide morphological variation, particularly in the case of E. reniformis. Reviewing the literature on symbiotic partners, it is clear that the morphology of algae in the Choricystis group (sensu Leliaert et al. 2012) may present considerable challenges until enough sequenced reference strains are available for development of more robust hypotheses.
In addition, more than a single photobiont lineage may exist in lichen thalli (Piercey-Normore Reference Piercey-Normore2006; Mansournia et al. Reference Mansournia, Wu, Matsushita and Hogetsu2012; Park et al. Reference Park, Kim, Elvebakk, Kim, Jeong and Hong2014), and sometimes might be expected (Casano et al. Reference Casano, del Campo, Garcia-Breijo, Reig-Arminana, Gasulla, del Hoyo, Guera and Barreno2011). Two photobionts from M. melanoloba (= M. prasina) AV12 were reported in Voytsekhovich et al. (Reference Voytsekhovich, Dymytrova and Nadyeina2011a ), E. subsphaerica and Pseudococcomyxa sp.; only the Pseudococcomyxa strain was available for sequencing. Likewise, both E. reniformis and E. subsphaerica were reported from M. peliocarpa AV14, but only the former was available for sequencing. This strain appears morphologically very distinct, but the presence of a single type of autospore (of elongated shape) and the formation of an irregular cell shape can justify classification of this strain into the genus Coccomyxa. Despite careful cleaning methods, the difficulty in direct amplification of photobionts could be a result of either closely attached epibionts, or the presence of more than a single photobiont.
The Micarea species included in this study are all members of the Pilocarpaceae, either related to the type species of Micarea prasina (e.g. M. byssacea (Th. Fr.) Czarnota et al., and M. micrococca (Körb.) Gams ex Coppins), or in a separate clade related to M. denigrata (Fr.) Hedl. (e.g. M. nitschkeana (J. Lahm ex Rabenh.) Harm., M. leprosula (Th. Fr.) Coppins & A. Fletcher, M. lignaria (Ach.) Hedl. and M. peliocarpa (Anzi) Coppins & R. Sant.; Andersen & Ekman Reference Andersen and Ekman2005). The recently segregated genus Brianaria was not studied here, though earlier morphological observations point to different photobionts as members of that symbiotic association (Coppins Reference Coppins1983).
The diversity of species interactions has been suggested to be one of the major forces driving diversification in diverse lineages, from insect pollinators of flowering plants (Thompson Reference Thompson2009) to specialization of fungal endophytes on lichens (Arnold et al. Reference Arnold, Miądlikowska, Higgins, Sarvate, Gugger, Way, Hofstetter, Kauff and Lutzoni2009). The evolutionary mechanisms of some species interactions have been studied in detail for only relatively few systems, permitting inferences about processes driving these patterns. In most groups, carefully planned studies of ecologically- and taxonomically-stratified samples are lacking, meaning that for the majority of descriptive studies so far completed, testable inferences for what mechanisms might be at work are impossible (e.g. Mueller Reference Mueller2012). Nevertheless, patterns of association between symbionts are being amassed at an increasing rate, and trends among studies can be compared. For example, reciprocal specificity (sensu Smith & Douglas Reference Smith and Douglas1987) between fungi and their photobionts tends to be rare (Otálora et al. Reference Otálora, Martínez, O’Brien, Molina, Aragón and Lutzoni2010), and most fungi can associate with several related strains of their photobiont hosts, which may be adapted to their ecological setting rather than their fungal host. In this first sequence-based glimpse at the diversity of photobionts within Micarea, patterns of association seem to be supporting the general trends of genus-level specificity of fungi for photobionts (i.e. Micarea with the Elliptochloris clade, sensu Pröschold et al. Reference Pröschold, Darienko, Silva, Reisser and Krienitz2011), low specificity within fungal species, and unrecognized diversity of photobionts. The detailed genetic structure of this association, including symbiotic specificity, requires a carefully conceived study.
Brian Coppins is warmly thanked for his assistance in the field and in checking determinations of specimens. Mark Powell provided several fresh specimens from England for this study. Sally Eaton, Laura Forrest and Michelle Hollingsworth offered technical help and/or training in the molecular laboratory. Two anonymous reviewers provided helpful feedback on an earlier draft of this manuscript.
Supplementary Material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0024282915000341