Introduction
Across all domains of life, there arguably exists no single class of traits with more of an impact on the origin and fate of evolutionary lineages than those related to reproductive biology (Stebbins Reference Stebbins1957; Grant Reference Grant1958; Gomez-Mestre et al. Reference Gomez-Mestre, Pyron and Wiens2012; Van der Niet et al. Reference Van der Niet, Peakall and Johnson2014). Reproductive traits determine the trajectory of a vast array of population-level processes and scale up to explain major macroevolutionary patterns (Fisher Reference Fisher1941; Maynard Smith Reference Maynard Smith1978; Tripp & Manos Reference Tripp and Manos2008; Kerr et al. Reference Kerr, Baird and Hughes2011). Among eukaryotes, fungi exhibit some of the greatest diversity in reproductive strategies, making them excellent model organisms for understanding the evolution and diversification of reproductive systems. Whereas most lineages of eukaryotes reproduce primarily through sexual means or asexual means with short-lived sexual cycles (but see Dacks & Roger Reference Dacks and Roger1999; Redecker Reference Redecker2002; Ramesh et al. Reference Ramesh, Malik and Logsdon2005; Asplen et al. Reference Asplen, Whitfield, De Boer and Heimpel2009), a sizeable proportion of the 1·5 million species of fungi on the planet (Hawksworth Reference Hawksworth2001) reproduce both sexually and asexually during time periods commonly referred to as teleomorphic and anamorphic phases.
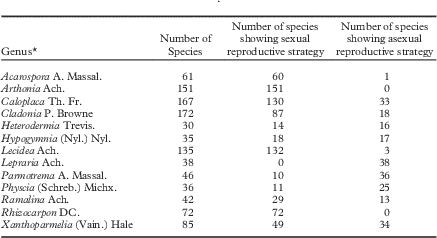
Lichenized fungi (hereafter lichens) constitute one-fifth of all fungi on Earth and approximately half of all ascomycetes (Honegger Reference Honegger1997; Jaklitsch et al. Reference Jaklitsch, Baral, Lücking, Lumbsch and Frey2016). An attractive hypothesis to explain their diversity and success worldwide, including in extreme or novel environments, is their plurality of reproductive strategies. Lichens are somewhat unusual among fungi in that teleomorphic and anamorphic stages are commonly persistent throughout the duration of an individual’s lifetime (rather than ephemeral or in phases; see Table 1 in Bowler & Rundel Reference Bowler and Rundel1975) and involve such divergent morphological forms that mode of reproduction has for the last half century or so been associated with identification of species (recently discussed in Brodo & Lendemer Reference Brodo and Lendemer2012). Diverse reproductive strategies in these organisms must in part be related to added complexity associated with being lichenized and may be driven by selection to maintain and enrich the symbiosis (see mechanisms discussed in Kroken & Taylor 2001 Reference Kroken and Taylora and Buschbom & Mueller Reference Buschbom and Mueller2005). Across lichen trees of life (e.g. Arnold et al. Reference Arnold, Miadlikowska, Higgins, Sarvate, Gugger, Way, Hofstetter, Kauff and Lutzoni2009; Miadlikowska et al. Reference Miadlikowska, Kauff, Högnabba, Oliver, Molnár, Fraker, Gaya, Hafellner, Hofstetter and Gueidan2014), individual species to whole clades of species are obligate sexual (e.g. Toninia A. Massal.; Timdal Reference Timdal1991), obligate asexual (Lepraria Ach.; Lendemer Reference Lendemer2013), almost entirely asexual with a few sexual species (e.g. Chrysothrix Mont.), almost entirely sexual with a few asexual species (e.g. Rinodina (Ach.) Gray; Sheard Reference Sheard2010) or more uncommonly, facultative asexual and/or sexual (e.g. Roccella galapagoensis Follmann; Tehler et al. Reference Tehler, Irestedt, Bungartz and Wedin2009; Lobaria pulmonaria (L.) Hoffm.; Zoller et al. Reference Zoller, Lutzoni and Scheidegger1999). North America, as one example, is home to genera with considerable variation in ratios of sexual to asexual species: some lineages have no variation in mode of reproduction whereas others approximate to a 50:50 ratio (Table 1).
Table 1 An example of reproductive strategies among genera of lichens in North America. Data derive from unpublished research (E. Tripp & J. Lendemer, unpublished data), using version 17 of Esslinger’s North American Lichen Checklist as a basis for taxon identification and enumeration
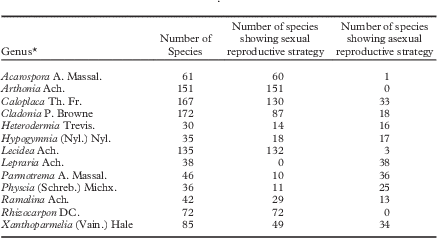
*Taxonomic authorities follow Esslinger (Reference Esslinger2015).
A given lineage of lichens such as Porpidia Körber (Buschbom & Barker Reference Buschbom and Barker2006), Letharia (Th. Fr.) Zahlbr. (Kroken & Taylor 2001 Reference Kroken and Taylorb ; Altermann et al. Reference Altermann, Leavitt, Goward, Nelsen and Lumbsch2014), or Dirina (Tehler et al. Reference Tehler, Ertz and Irestedt2013) commonly contains close relatives marked by either sexual or asexual reproduction. This suggests either a potentially strong correlation between mating system evolution and speciation in lichens, or widespread inaccuracy in taxonomic systems that have been in place for the last half century. We now know the latter to be untrue in numerous cases because studies have demonstrated strongly supported reciprocal monophyly of asexual versus sexual entities coupled with ecological or other forms of divergence typical of separately evolving lineages (e.g. Vulpicida pinastri (Scop.) J.-E. Mattsson & M. J. Lai vs. V. juniperinus (L.) J.-E. Mattsson & M. J. Lai: Saag et al. Reference Saag, Mark, Saag and Randlane2014; Porpidia degelii (H. Magn.) Lendemer vs. P. albocaerulescens (Wulfen) Hertel & Knoph: Lendemer & Harris Reference Lendemer and Harris2014; various groups within the Lobaria meridionalis clade: Cornejo & Scheidegger Reference Cornejo and Scheidegger2015; in contrast see Buschbom & Mueller Reference Buschbom and Mueller2005 and Tehler et al. Reference Tehler, Ertz and Irestedt2013). Such observations have long been of interest to lichenologists and underlie the classical notion of ‘species pairs’, in which a sexually reproducing species commonly has a closely related asexual species counterpart (Du Rietz Reference Du Rietz1924; Poelt Reference Poelt1970; Hale Reference Hale1976), not dissimilar to species pairs consisting of self-compatible and self-incompatible plants (reviewed in Charlesworth Reference Charlesworth2006). The observation that the sexual species is ancestral evolutionarily, which then gives rise to asexual derivatives (self-incompatibility to self-compatibility in plants), has for many years been orthodox (Poelt Reference Poelt1970; Bowler & Rundel Reference Bowler and Rundel1975; Tehler Reference Tehler1982; Takebayashi & Morrell Reference Takebayashi and Morrell2001). This topic has been debated extensively (Tehler Reference Tehler1982; Buschbom & Mueller Reference Buschbom and Mueller2005), with the last 20 years or so yielding the phylogenetic methods as well as taxon sampling needed to advance a more quantitative understanding of the subject. In particular, a modern view has begun to emerge in which mostly asexual lineages may be viable for the long term evolutionarily, and may give rise to sexual lineages (Kroken & Taylor 2001 Reference Kroken and Taylorb ; Buschbom & Barker Reference Buschbom and Barker2006; Cornejo et al. Reference Cornejo, Chabanenko and Scheidegger2009).
The tremendous expansion of published lichen phylogenies over these two or more decades has yielded ample fodder for re-examining dogma pertaining to reproductive evolution in lichenology. More generally, probable links between reproductive modes and diversification dynamics in lichens (e.g., speciation, extinction, maintenance of existing and exploitation of new symbioses) indicate that trends in sexual versus asexual reproduction in lichens are of broad interest to evolutionary biologists. Without attempts at answering how and why diverse reproductive strategies evolve and are, or are not, maintained, as well as patterns in the frequency and directionality of character transitions, empirical and theoretical syntheses of reproductive biology across multiple domains of life will continue to be advanced without a lichenological perspective (see recent examples of syntheses from insects (Shuker & Simmons Reference Shuker and Simmons2014), fish (Wootton & Smith Reference Wootton and Smith2014) and plants (Goodwillie et al. Reference Goodwillie, Kalisz and Eckert2005; Karron et al. Reference Karron, Ivey, Mitchell, Whitehead, Peakall and Case2012; Pierre-Olivier Reference Pierre-Olivier2012)). The present contribution seeks to make a first attempt at synthesizing knowledge of reproductive character evolution in lichens by explicit, model-based reconstruction of the frequency and polarity of evolutionary transitions between sexual and asexual reproduction. Here, asexuality as a dead end is defined as meeting two criteria: 1) that it is only a derived state and 2) that it is transient evolutionarily. I specifically aim to test whether dogma holds true and asexual reproduction is an evolutionary dead end in lichens, which has been argued in other lineages of life (Maynard Smith Reference Maynard Smith1978; Takebayashi & Morrell Reference Takebayashi and Morrell2001; Poulíčková et al. Reference Poulíčková, Sato, Evans, Chepurnov and Mann2014), or conversely whether asexual lineages are a source for evolutionary innovation.
Materials and Methods
General strategy
The present investigation is based on ancestral state reconstructions of mode of reproduction. To accomplish this, only previously published phylogenies (i.e., tree files) with adequate taxon sampling and branch support were utilized (see below). Justification for using only published trees without further modification through new analyses of original datasets is that the enormous amount of work, knowledge, and expertise that accompanies any single phylogenetic study suggests that the best expert opinion regarding taxon sampling and phylogenetic interpretations is that conveyed in the original manuscript itself. Moreover, the present study is not focused on reassessing relationships presented in more than 20 prior works. As such, I refrain here from making any assertions or claims about what a given phylogeny ‘should’ look like, but rather make use of datasets that fit the criteria for analyses (see below).
Reproductive definitions
In the present study, sexually reproducing lichens are defined as species reproducing primarily through mycobiont spores that are products of meiosis (ascospores); these spores must then go on to encounter a suitable photobiont through various means (free-living, borrowed from a nearby thallus, etc.). Primary sexual structures in lichens are termed ascomata (specifically, apothecia (disc-like) or perithecia (flask-like)). Asexually reproducing lichens are defined here as species reproducing primarily through specialized propagules that are lichenized containing both the mycobiont and photobiont and are derived from mitosis. Primary asexual structures in lichens are termed soredia (ecorticate spherical bundles of hyphae and algae that usually form in the photobiont layer, ~20–100 µm diam.; Bowler & Rundle 1975), isidia (corticate finger-like outgrowths of the thallus containing hyphae and algae, variable in shape and size but typically larger than soredia) and phyllidia or schizidia and other outgrowths of the thallus that contain both symbionts, these often occurring along margins of lobes. A separate class of mitotic propagules occurs in lichens (conidia) but these disperse only the mycobiont (i.e. are non-lichenized) and are thought to function primarily in diploidization of sexually reproducing species but may also make contact with a suitable photobiont and establish new lichen thalli; as such, conidia are excluded from further consideration in this study.
Taxon sampling
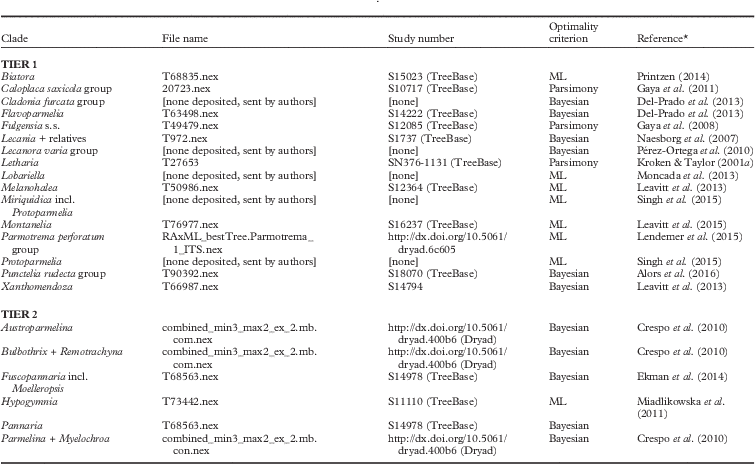
It is widely appreciated that the single largest feature that has an impact on ancestral state reconstructions is taxon sampling (Salisbury & Kim Reference Salisbury and Kim2001). In this study, I attempted to overcome this challenge as much as is possible, first by selecting lineages (clades, for the most part at the rank of genus and below) in which >50% of the total number of currently recognized species were included in phylogenetic sampling (Tier 1 analyses). The Lecanora varia (Hoffm.) Ach. group was included in this tier because, even though it was not possible to find published estimates of total clade size, it is likely that Pérez-Ortega et al.’s (Reference Pérez-Ortega, Spribille, Palice, Elix and Printzen2010) sampling of 33 species exceeds 50% of the total. However, because of the general paucity of studies that fit this criterion (this paucity is exacerbated by the lack of availability of data files in online repositories), a second threshold of taxon sampling was explored in which a minimum of 25% of species were included in the study (Tier 2 analyses). The present investigation represents a first attempt at understanding the generality of transitions in reproductive modes, and future studies with more complete taxon sampling are needed to fully explore the question at hand.
Five additional criteria were applied to both Tier 1 and Tier 2 analyses in selecting datasets suitable for analyses: 1) the group was monophyletic (e.g. for lineages in which non-monophyly has recently been demonstrated, such as Hypotrachyna (Vain.) Hale (Divakar et al. Reference Divakar, Lumbsch, Ferencova, Del Prado and Crespo2010), only a monophyletic ingroup was analyzed, e.g., Remotrachyna Divakar & A. Crespo + Bulbothrix Hale); 2) the focal ingroup was composed of species with more or less clearly delimited modes of reproduction either sexual or asexual. Trees containing species commonly marked by mixed modes of reproduction such as Lobaria pulmonaria (L.) Hoffm. were not included in this study but warrant future investigation; 3) ingroup taxa displayed a diversity in reproductive mode. Well-sampled phylogenies such as those of Psora Hoffm. (Ekman & Blaalid Reference Ekman and Blaalid2011) and Polyblastia A. Massal. (Savíc et al. Reference Savíc, Tibell, Gueidan and Lutzoni2008) were not included because reproductive mode is fixed among taxa; 4) phylogenies contained several (but not necessarily all) branches that were strongly supported by bootstrap analysis or posterior probabilities; 5) a tree file containing a phylogeny derived from parsimony, maximum likelihood (ML), or Bayesian analyses was available in Dryad, TreeBase, or via email contact with the authors of the study.
Outgroup selection
Like taxon sampling, it is widely understood that outgroup selection can and does have a major impact on ancestral state reconstructions and has thus been the subject of extensive investigation (Salisbury & Kim Reference Salisbury and Kim2001; Brady et al. Reference Brady, Litman and Danforth2011). However, rigorous and detailed exploration of this topic across the diversity of lineages included here, which would best be addressed through a combination of empirical and simulation studies, is beyond the scope of this investigation. I selected two or more of the closest relatives to the ingroup based on the published phylogeny. In rare cases, tree files were unrooted; in these instances, rooting on the dataset was imposed using the phylogenetic results presented in the literature.
Character matrices and tree files
One of two major challenges facing this study was scoring characters. Several (to my knowledge, there has never been a published estimate) asexually reproducing species are known to pass through a sexual phase at some point during an individual’s lifetime, such as the widespread Pyxine sorediata (Ach.) Mont. in eastern North America. In contrast, most sexual species are not known to pass through an asexual phase, but see Hestmark (Reference Hestmark1991) who reported contemporaneous phases present in numerous species of Umbilicaria Hoffm. Moreover, we lack data regarding how commonly polymorphisms occur across multiple populations of a given species, across multiple species in a given clade, and how reproductive structures develop over the course of an individual lichen’s entire life cycle; elegant examples of documentation of this can be found in Denison (Reference Denison2003) and Sanders (Reference Sanders2014). As such, currently available data on intrathalline polymorphisms are not amenable to phylogenetic reconstruction of this feature across most lineages of lichens. This limitation directed a discrete rather than continuous character approach. I thus coded species according to their primary mode of reproduction, defined as the mode of reproduction most commonly encountered in the field, herbarium, and/or literature. Extensive effort was made to reference species protologues and/or images of original material (available on JSTOR Plants) when possible to score reproductive mode as sexual (0) or asexual (1).
The second major challenge facing this study was the availability of datasets that are deposited in public repositories. Many academic publications still do not require this from authors and, as such, approximately half of all studies identified as suitable for this investigation lacked available data online. In several instances, I was able to retrieve tree files from generous authors. Nonetheless, this deficiency limited the scope of the present study. Tree files used for reconstructions are described in Table 2.
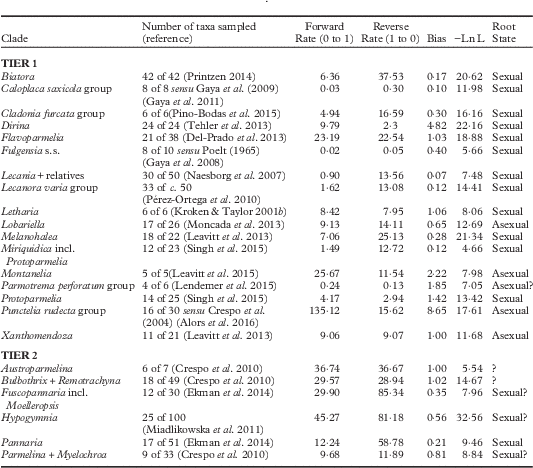
Table 2 Inferred numbers and rates of transitions in mode of reproduction (sexual, asexual) for 23 clades of lichens included in this study. Ancestral state reconstructions were implemented via maximum likelihood methods utilizing a two-rate (asymmetric) model of evolution. The number of estimated taxa in a given clade was derived from the reference included in parenthesis. Log likelihoods of optimized reconstruction under the asymmetric model are provided.See Materials and Methods for explanation of terms
Ancestral state reconstructions
Reconstructions were conducted on tree files available in public repositories. These datasets provided a range of possible tree files, including a single type of file derived from only one analysis to multiple types of tree files derived from analyses using different optimality criteria. Only a fraction of datasets included posterior distributions of highly likely trees (i.e. tree sets) derived from Bayesian analyses. Reconstructing ancestral states of traits on posterior distributions of trees yields the ability to explore uncertainties in their topologies (Tripp & Manos Reference Tripp and Manos2008). However, the limited availability of this file type prevented widespread exploration of topological uncertainty. As such, and to enable easier comparison of patterns among all datasets, reconstructions were conducted on only a single tree including a most likely tree derived from ML, a maximum clade credibility tree derived from Bayesian analysis, or a consensus summary tree derived from parsimony analysis (Table 2). In cases where more than one tree file was available, reconstructions were conducted on the most favoured phylogeny presented in the published study. For likelihood-based studies (ML, Bayesian), ancestral state reconstructions made use of estimated branch lengths derived from the original study.
The only manipulation made to tree files prior to analysis was the removal of taxa not included in the ingroup or retained as outgroups for rooting trees. For example, for the Fulgensia A. Massal. & De Not. analysis all taxa in Gaya et al. (Reference Gaya, Navarro-Rosinés, Llimona, Hladun and Lutzoni2008) were deleted except sampled Fulgensia plus the two nearest outgroups (Letrouitia domingensis (Pers.) Hafellner & Bellm. and Letrouitia parabola (Nyl.) R. Sant & Hafellner); from this matrix Fulgensia australis (Arnold) Poelt. was further pruned because the phylogenetic results in Gaya et al. (Reference Gaya, Navarro-Rosinés, Llimona, Hladun and Lutzoni2008) demonstrated that this taxon was not resolved within the otherwise monophyletic Fulgensia s. str. clade. Other than taxon pruning, no matrices or tree files were manipulated prior to analyses except in two instances where this was necessary, thus enabling a direct comparison of character evolution derived from this study with previously presented phylogenetic data. Firstly, no published phylogeny of Punctelia rudecta (Ach.) Krog exists but both a matrix and a tree file were available on TreeBase. However, because the tree file lacked branch support, the original matrix was used to conduct a parsimony bootstrap search in PAUP* using default settings and 100 replicates. Secondly, no online data were available for Tehler et al.’s (Reference Tehler, Ertz and Irestedt2013) phylogenetic study of Dirina Fr., but A. Tehler kindly provided the DNA matrix used in that publication; this was used to conduct an ML search and ML bootstrap search (100 replicates) in Garli (default settings). Finally, names of taxa/terminals were not altered prior to analysis except in instances where terminals contained no information indicative of species identification. In these cases, I added the specific epithet plus an underscore to the beginning of the name of the OTU. Information on data matrices, tree file names, and public repositories is provided in Appendix 1.
Rates of transitions
Using marginal reconstructions and comparative methods based on Brownian motion (cf. Felsenstein Reference Felsenstein2012) implemented in Mesquite v3.04, reproductive modes were estimated across nodes in target phylogenies using a simple Q matrix for a single character with two discrete states. This matrix contains four terms that describe stasis in either of the states (q00, q11) plus two instantaneous transition rates between the states: one forward and one reverse (q01, q10); the present study focused only on rates associated with transitions, not those associated with stasis. I used an asymmetric Markov continuous model of character evolution that permitted different rates for q01 (forward rate of change) versus q10 (reverse rate of change). The forward rate is calculated as the overall rate × square root of the bias whereas the reverse rate is calculated as the overall rate/square root of the bias. The overall bias in transition rates was calculated as the rate of gain of asexual reproduction (q01) / the rate of gain of sexual reproduction (q10). Thus, values >1 indicate higher rates of gain of asexual reproduction from sexual ancestors whereas values <1 indicate higher rates of gain of sexual reproduction from asexual ancestors. A two-rate model was used instead of a one-rate model (where forward and reverse transition rates are equal) because the former yielded reconstructions with higher likelihoods in all cases, except in two instances in which the log likelihood estimated under the two different models was the same. The root state was optimized under a model that assumes root transition rates consistent with the overall model (i.e. the ‘Root State Frequencies Same as Equilibrium’ option in Mesquite).
Numbers of transitions
The overall number of state transitions (forward and reverse) was estimated as follows. The proportional likelihoods of states 0 and 1 for a given node were calculated and statistical support for each reconstructed node was based on the difference in log likelihood between the two character states: a difference >2 was taken to indicate support for the reconstruction. As a qualitative assessment, a 50% proportional likelihood cut-off was used to estimate whether a given node was most likely sexually or asexually reproducing. However, tallies of numbers of transitions were considered significant only if two conditions were met: 1) proportional likelihoods themselves were significant in that the difference between the two states was >2 log likelihood units, and 2) relevant branches were supported because they were ≥70% likelihood bootstrap (LB), ≥70% parsimony bootstrap (PB), and/or ≥95% posterior probability (PP)). Ancestral states were depicted in the figures only for nodes relevant to transitions (q01 or q10) rather than on every node in a phylogeny (instances of q00 or q11). Root state was inferred as the state reconstructed with the higher proportional likelihood but was not reported as significant unless the difference was >2 log likelihood units. The resulting files upon which ancestral state reconstructions are based have been deposited in Dryad (doi:10.5061/dryad.29h6k).
Results
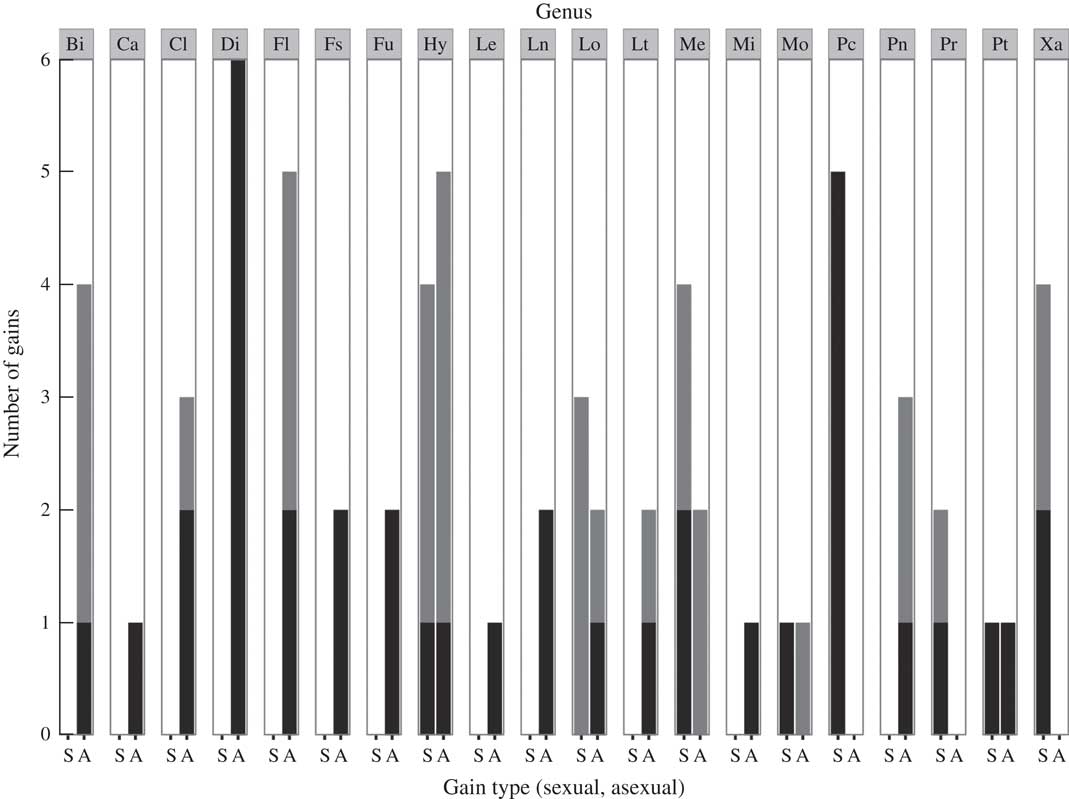
A total of 23 datasets were utilized in the analyses: 17 comprised Tier 1 and six comprised Tier 2. Overall, transition rates were not predictive of numbers of inferred gains and losses of asexuality (Table 2, Fig. 1). Most lineages displayed higher rates of gain of sexual reproduction from asexual ancestors (i.e. reverse transitions with biases <1), despite the fact that in these lineages there were generally greater numbers of inferred gains of asexuality than losses (Fig. 1).
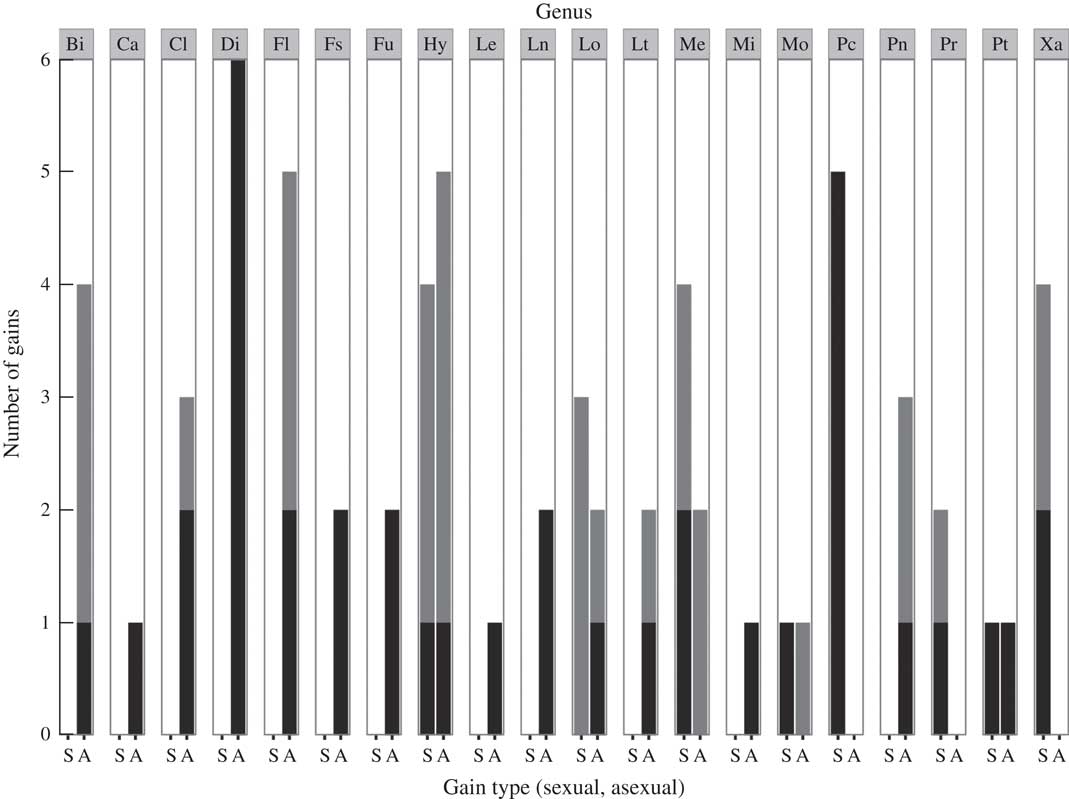
Fig. 1 Summary of numbers of inferred transitions from reconstructions of ancestral state of mode of reproduction in lichens. For each lineage, forward transitions, or gains of asexuality from sexual ancestors, are shown on the right (A) and reverse transitions, or gains of sexuality from asexual ancestors, are shown on the left (S). Black columns indicate significant transitions whereas grey columns indicate potentially additional but non-significant transitions. Data from 20 of 23 clades are presented; Austroparmelia, Bulbothrix and Parmelina were omitted because directionality of transition cannot be inferred from ancestral state reconstruction. Lineages are labelled by focal genus. From left to right, these are: Biatora (Bi), Caloplaca (Ca), Cladonia (Cl), Dirina (Di), Flavoparmelia (Fl), Fulgensia (Fs), Lecania (Le), Lecanora (Ln), Lobariella (Lo), Letharia (Lt), Melanohalea (Me), Miriquidica (Mi), Montanelia (Mo), Punctelia (Pc), Pannaria (Pn), Parmotrema (Pr), Protoparmelia (Pt), and Xanthomendoza (Xa).
Across all 23 datasets, ancestral state reconstructions indicated dynamic histories of mode of reproduction in lichens (Figs 1–5; Supplementary Material Figures 1–19, available online). Gains of asexual reproduction from sexual ancestor was the more common pattern of transition but this transition was not irreversible; in several lineages including Hypogymnia (Nyl.) Nyl., Melanohalea O. Blanco, Montanelia Divakar, Parmotrema A. Massal., Protoparmelia M. Choisy, Punctelia Krog, and Xanthomendoza S. Y. Kondr. & Kärnefelt, statistically supported gains of sexual reproduction from asexual ancestors were documented (Table 2). The overall total number of gains of asexual reproduction from sexual ancestors was 26 (supported), plus additional unsupported gains (Fig. 1). The total number of gains of sexual reproduction from asexual ancestors was 14 (supported), plus additional unsupported gains (Fig. 1).
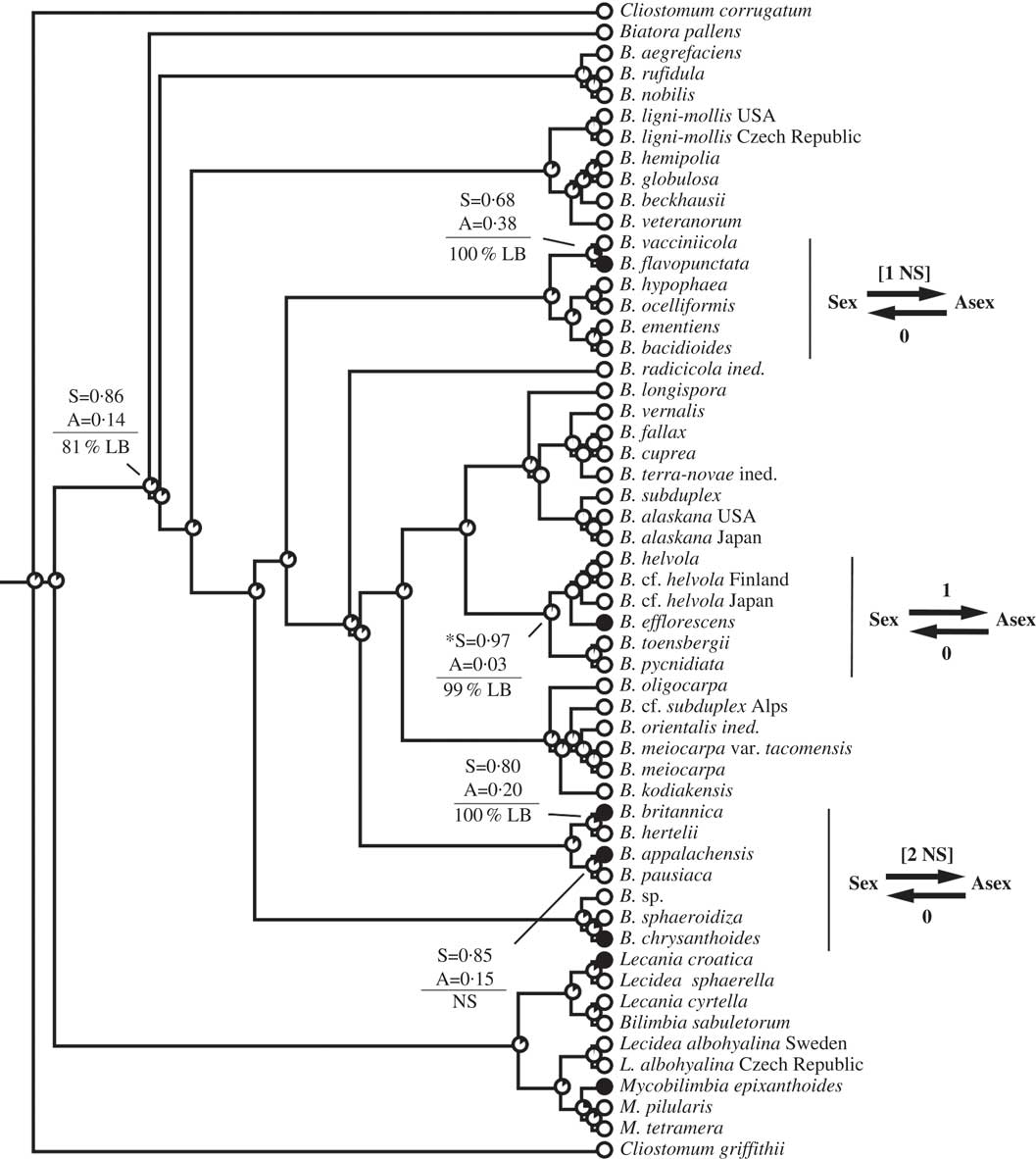
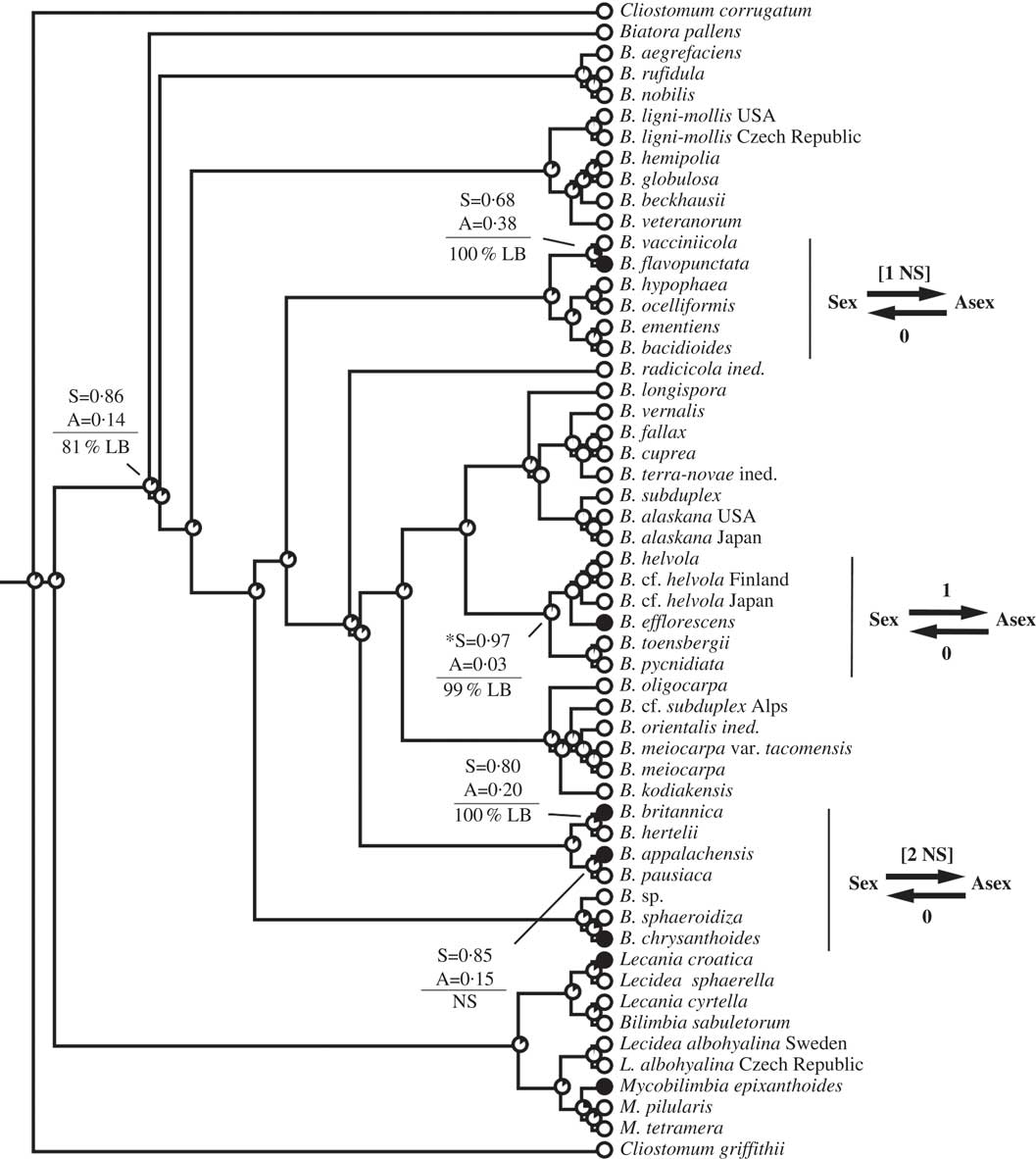
Fig. 2 Ancestral state reconstructions of reproductive mode using the Biatora clade (Tier 1 analysis). Proportional likelihoods of reconstructed state shown in circles on nodes, white=sexual; black=asexual. Nodes pertinent to transitions are labelled as follows: above horizontal lines are relative proportional likelihoods for sexual (“S”) or asexual (“A”) states, and an asterisk indicates reconstruction was significant for that state; below horizontal lines are support values (PP=Posterior Probability, LB=Likelihood Bootstrap, PB=Parsimony Bootstrap, NS=non-significant (i.e. <95% PP or <70% LB or PB)). Numbers of inferred transitions and directionality of transitions given to the right of taxon labels. NS indicates that the reconstruction was non-significant (i.e. that it did not meet both criteria for significance as described under Materials and Methods).
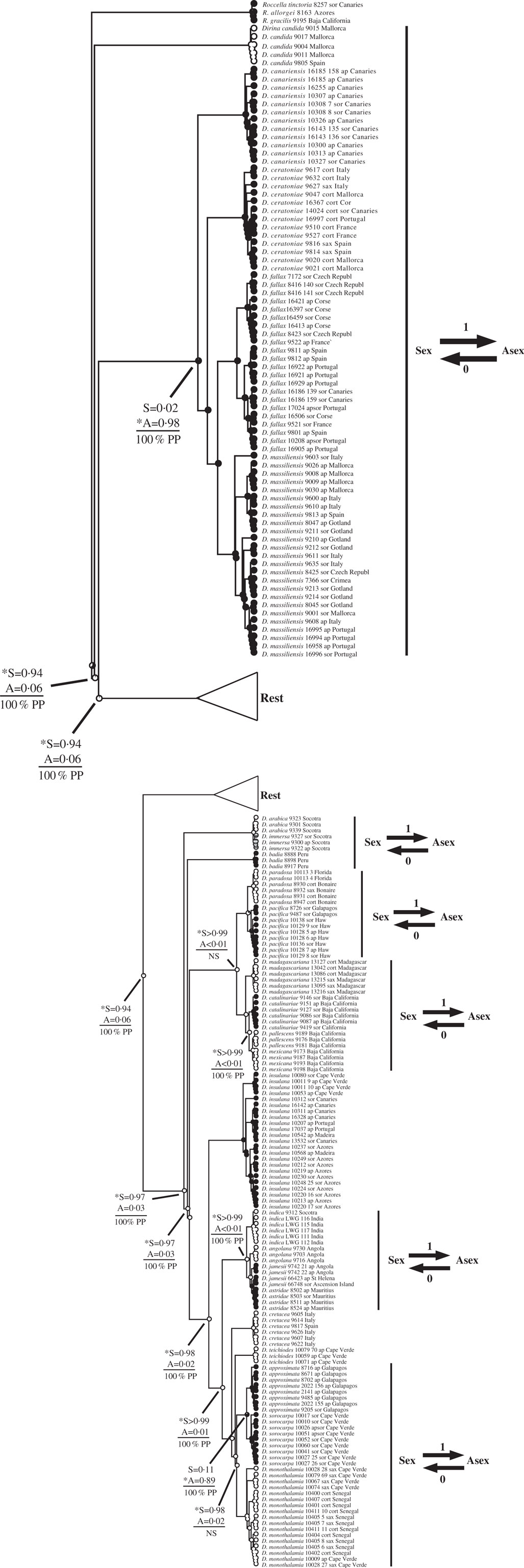
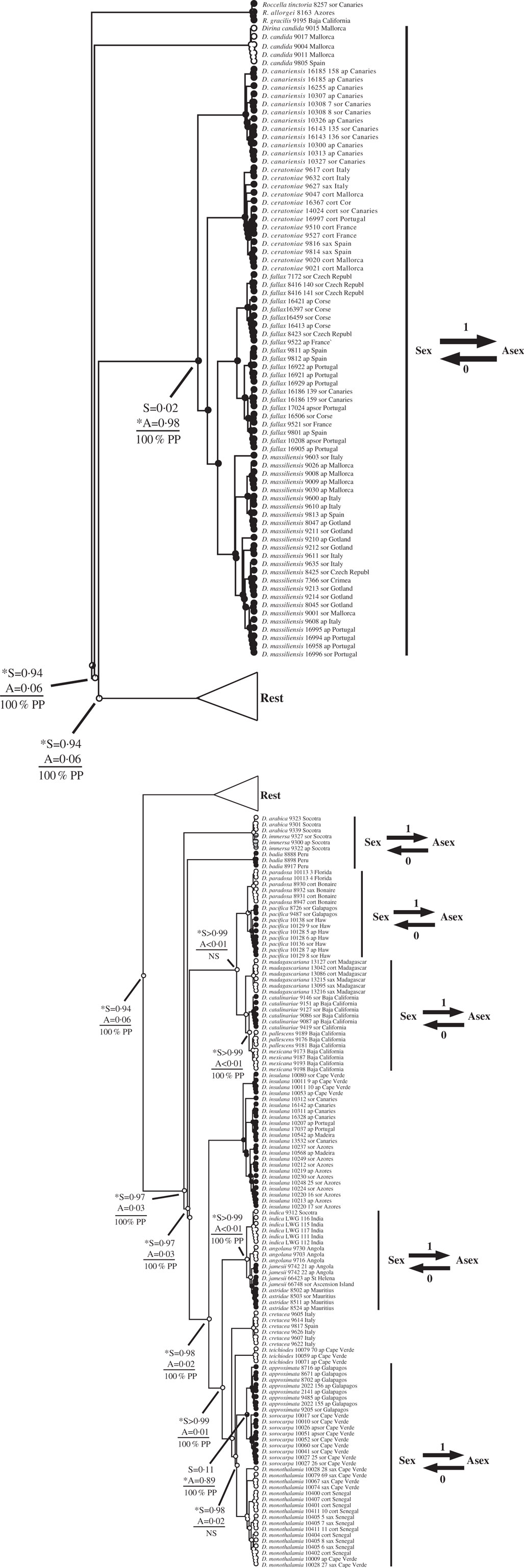
Fig. 3 Ancestral state reconstructions of reproductive mode using the Dirina clade (Tier 1 analysis). The genera Dirina and Roccella are presented within this clade. Proportional likelihoods of reconstructed state shown in circles on nodes: white=sexual; black=asexual; grey=ambiguous reconstruction owing to missing data. Nodes pertinent to transitions are labelled as follows: above horizontal lines are relative proportional likelihoods for sexual (“S”) or asexual (“A”) states, and an asterisk indicates reconstruction was significant for that state; below horizontal lines are support values (PP=Posterior Probability, LB=Likelihood Bootstrap, PB=Parsimony Bootstrap, NS=non-significant (i.e. <95% PP or <70% LB or PB)). Numbers of inferred transitions and directionality of transitions given to the right of taxon labels. Key: ap=apotheciate; sor=sorediate; cort=corticolous; sax=saxicolous.
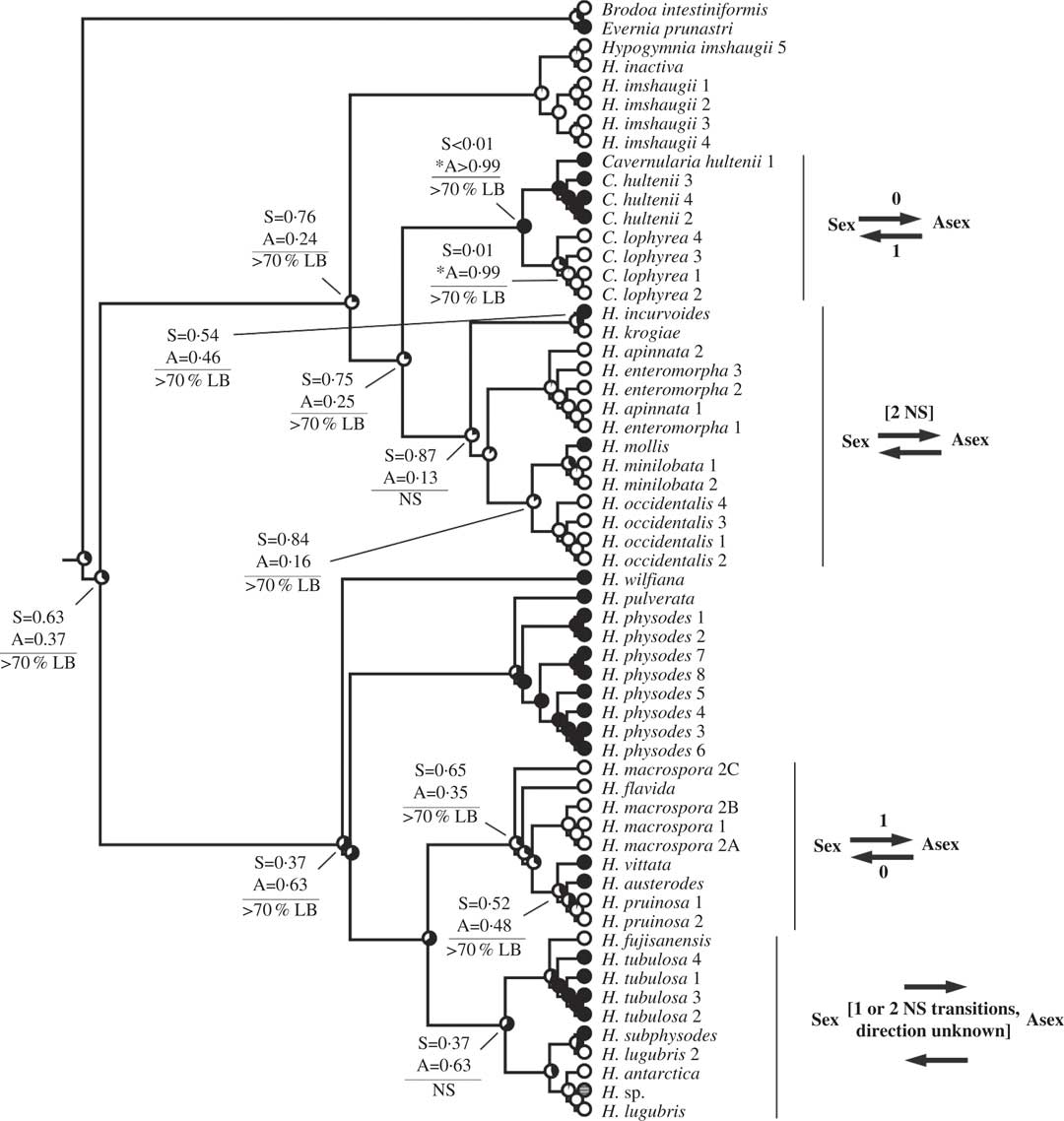
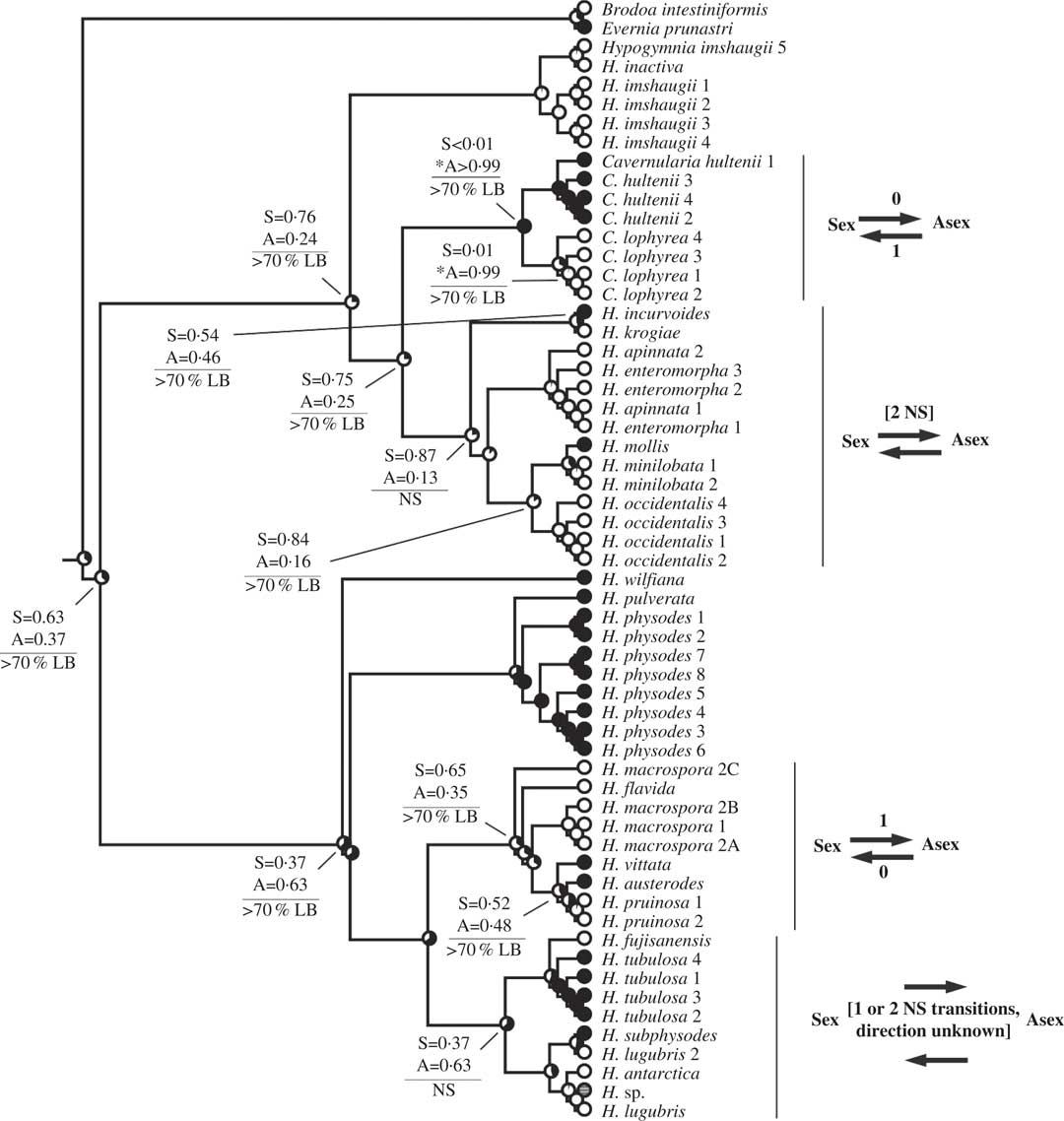
Fig. 4 Ancestral state reconstructions of reproductive mode using the Hypogymnia clade (Tier 2 analysis). Proportional likelihoods of reconstructed state shown in circles on nodes: white=sexual; black=asexual; grey=ambiguous reconstruction owing to missing data. Nodes pertinent to transitions are labelled as follows: above horizontal lines are relative proportional likelihoods for sexual (“S”) or asexual (“A”) states, and an asterisk indicates reconstruction was significant for that state; below horizontal lines are support values (PP=Posterior Probability, LB=Likelihood Bootstrap, PB=Parsimony Bootstrap, NS=non-significant (i.e. <95% PP or <70% LB or PB)). Numbers of inferred transitions and directionality of transitions given to the right of taxon labels. NS indicates that the reconstruction was non-significant (i.e. it did not meet both criteria for significance as described in Materials and Methods).
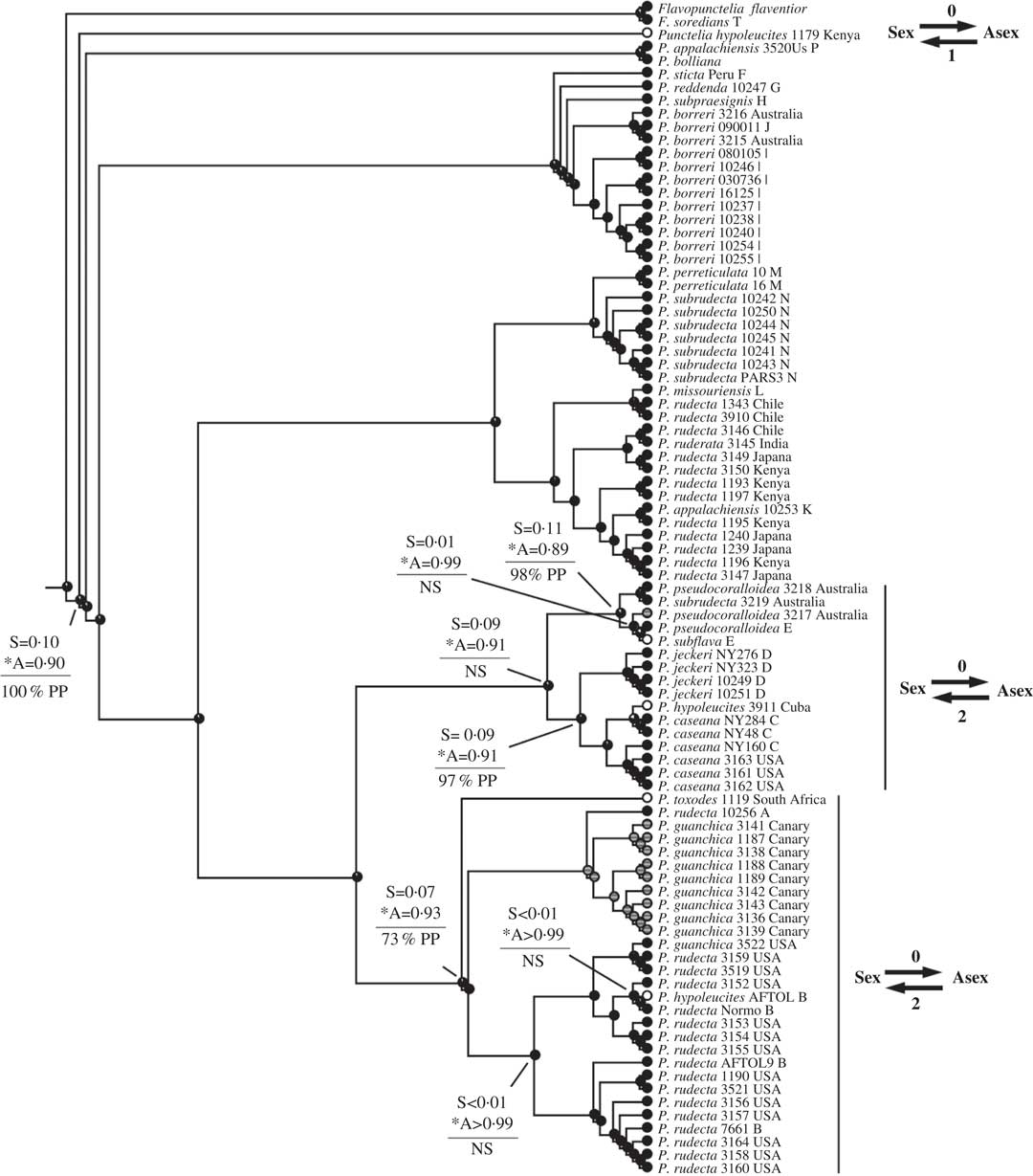
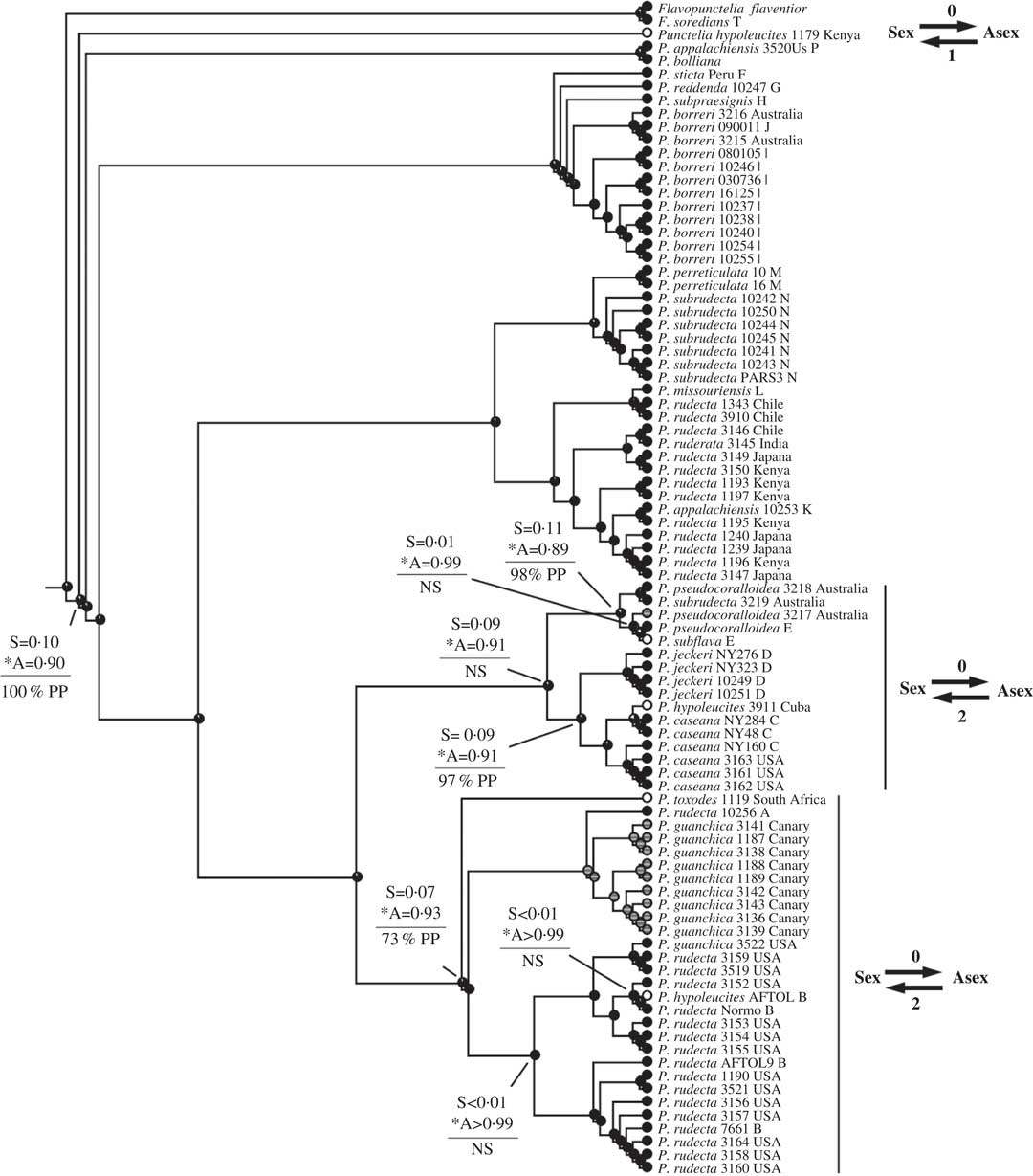
Fig. 5 Ancestral state reconstructions of reproductive mode using the Punctelia clade (Tier 1 analysis). Proportional likelihoods of reconstructed state shown in circles on nodes, white=sexual; black=asexual; grey=ambiguous reconstruction owing to missing data. Nodes pertinent to transitions are labelled as follows: above horizontal lines are relative proportional likelihoods for sexual (“S”) or asexual (“A”) states, and an asterisk indicates reconstruction was significant for that state; below horizontal lines are support values (PP=Posterior Probability, LB=Likelihood Bootstrap, PB=Parsimony Bootstrap, NS=non-significant (i.e. <95% PP or <70% LB or PB)). Numbers of inferred transitions and directionality of transitions given to the right of taxon labels.
Discussion
Reproductive traits are among the most important and thus frequently studied aspects of the ecology and life history of organisms (Tehler Reference Tehler1982; Case et al. Reference Case, Graham, Macfarlane and Barrett2008; Asplen et al. Reference Asplen, Whitfield, De Boer and Heimpel2009; Gomez-Mestre et al. Reference Gomez-Mestre, Pyron and Wiens2012; Poulíčková et al. Reference Poulíčková, Sato, Evans, Chepurnov and Mann2014; Oliveira et al. Reference Oliveira, Oi, do Nascimento, Vollet-Neto, Alves, Campos, Nascimento and Wenseleers2015). In particular, the question of whether asexuality (including ‘selfing’ in vascular plants) represents a terminal versus ancestral state and a stable versus transient state evolutionarily is both longstanding and pervasive across the tree of life (Bowler & Rundel Reference Bowler and Rundel1975; Judson & Normark Reference Judson and Normark1996; Goodwillie Reference Goodwillie1997; Normark et al. Reference Normark, Judson and Moran2003; Igic et al. Reference Igic, Bohs and Kohn2006; Gioti et al. Reference Gioti, Stajich and Johannesson2013; Castagnone-Sereno & Danchin Reference Castagnone-Sereno and Danchin2014; Hespeels et al. Reference Hespeels, Flot, Derzelle and Van Doninck2014). In this study, I amassed published datasets to explicitly reconstruct the frequency and polarity of evolutionary transitions between sexual and asexual reproduction in lichens. The resulting analyses indicate that gains of asexuality from sexual ancestors, consistent with dogma in lichenology, are indeed common and widespread across divergent groups of lichens. Summarizing the 23 clades studied here, the total number of forward transitions ranged from 26 (supported only) to 44 (including non-supported transitions). However, the analyses also yielded numerous reverse transitions: from 14 (supported) to 25 (including non-supported transitions) (Table 2). There are a few instances of non-supported transitions in which the direction of change is unknown. Thus, the numerous ‘reverse transitions’ from asexual ancestors to sexual derivatives documented here indicate ample diversity in reproductive mode evolution in lichens.
Asexuality is not a dead end in lichens
The results presented here reinforce a theme that has begun to emerge in lichenology in recent years, with several studies documenting the evolutionary potential of asexual lineages (e.g. Cornejo et al. Reference Cornejo, Chabanenko and Scheidegger2009; Lendemer Reference Lendemer2013). The work of Buschbom & Barker (Reference Buschbom and Barker2006) was one of the first and most important demonstrations that sexual lineages can arise from asexual ancestors. Through model-based reconstructions of reproductive mode in Porpidia s. lat. and other phylogenetic methods, these authors found evidence for the presence of traditional species pairs, statistical support for the non-monophyly of different asexual lineages, extremely high rates of gain of sexual reproduction from asexual ancestors, and high (although non-significant) conditional probabilities of asexuality serving as the ancestral state for four of six key nodes.
Clearly, asexual reproduction in lichens does not represent an evolutionary dead end based on Criterion 1 laid out in the Introduction, that it is a derived state only; instead, asexuality also serves as an ancestral state. Criterion 2, that whole lineages are known to be entirely asexual (Lepraria (57 spp.): Saag et al. Reference Saag, Saag and Randlane2009; Lendemer Reference Lendemer2013) or nearly entirely asexual (Chrysothrix (c. 20 spp.): Nelsen et al. Reference Nelsen, Lücking, Grube, Mbatchou, Muggia, Plata and Lumbsch2009; Leprocaulon s. str. Lamy (c. 12 spp.): Lendemer & Hodkinson Reference Lendemer and Hodkinson2013; J. Lendemer, pers. comm.) indicates that asexual lineages obviously undergo speciation and thus cannot be described as transient but are rather stable evolutionarily. However, the degree to which asexual versus sexual lineages persist remains an open question that will be among the most difficult to answer in the coming years. One potential means of exploring this question would be through more complete taxon sampling and analyses that account for time, for example, in a divergence time framework that allows for the calculation of dates of gain of asexuality as well as an estimation of longeveity of asexuality (the latter could then be compared to longeveity of sexuality). Such an approach would benefit from datasets in which authors have explicitly delimited phylogenetic species through gene tree-species tree reconciliation approaches, such as those made possible through coalescent based inference (e.g. Leavitt et al. Reference Leavitt, Divakar, Ohmura, Wang, Esslinger and Lumbsch2015; Singh et al. Reference Singh, Dal Grande, Divakar, Otte, Leavitt, Szczepanska, Crespo, Rico, Aptroot and Cáceres2015).
Whether reverse transitions are truly reflective of a complete regaining of sexuality rather than representing shifts from predominantly asexual to predominantly sexual states merits discussion. Regarding evolutionary trait reversals in other lineages, the genetic architecture underlying the trait of interest might not always be fully ‘lost’, so that the term ‘gain’ could be misleading (reviewed and discussed extensively in Collin & Miglietta Reference Collin and Miglietta2008). Instead, sexual reproduction could be suppressed (functional and under selection, but down-regulated) via one or more regulatory mechanisms for some evolutionary time period. If accurate, regains of sexual reproduction from asexual ancestors might instead reflect ‘latent homology’ (Osborn Reference Osborn1902; Carroll Reference Carroll2008) of sexuality, in which case the terms gain and loss should be interpreted with caution. Lichens that commonly display both asexual and sexual modes of reproduction contemporaneously (e.g. Leptogium dactylinum Tuck.; E. Tripp, pers. obs. (Lendemer, Tripp, et al. 30272, NY)) or at different life stages (e.g. Lasallia pustulata (L.) Mérat; Hestmark Reference Hestmark1992) lend evidence to support this hypothesis (see Hestmark et al. Reference Hestmark, Miadlikowska, Kauff, Fraker, Molnar and Lutzoni2011 for further discussion). In lichens, I am not aware of any study that has described the genetic architecture underlying sexual versus asexual reproduction, but I predict that a complete loss of sexual reproduction would be an exception rather than the rule (the genus Lepraria representing one possible exception).
Selfing as a dead end in other lineages?
To date, the majority of research that has explored micro- and macroevolutionary consequences of reduced outcrossing has done so from a vascular plant perspective of ‘selfing’, most commonly sporophytic selfing (and thus not selfing in the gametophytic or truly asexual sense). That selfing evolves from self-incompatible ancestors is axiomatic in plant reproductive biology and has been intensively investigated ever since Fisher’s (Reference Fisher1941) earliest theoretical models describing reduced fitness but transmission advantage of selfers over outcrossers. An exhaustive review of selfing as a dead end in plants was undertaken by Takebayashi & Morrell (Reference Takebayashi and Morrell2001), who concluded that the evidence to date was largely consistent with the dead-end hypothesis but recognized that directionality in plant mating system evolution may be more dynamic than previously appreciated. Since that review, evidence from plants as well as other organisms has cast doubt on the robustness of the dead end dogma. In an exhaustive study involving 571 taxa of Asteraceae, Ferrer & Good-Avila (Reference Ferrer and Good-Avila2007) used phylogenetic methods to demonstrate both forward and reverse transitions between self-incompatibility and self-compatibility, suggesting that selfing is not a dead end. In nematodes, unique genomic rearrangements are associated with mitotic, highly successful clades of asexual parasites (Castagnone-Sereno & Danchin Reference Castagnone-Sereno and Danchin2014), similarly suggesting that selfing is not a dead end. In an extensive review of estimates of the ages of asexual lineages from across the tree of life, Neiman et al. (Reference Neiman, Meirmans and Meirmans2009) demonstrated that asexual lineages are not always short-lived, as has been commonly held.
One limitation of the above comparison is that asexual reproduction in lichens is not equivalent to (sporophytic) selfing in vascular plants. A more appropriate comparison would be that of homothallism and heterothallism in lichens to self-compatibility and self-incompatibility in vascular plants, but data available on homo/heterothallism in lichens are far more scarce (before Scherrer et al. Reference Scherrer, Zippler and Honegger2005; Singh et al. Reference Singh, Dal Grande, Cornejo, Schmitt and Scheidegger2012) than the basic knowledge of sexual versus asexual modes of reproduction. Nonetheless, taken together, evidence from lichens, plants, and other organisms suggests that re-examination of the dead-end hypothesis is needed across the board: primarily asexual and/or selfing lineages can and do undergo speciation, may be old evolutionarily, and can and do give rise to primarily sexual lineages.
Origins, maintenance, and success of asexual lichens
The origin, maintenance, and success of asexuality or selfing as a primary reproductive strategy call for efforts to understand the underlying genetic mechanisms and population-level processes. Regarding origins of asexuality, the loss of mitochondria from conidia, which has been documented in some Xanthoria (Fr.) Th. Fr., effectively renders these propagules (that presumably function in fertilization of sexual species) functionless, without the capacity to germinate (Honegger Reference Honegger1984). Such a mechanism may be related to the complete loss of sexual reproduction in these organisms. Secondly, origins of asexuality at a population level may be explained by an imbalance in mating type alleles among individuals wherein heterothallic species fail to form sexual structures because of a paucity or lack of compatible mating types (Zoller et al. Reference Zoller, Lutzoni and Scheidegger1999; Singh et al. Reference Singh, Dal Grande, Cornejo, Schmitt and Scheidegger2012; see below for further discussion). Regardless of the mechanisms of the origins of asexuality, it must be remembered that sexual reproduction may in fact rarely be fully ‘lost’ (see above). Future studies at the genetic level are needed to clarify the molecular basis for sexual trait expression in lichens.
Maintenance of asexuality is likely explainable through some mechanism or set of mechanisms to acquire new genetic variation, perhaps to a degree greater than previously appreciated. First, both Kroken & Taylor (2001 Reference Kroken and Taylora ) and Buschbom & Mueller (Reference Buschbom and Mueller2005) cited recombination in asexual lineages of lichens, perhaps made possible through rare sexual reproduction, and similar evidence derives from non-lichenized fungi (Burt et al. Reference Burt, Carter, Koenig, White and Taylor1996; Geiser et al. Reference Geiser, Pitt and Taylor1998). Second, new variation may additionally be acquired through mutation. Dal Grande et al. (Reference Dal Grande, Widmer, Wagner and Scheidegger2012) found that, among 2229 thalli spanning 62 populations of Lobaria pulmonaria, 15% of genetic diversity in the mycobiont might be explainable by somatic mutations; more generally, appreciation for a role for somatic mutations in generating new genetic diversity is growing (Frank Reference Frank2010; Yong Reference Yong2012). Third, fungi may acquire new genetic material through non-sexual processes such as parasexuality, or the fusion of vegetative cells from adjacent thalli (Clutterbuck Reference Clutterbuck1996).
Success of asexual lineages has been addressed in a variety of previous works. In general, co-dispersal of both symbionts in vegetative propagules of asexual species is a highly efficient means of reproduction in lichens (Honegger Reference Honegger1984), and some asexually reproducing species have geographical ranges that are nearly twice as large as those of sexual species (Tripp et al. Reference Tripp, Lendemer, Barberán, Dunn and Fierer2016). Furthermore, Bowler & Rundel (Reference Bowler and Rundel1975) speculated that species with asexual reproduction have, on the whole, broader ecological amplitudes, such as the capacity of sorediate taxa to colonize both hardwoods and conifers (vs. some sexual taxa that colonize only hardwoods). Such ‘generalist’ strategies may correlate to the evolutionary longevity of asexual lineages. Other authors have hypothesized that asexuality is an effective mode of reproduction in degraded landscapes such as urban areas or industrial sites (Howe & Lendemer Reference Howe and Lendemer2010). For example, LeBlanc & De Sloover (Reference LeBlanc and De Sloover1970) found increased production of soredia associated with atmospheric pollution. Nonetheless, we currently lack a large-scale metasynthesis of correlations among environmental conditions, mode of reproduction, and other lichen functional traits. Future studies are needed to help understand the repeated origins, maintenance, and successes of asexual lineages.
Discrepancy in transition rates versus transition numbers
This study reiterates caveats associated with inferring rates of evolution during ancestral state reconstructions of character states. In particular, taxon sampling, branch lengths, models of evolution, and variable rates of evolution are among the many features that have an impact on reconstructions (Salisbury & Kim Reference Salisbury and Kim2001; Wiens et al. Reference Wiens, Kuczynski, Duellman and Reeder2007; Cusimano & Renner Reference Cusimano and Renner2014). In this study, I utilized an asymmetric two-rate model of evolution that was favoured over a symmetric one-rate model. However, the model implemented assumed constant rates of character evolution through time, no differences in rates of evolution across branches, and no differences in extinction rates among lineages. It is likely that the violation of one, two, or all three of these assumptions contributed to the discrepancy between transition rates and transition numbers documented here. For example, the Dirina and Biatora Fr. analyses yielded qualitatively similar patterns in transitions, in that both showed gains of asexuality from sexual ancestors and none in the reverse direction, but yielded very different transition rate biases (biases >1 in Dirina but <1 in Biatora). These two datasets additionally differed in their distribution of transitions: gains of asexuality were inferred only among extreme tips of the Biatora phylogeny, whereas gains of asexuality characterized deeper nodes of the Dirina phylogeny. Because of limitations of the method of ancestral state reconstruction implemented here, I have erred on the side of caution and emphasize inferred numbers of transitions over rates of transition in interpreting results. Future studies on lichen reproductive evolution should explore the impacts of different methods on ancestral state reconstructions, including methods that account for differential rates of character evolution and extinction, as well as those that account for relative time (e.g. rate smoothing approaches) or absolute time (e.g. divergence time analyses calibrated preferably by primary fossils).
Additional limitations
Interpretations from the present study rely on the robustness of the patterns documented here. Namely, it is well known that ancestral state reconstructions are complex analyses that depend heavily on several parameters in addition to those described above, not least of which is working with trees that reflect true evolutionary history (Duchêne & Lanfear Reference Duchêne and Lanfear2015). Igic et al. (Reference Igic, Bohs and Kohn2006) demonstrated that spurious results can arise from analyses that use only character states of extant taxa to infer ancestral states. At present, inference of major macroevolutionary patterns among lichens is limited by current availability of thoroughly (to completely) sampled phylogenies of extant taxa that form monophyletic groups; only with focused efforts to build such datasets can we begin to better understand the extent to which incomplete taxon sampling alters our assessment of these patterns. Both taxon and molecular (genetic) sampling similarly affect branch lengths, which are also well known to influence ancestral state reconstructions (Pagel Reference Pagel1999; Litsios & Salamin Reference Litsios and Salamin2012). The impact of basing reconstructions on raw phylogenetic distances that account only for substitutions instead of ultrametricized distances that additionally account for time has been debated, but recent studies have suggested that the two methods might yield qualitatively similar results more often than not (Cusimano & Renner Reference Cusimano and Renner2014). Here, I used trees based on raw phylogenetic distances not transformed in any way to account for relative or absolute time; further study is needed to explore the impact of reconstructions conducted on ultrametricized trees, if any.
In summary, although taxon sampling, extinction, variable rates of evolution, and many other factors are well-known complicators of phylogenetic and character evolution inferences (Salisbury & Kim Reference Salisbury and Kim2001; O’Meara Reference O’Meara2012), much knowledge can still be gained through ancestral state reconstructions and I predict that the overall dynamic histories of lichen reproductive evolution herein documented will hold through time.
What’s next?
In lichens, as well as in many non-lichenized fungal lineages, genetic research on reproductive biology has focused on homothallism and heterothallism, roughly equivalent to self-compatibility versus incompatibility in flowering plants. Even though not as exhaustively researched for non-lichenized ascomycetes, it is well understood that lichens have two extremely divergent mating type alleles found at a single locus (MAT-1, MAT-2) that determine reproductive compatibility (Coppin et al. Reference Coppin, Debuchy, Arnaise and Picard1997; Honegger et al. Reference Honegger, Zippler, Gansner and Scherrer2004; Rydholm et al. Reference Rydholm, Dyer and Lutzoni2007). Heterothallic lichens reproduce sexually when two nuclei containing different alleles fuse, whereas homothallic lichens reproduce sexually when nuclei containing the same mating type allele fuse (note that some homothallic species harbour both alleles; Scherrer et al. Reference Scherrer, Zippler and Honegger2005; Singh et al. Reference Singh, Dal Grande, Cornejo, Schmitt and Scheidegger2012). Future research in this area will undoubtedly yield important insights into mating system evolution, especially studies that combine next generation sequencing technologies with careful laboratory work such as analyses of single sporelings (Honegger et al. Reference Honegger, Zippler, Gansner and Scherrer2004). What remains to be explored in any detail is the relationship between mode of reproduction (sexual, asexual) and breeding system (homothallic, heterothallic). It seems clear that sexual versus asexual reproduction in lichens is largely one with genetic underpinnings rather than environmental plasticity. Yet the genetic basis of reproductive structures in lichens has never been studied. This is the most obvious area of extension for further research in lichen reproductive biology and is, to my knowledge, essentially wide open.
Conclusions
The present study represents a first attempt to synthesize general patterns in macroevolutionary transitions in mode of reproduction across disparate groups of lichenized fungi. Ancestral state reconstructions on phylogenies from several different groups indicate long-held assumptions about directionality in reproductive evolution are supported by empirical data in some cases, but not in others. In fact, that asexual lineages are long-lived evolutionarily and can give rise to sexual lineages (i.e. are not evolutionary dead ends) has been minimally appreciated in the past. Ancestral state reconstruction remains a powerful approach to understanding major trends in character evolution across groups. However, methods are well known to be sensitive to a wide variety of parameters including those pertaining to taxon sampling, character coding, model of character evolution, branch lengths, and rate heterogeneity among branches and through time (Ekman et al. Reference Ekman, Andersen and Wedin2008; Cusimano & Renner Reference Cusimano and Renner2014; King & Lee Reference King and Lee2015). A fully elucidated picture of lichen reproductive trait evolution awaits more densely sampled phylogenies and rigorous exploration of the impact of alternative reconstruction methods and models of evolution. For now, we can appreciate that asexuality in lichens may not be the evolutionary end point it was once viewed as.
I thank Ernie Brodo for his many contributions to lichenology that have provided phylogenetic fodder for the analyses conducted here and have also inspired my own interest in these remarkable organisms. I thank James Lendemer for our numerous discussions on lichen reproductive biology and for offering feedback on an earlier version of this manuscript. Kyle Dexter, François Lutzoni, and two anonymous reviewers similarly provided critical feedback on an earlier version, which improved the text. Much of this work has been inspired by the careful developmental and reproductive work conducted by Rosmarie Honegger and colleagues over recent decades, and she is here acknowledged for helping motivate a plant biologist to think more critically about lichen biology. The following individuals graciously answered queries and provided me with data files to enable my analyses: Mika Bendiksby, Pradeep Divakar, Damien Ertz, Klaus Kalb, James Lendemer, Robert Lücking, Thorsten Lumbsch, Rikke Naesborg, Sergio Pérez-Ortega, Raquel Pino-Bodas, Tiina Randlane, Philipp Resl, Lauri Saag, Garima Singh, Toby Spribille, Anders Tehler, and Martin Westberg. Finally, I am grateful to Peter Crittenden, James Lendemer, and François Lutzoni for their many efforts in organizing this special issue. This research was supported by an NSF Dimensions of Biodiversity Grant to Erin Tripp, James Lendemer, Nolan Kane, and Christy McCain (Award #1432629 & #1542639).
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0024282916000335
Appendix 1
Information on datasets used in this study including original file name as provided in TreeBase or DRYAD, study number provided in TreeBase or DRYAD, optimality criterion used to construct phylogeny and reference from which tree file was derived