Introduction
One of the challenges confronting lichenologists in relatively poorly documented regions such as Australia is to link the many disused species names, mostly described in the 19th century and in an extremely concise way, to modern collections and comprehensive descriptions, and to assign them to currently accepted genera. This particularly applies to names placed in the old Zahlbruckner ‘form’ genera such as Bacidia, Catillaria and, in particular, Lecidea. A reappraisal of these groups has now been underway for several decades, initially based principally on anatomical characters, especially ascus structure (e.g. Hafellner Reference Hafellner1984; Timdal Reference Timdal1984; Hertel & Rambold Reference Hertel and Rambold1987, Reference Hertel and Rambold1990; Kantvilas & Elix Reference Kantvilas and Elix1994; Printzen Reference Printzen1995, Reference Printzen1999; Rodriguez Flakus Reference Rodriguez Flakus2020), and, more recently, with the added phylogenetic evidence from DNA sequence data (e.g. Printzen & Kantvilas Reference Printzen and Kantvilas2004; Printzen et al. Reference Printzen, Spribille and Tønsberg2008; Stenroos et al. Reference Stenroos, Huhtinen, Lesonen, Palice and Printzen2009; Kalb et al. Reference Kalb, Rivas Plata, Lücking and Lumbsch2011; Schmull et al. Reference Schmull, Miadlikowska, Pelzer, Stocker-Wörgötter, Hofstetter, Fraker, Hodkinson, Reeb, Kukwa and Lumbsch2011; Rodriguez Flakus & Printzen Reference Rodriguez Flakus and Printzen2014; Spribille et al. Reference Spribille, Fryday, Pérez-Ortega, Svensson, Tønsberg, Ekman, Holien, Resl, Schneider and Stabentheiner2020). Thus, Lecidea in the strictest sense is today considered an exclusively saxicolous genus, characterized by, inter alia, distinctive 8-spored asci (Lecidea-type, after Hafellner (Reference Hafellner1984)) with simple, hyaline, non-halonate ascospores (e.g. see Hafellner Reference Hafellner1984; Hertel Reference Hertel1984; Fryday & Hertel Reference Fryday and Hertel2014) and, consequently, the corticolous taxa currently classified in this genus do not belong here. This is also the case for most of the species listed under Lecidea in the Australian lichen checklist (McCarthy Reference McCarthy2020), whereas for New Zealand, Galloway (Reference Galloway2007) explicitly noted that at least 11 of the 26 species of Lecidea recognized are misplaced.
The beginnings of the present study lay in the quest to establish the taxonomic affinities for a widespread, frequently collected corticolous Australian species that has gone under the name of Lecidea immarginata R. Br. ex Cromb. (Kantvilas & James Reference Kantvilas and James1991). This was one of the first lichens to be collected in Australia, by the botanist explorer Robert Brown who accompanied the expedition of the navigator Matthew Flinders in 1801–1803 to circumnavigate the continent of Australia, and who was present in 1804 at the founding of the settlement in Tasmania that was to become Hobart. The species was not formally described until much later, when the British lichenologist Rev. James Crombie reviewed Brown's collections, by then housed in London's Natural History Museum (Crombie Reference Crombie1880). Our examination of numerous Lecidea taxa based on Australasian types revealed that L. immarginata has been described multiple times. Its curious ascus type led to an investigation of several other genera, most notably Malcolmiella Vĕzda (Vĕzda Reference Vĕzda1997), Malmidea Kalb et al. (Kalb et al. Reference Kalb, Rivas Plata, Lücking and Lumbsch2011) and Myochroidea Printzen et al. (Printzen et al. Reference Printzen, Spribille and Tønsberg2008).
The genus Malcolmiella was introduced for the single species M. cinereovirens by Vĕzda (Reference Vĕzda1997), who described two varieties, differing by the putative presence of isidia. His description was scant and made no particular reference to the peculiar asci of these lichens. The genus was soon taken up by Lücking & Kalb (Reference Lücking and Kalb2000) who added four taxa and noted that the critical features of Malcolmiella were the asci having a ring structure, the paraplectenchymatous exciple and the halonate ascospores. Over the following years, Malcolmiella was gradually expanded by the addition of further, exclusively tropical taxa (Kalb Reference Kalb2004; Aptroot et al. Reference Aptroot, Saipunkaew, Sipman, Sparrius and Wolseley2007; Cáceres Reference Cáceres2007; Lücking Reference Lücking2008; Kalb et al. Reference Kalb, Buaruang, Papong and Boonpragob2009). Interestingly, some of these authors specifically referred back to the particular asci that were present in Vĕzda's type species from the cool temperate latitudes of New Zealand, even though the taxa they were studying did not possess this character.
At length, Kalb et al. (Reference Kalb, Rivas Plata, Lücking and Lumbsch2011) studied these taxa using molecular and anatomical data and found that the tropical species, all essentially those referred to historically as the Lecidea piperis-group, were in fact unrelated to Malcolmiella and erected the genus Malmidea within a new family, the Malmideaceae. With respect to anatomy, Malmidea differs from Malcolmiella by its exciple of radiating, thick hyphae encrusted with hydrophobic granules, a dark pigmented hypothecium, a coherent hymenium with thin, entangled, branched paraphyses, asci with a tholus that lacks any internally differentiated structures, thinly halonate ascospores, sometimes with an apically thickened wall, and a thallus chemistry usually consisting of atranorin and other substances. Molecular data supported Malmidea as sister to the Pilocarpaceae, whereas Malcolmiella was, curiously, placed as sister to the Teloschistaceae, even though there were no supporting anatomical similarities between the two (Kalb et al. Reference Kalb, Rivas Plata, Lücking and Lumbsch2011). Malmidea is now a generally accepted genus of some 50 species (Breuss & Lücking Reference Breuss and Lücking2015) whereas Malcolmiella is monotypic. McCarthy (Reference McCarthy2020) records four species of Malmidea for Australia, chiefly from tropical latitudes, but it is likely that further species may be lurking amongst Australasian species and herbarium specimens currently classified in Lecidea.
Myochroidea was described by Printzen et al. (Reference Printzen, Spribille and Tønsberg2008) for a group of corticolous, lecideoid lichens with reddish brown, biatorine apothecia and 8-spored, Micarea-type asci. All four species are known only from the temperate Northern Hemisphere.
In the present study, these and other superficially similar genera are compared and the new genus Australidea is described to accommodate Lecidea immarginata under an older name, L. canorufescens. A comprehensive anatomical and morphological description of Malcolmiella is also provided and this genus is recorded for Tasmania for the first time. Several names based on Australasian types are reduced to synonymy.
Materials and Methods
Morphology and anatomy
The study is based mainly on the first author's collections, housed in the Tasmanian Herbarium (HO), and on reference material in other herbaria as cited, chiefly London's Natural History Museum (BM), the Finnish Museum of Natural History (H), the Museum of Evolution, Uppsala University (UPS), and the Swedish Museum of Natural History (S). Investigations were undertaken on hand-cut sections of thalli and apothecia, using standard methods, reagents and stains: water, 10% KOH (K), lactophenol cotton blue and Lugol's iodine (I).
Measurements of ascospores are based on 60–100 observations of each taxon and are presented in the format 5th percentile–average–95th percentile, with outlying values in brackets and n the number of observations. Routine thin-layer chromatographic analysis (TLC) was undertaken using standard methods, with solvent A as the preferred medium (Orange et al. Reference Orange, James and White2010).
Observing and interpreting the structure of the exciple and asci can present technical difficulties. Interpretation is rarely arrived at after a single observation or even several, but may require multiple sections, experimentation with different concentrations of key reagents, and other manipulations. Such complications should not act as a deterrent, especially when, as illustrated in this study, many of the taxa in question are represented entirely by old specimens which do not offer usable DNA and anatomical characters are all that are available.
Comparative herbarium material examined for anatomical studies
Type material studied is cited in the main text. Other specimens studied are listed here:
Biatora subduplex (Nyl.) Printzen. Austria: Oetztal, Obergurgl, 2400−2700 m, 1993, A. Vĕzda & F. Ceni (A. Vĕzda: Lich. Rar. Exs., 112) (HO).
Biatora vernalis (L.) Fr. Sweden: Jämtland, Undersåker, Vällista, 1912, G. O. Malme (Malme: Lich. Suec. Exs., 285a) (UPS).
Japewiella pruinosa (Müll. Arg.) Kantvilas. Australia: Tasmania: St Crispins Well, 42°56′S, 147°13′E, 640 m, 1987, G. Kantvilas 69/87 (HO).
Malmidea granifera (Ach.) Kalb et al. Brazil: Rio de Janeiro: M° Canos da Carioca, 1892, G. O. Malme 144 (S).
Malmidea leptoloma (Müll. Arg.) Kalb et al. Solomon Islands: Guadalcanal: Umasani River, 50–150 m, 1965, D. J. Hill 8072 (BM).
Malmidea piperis (Spreng.) Kalb et al. Peru: Prov. San Martin: Cerro Escalera (c. 20 km NE of Tarapoto, 6°27′S, 76°15′W, 900–1100 m, 1981, R. Santesson & G. Thor P72:29 (S).—Paraguay: Concepción: Colonia Risso, 1893, G. O. Malme 1942 (S).—Australia: Queensland: Cape Tribulation, 16°05′S, 145°29′E, 1991, W. H. Ewers 8342 (CANB, HO). Northern Territory: Channel Point, 13°07′S, 130°13′E, 10 m, 1991, J. A. Elix 27700, H. T. Lumbsch & H. Streimann (CANB, HO).—USA: Louisiana: Burden Research Plantation, Essen Lane, Baton Rouge, 1984, S. Tucker 26667 (HO).
Malmidea psychotrioides (Kalb & Lücking) Kalb et al. Cuba: Prov. Oriente: Bayate, 1917, E. L. Ekman s. n. (S L6964).
Myochroidea porphyrospoda (Anzi) Printzen et al. Sweden: Jämtland: Mt Täljstensvalen, 63°15′N, 12°27′E, 750 m, 2005, M. Svensson 549 (HO, UPS).
Myochroidea rufofusca (Anzi) Printzen et al. Sweden: Torne Lappmark, southern slope of Mt Latnjačorru, 1935, G. E. Du Rietz (HO, UPS).
DNA extraction, amplification and sequencing
Total DNA was extracted using the Plant DNA Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. The mrSSU rDNA was amplified and sequenced with the primers mrSSU1 and mrSSU3R (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999). The nuLSU rDNA was amplified and sequenced using the primers nu-LSU-155-5′ (Döring et al. Reference Döring, Grube, Clerc and Wedin2000) or LRlecF (Schneider et al. Reference Schneider, Resl, Westberg and Spribille2015), or in combination with either LR5 (Vilgalys & Hester Reference Vilgalys and Hester1990) or LRlecR (Schneider et al. Reference Schneider, Resl, Westberg and Spribille2015).
Amplifications were performed with the initial denaturation at 95 °C for 5 min, followed by 35 (mrSSU) or 40 (nuLSU) cycles of 95 °C for 45 s, 57 °C for 45 s, 72 °C for 1 min 45 s, and a final extension at 72 °C for 10 min. PCR products were subsequently purified with Exonuclease I and FastAP Thermosensitive Alkaline Phosphatase (Thermo Scientific™). For mrSSU, sequencing reactions were carried out using the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Warrington, Cheshire, UK), and fragments were separated on an ABI 3130xl Genetic Analyzer (Applied Biosystems). For LSU, purified PCR products were sequenced by Macrogen Europe B.V. (Amsterdam, The Netherlands).
Taxon sampling
BLAST searches and preliminary analyses suggested that Australidea canorufescens is a member of the Malmideaceae. To further assess the phylogenetic position of this species within the family, sequences from representatives of all genera known to belong to Malmideaceae were downloaded from GenBank: Cheiromycina (Muggia et al. Reference Muggia, Mancinelli, Tønsberg, Jablonska, Kukwa and Palice2017), Crustospathula (Sodamuk et al. Reference Sodamuk, Boonpragob, Mongkolsuk, Tehler, Leavitt and Lumbsch2017; Kistenich et al. Reference Kistenich, Timdal, Bendiksby and Ekman2019), Malmidea (Kalb et al. Reference Kalb, Rivas Plata, Lücking and Lumbsch2011), Puttea (Spribille et al. Reference Spribille, Fryday, Pérez-Ortega, Svensson, Tønsberg, Ekman, Holien, Resl, Schneider and Stabentheiner2020), Savoronala (Ertz et al. Reference Ertz, Fischer, Killmann, Razafindrahaja and Sérusiaux2013), Sprucidea (Cáceres et al. Reference Cáceres, Aptroot, Mendonça, Santos and Lücking2017) and Zhurbenkoa (Flakus et al. Reference Flakus, Etayo, Pérez-Ortega, Kukwa, Palice and Rodriguez2019). For all these genera, mitochondrial SSU was available in GenBank; we also included LSU when that was available (see Table 1). Species of unclear generic affinities (e.g. Lecidea cyrtidia, Psoroma karstenii, Toninia thiopsora) that might belong in the Malmideaceae (e.g. Ertz et al. Reference Ertz, Fischer, Killmann, Razafindrahaja and Sérusiaux2013; Kistenich et al. Reference Kistenich, Timdal, Bendiksby and Ekman2019) were not included. The only available LSU sequence of Puttea margaritella (GenBank Accession no.: EU940111) was found to blast close to species of Phacidium and was therefore not included. As outgroup, we selected representatives from the Sphaerophorinae, including the families Pilocarpaceae, Psoraceae, Ramalinaceae and Sphaerophoraceae. This decision was based on Malmideaceae being sister to Pilocarpaceae in the analysis by Kalb et al. (Reference Kalb, Rivas Plata, Lücking and Lumbsch2011) and as sister to the Sphaerophorinae in fig. 10 of Spribille et al. (Reference Spribille, Fryday, Pérez-Ortega, Svensson, Tønsberg, Ekman, Holien, Resl, Schneider and Stabentheiner2020). The recently described genus Kalbionora was placed in the Malmideaceae by its authors (Sodamuk et al. Reference Sodamuk, Boonpragob, Mongkolsuk, Tehler, Leavitt and Lumbsch2017) but, in a subsequent analysis using wider taxon sampling and more loci, a placement of Kalbionora within Malmideaceae was not supported (Spribille et al. Reference Spribille, Fryday, Pérez-Ortega, Svensson, Tønsberg, Ekman, Holien, Resl, Schneider and Stabentheiner2020). Consequently, we excluded Kalbionora from our analyses. Furthermore, we note that the only available mrSSU sequence from Kalbionora palaeotropica (GenBank Accession no.: KY926784), and which was used in the earlier analyses, blasts with members of the Chaeothyriales. No sequences for Myochroidea were available, nor could recent collections suitable for sequencing be obtained.
Table 1. Sequence data used for the phylogenetic study of Australidea canorufescens, with GenBank Accession numbers and voucher information. Newly obtained sequences are in bold.

Since the earlier analysis of Kalb et al. (Reference Kalb, Rivas Plata, Lücking and Lumbsch2011) placed Malcolmiella as sister taxon to Teloschistaceae, and as our preliminary analyses indicated that it was indeed only distantly related to the Malmideaceae, we performed a separate analysis to assess the phylogenetic position of this genus. BLAST searches and preliminary analyses indicated a possible relationship with the Lecideaceae, and we thus selected a number of genera from this family in a broad sense. We retained Teloschistaceae in the analysis and also included two genera each from the Peltigerinae and Collematinae, since these are probably more closely related to Lecideaceae than to Teloschistaceae (Miadlikowska et al. Reference Miadlikowska, Kauff, Högnabba, Oliver, Molnár, Fraker, Gaya, Hafellner, Hofstetter and Gueidan2014). We selected Rhizocarpaceae as outgroup, as this family appears basal to the Lecanoromycetidae (Miadlikowska et al. Reference Miadlikowska, Kauff, Högnabba, Oliver, Molnár, Fraker, Gaya, Hafellner, Hofstetter and Gueidan2014), which includes all other genera and families used in the analysis. For the selected genera and species, we downloaded sequences of mrSSU, LSU, ITS, RPB1 and RPB2 from GenBank (Table 2).
Table 2. Sequence data used for the phylogenetic study of Malcolmiella interversa, with GenBank Accession numbers and voucher information. Newly obtained sequences are in bold.

Sequence alignment, partitioning scheme and phylogenetic analysis
For both the Malmideaceae and Malcolmiella phylogenies, we estimated separate alignments for mrSSU, LSU and (for the Malcolmiella analysis) ITS using PASTA (Mirarab et al. Reference Mirarab, Nguyen, Guo, Wang, Kim and Warnow2015), with the mask option activated, MAFFT (algorithm L-INS-i) for alignment, OPAL for the pairwise merging, and FastTree as the tree estimator, with GTR + Γ as the model for molecular evolution. As PASTA is an iterative method that optimizes the alignment under a maximum likelihood (ML) framework, we did no further manual adjustment or filtering of ambiguous regions of the resulting alignments. For RPB1 and RPB2, we estimated the alignment using MAFFT (algorithm E-INS-i; Katoh et al. Reference Katoh, Rozewicki and Yamada2019). After aligning the sequences, we identified several non-coding introns in the RPB1 alignment and removed these before any further analysis was performed.
To check for possible conflicting phylogenetic signals between datasets, we performed a separate ML analysis of each alignment using IQ-TREE (Nguyen et al. Reference Nguyen, Schmidt, von Haeseler and Minh2015), assessing branch support with ultrafast bootstrap (Hoang et al. Reference Hoang, Chernomor, von Haeseler, Minh and Vinh2018), running 2000 replicates. We evaluated models of molecular evolution using the version of ModelFinder (Kalyaanamoorthy et al. Reference Kalyaanamoorthy, Minh, Wong, von Haeseler and Jermiin2017) implemented in IQ-TREE and chose AICc as the criterion for estimation of model fit. For the Australidea analysis, GTR + F + I + G4 (mrSSU) and GTR + F + R3 (LSU) were selected as having the best model fit, whereas for the Malcolmiella analysis, the models TIM2 + F + I + G4 (ITS, RPB1), TIM2 + F + R3 (LSU), TVM + F + R3 (mrSSU) and TIM3 + F + I + G4 (RPB2) were selected. The single marker trees were then compared to locate any supported (> 80% bootstrap), conflicting results. As no such results were detected, in both cases (Malmideaceae + Malcolmiella) we decided to concatenate the separate datasets into one alignment. The final, concatenated alignments are deposited in TreeBase (TB-ID 28193).
Assessment of the division of the two different concatenated alignments into partitions was undertaken using PartitionFinder 2.1.1. (Lanfear et al. Reference Lanfear, Frandsen, Wright, Senfeld and Calcott2017), which also allows for simultaneous estimation of models of molecular evolution for the partitions. We restricted the estimation to models implemented in MrBayes 3.2.6. (which was used for subsequent phylogenetic analysis, see below), used AICc for model selection, assumed linked branched lengths, and used the ‘greedy’ algorithm (Lanfear et al. Reference Lanfear, Calcott, Ho and Guindon2012). For the Malmideaceae phylogeny, we assessed the division of the concatenated alignment into two partitions, mrSSU and LSU. The analysis recommended keeping two partitions, with GTR + Γ selected as the best model for mrSSU and GTR + Γ + I for LSU. For the Malcolmiella phylogeny, we assessed the division of the dataset into 11 partitions: five for mrSSU, LSU, ITS1, 58S and ITS2, and six for three independent codon positions of RPB1 and RPB2, respectively. The analysis recommended merging the 1st codon position of RPB1 and RPB2 into one partition. Furthermore, the model selection gave the best models for the partitions as GTR + Γ (ITS2, RPB2 2nd codon position, RPB2 3rd position), GTR + Γ + I (mrSSU, LSU, RPB1 2nd codon position, RPB1 + RPB2 1st codon positions combined), SYM + Γ (RPB1 2nd codon position) and K80 + Γ (ITS1).
We performed phylogenetic analyses on the concatenated, partitioned alignments using MrBayes 3.2.6. (Ronquist et al. Reference Ronquist, Teslenko, Mark, Ayres, Höhna, Larget, Liu and Huelsenbeck2012). We used flat Dirichlet priors for the substitution rates and state frequencies, and a uniform prior for invariant sites. We ran four Markov chain Monte Carlo (MCMC) chains, three incrementally heated (by a factor of 0.1) and one cold. The sample frequency was set to every 100th generation. The analysis was halted when convergence was reached, which was defined as an average standard deviation of split frequencies below 0.01. The fraction of trees discarded as burn-in was set to 25%. In addition to the Bayesian analysis, we also performed ML analyses of the concatenated alignments with IQTree, using the same partitioning scheme and models of molecular evolution as for the Bayesian analysis. We used edge-proportional partition models and assessed branch support by running 1000 non-parametric bootstrap replicates.
Results
The results of the molecular analysis of Malmideaceae (Fig. 1) show strong support for a placement of Lecidea canorufescens in this family, where the species is sister to the genus Malmidea (the type genus of the family). In addition to the phylogenetic results, L. canorufescens also differs from Malmidea and other genera of Malmideaceae in anatomical and morphological respects, and we thus describe the new genus Australidea to accommodate it.

Fig. 1. Majority-rule consensus tree based on a Bayesian MCMC analysis of mrSSU and LSU, showing the phylogenetic position of Australidea in the Malmideaceae. Branch support is given as posterior probability(PP)/bootstrap support (BS). Bootstrap support values are from a corresponding maximum likelihood analysis. Only BS values > 70% are shown. GenBank Accession numbers and voucher information are given in Table 1.
The analysis of the phylogenetic position of Malcolmiella (Fig. 2) shows that this genus is only distantly related to Teloschistaceae, where it had appeared as sister in earlier work (Kalb et al. Reference Kalb, Rivas Plata, Lücking and Lumbsch2011). The results indicate affinities with a group of genera (Bryobilimbia, Clauzadea, Romjularia and the Lecidea berengeriana-group) that is usually assigned to Lecideaceae. While our analysis does not offer any unequivocal conclusions regarding the phylogenetic position of this group (including Malcolmiella), the results clearly indicate that it, as well as the genus Lecidoma, do not belong in the Lecideaceae.
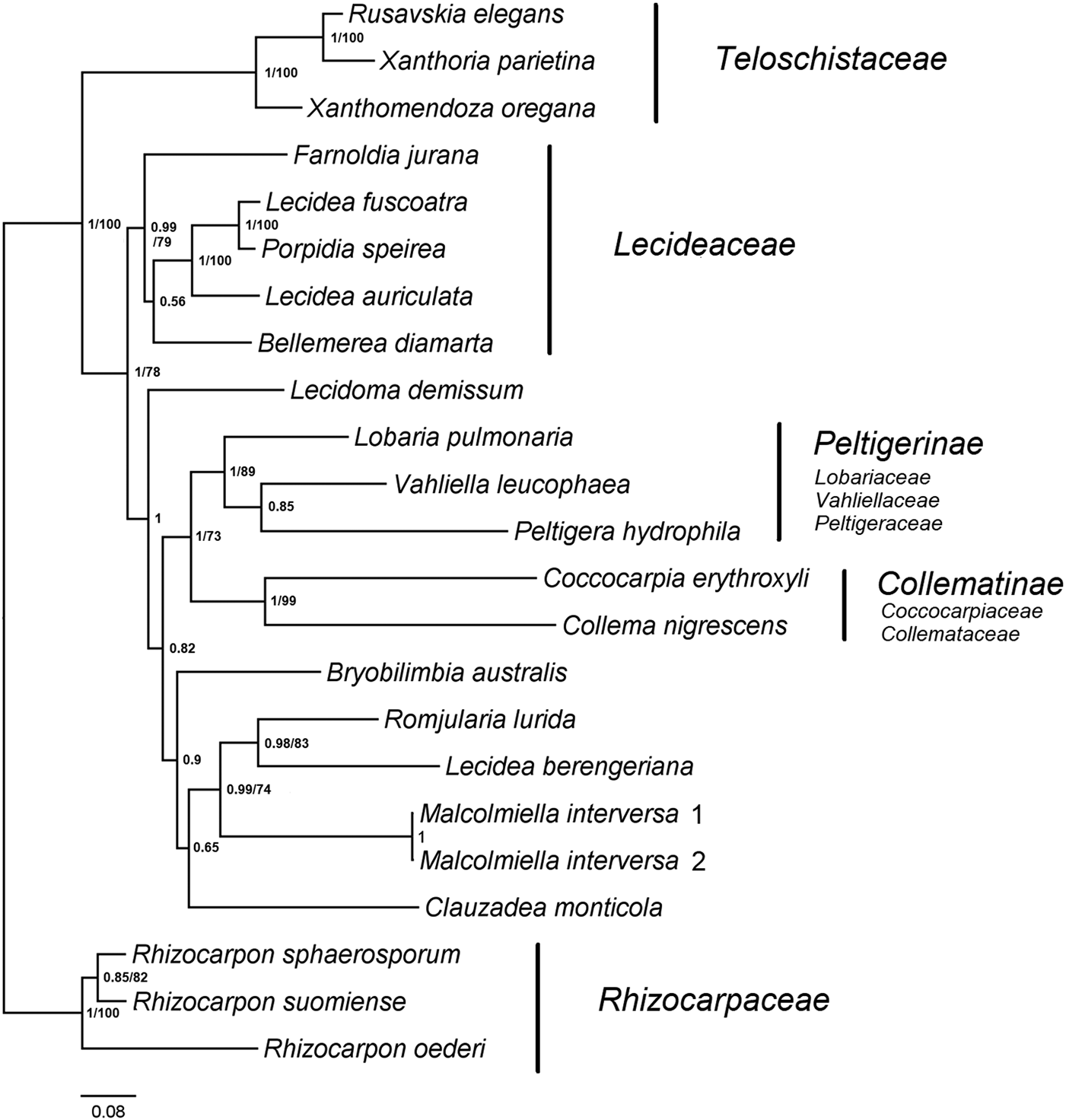
Fig. 2. Majority-rule consensus tree based on a Bayesian MCMC analysis of mrSSU, LSU, ITS, RPB1 and RPB2, showing the phylogenetic position of Malcolmiella. Branch support is given as posterior probability(PP)/bootstrap support (BS). Bootstrap support values are from a corresponding maximum likelihood analysis. Only BS values > 70% are shown. GenBank Accession numbers and voucher information are given in Table 2.
Taxonomy
Australidea Kantvilas, Wedin & M. Svensson gen. nov.
MycoBank No.: MB 839472
Thallus crustaceus, corticolus, algas virides unicellulares continens. Apothecia biatorina, excipulo proprio cupulato interne incolorato, ex hyphis ramosis anastomosantibusque constructo. Paraphyses plerumque simplices. Asci octospori typo Porpidiae pertinentes, ascosporis simplicibus hyalinis non-halonatis ovatis vel ellipsoideis.
Typus generis: Australidea canorufescens (Kremp.) Kantvilas, Wedin & M. Svensson.
Thallus crustose. Photobiont a unicellular green alga with globose cells 6–15 μm diam.
Ascomata apothecia, biatorine, basally constricted; proper exciple in section cupulate, hyaline within, not inspersed, composed of a loose reticulum of branched and anastomosing hyphae in a gel matrix. Hypothecium hyaline. Hymenium intensely KI+ blue, rather coherent in water and K. Paraphyses simple or, very occasionally, sparsely branched, not capitate. Asci clavate, 8-spored; tholus amyloid, with a darker-staining ring structure with parallel or diverging sides; ocular chamber not developed. Ascospores simple, hyaline, non-halonate, ovate to ellipsoid.
Pycnidia not found.
Chemistry
No substances detectable by TLC.
Etymology
The generic name is derived from ‘austral’, meaning ‘southern’ in geographical distribution, and ‘Lecidea’, the traditional placeholder genus for many crustose lichens with simple ascospores.
Remarks
The combination of the particular ascus type, with its intensely amyloid ring structure within the tholus, the anatomy of the exciple, the mostly simple, non-capitate paraphyses, and the relatively large, simple, non-halonate ascospores with a distinct wall of ±uniform thickness characterizes this genus and distinguishes it from other genera of Lecidea s. lat. It is the ascus in particular that best distinguishes Australidea from superficially similar genera with reddish brown, biatorine apothecia and relatively large ascospores, notably Japewiella and Myochroidea. The ascus is reminiscent of the Porpidia-type (Hafellner Reference Hafellner1984), which is known only from a complex of genera that are either exclusively saxicolous (e.g. Porpidia), terricolous or overgrow epiphytic bryophytes (e.g. Bryobilimbia). Many of the species in these genera also have intensely dark-pigmented apothecia and, at times, septate ascospores, and are clearly not closely related to the new genus. A comparison of the salient features of selected superficially similar genera is summarized in Table 3; asci are compared in Fig. 4.
Table 3. Comparison of salient features of some superficially similar crustose lichen genera with biatorine apothecia.

Australidea canorufescens (Kremp.) Kantvilas, Wedin & M. Svensson comb. nov.
MycoBank No.: MB 839489
Lecidea canorufescens Kremp., Verhandl. Zool.-Bot. Ges. Wien 26, 454 (1876); type: New Zealand, sine loco [probably Wellington], Charles Knight (M 24801!—lectotype, designated here, MBT10001035; M 24800!, M 24803!—isolectotypes).
Lecidea immarginata R. Br. ex Cromb., J. Linn. Soc., Bot. 17, 400 (1880); type: [Australia, New South Wales] amongst mosses on the bark of trees, bank of Grose River, R. Brown 513 (BM!—lectotype, designated here, MBT10001036; H-NYL 20464!—isolectotype).
Lecidea glandulosa C. Knight, Trans. N. Z. Inst. 12, 376 (1880); type: New Zealand, sine loco [probably Wellington], Charles Knight (BM!—lectotype, designated here, MBT10001037; H-NYL!, UPS!—isolectotypes).
Lecidea intervertens Nyl., Lich. Nov. Zel., 79 (1888); type: New Zealand, sine loco [probably Wellington], 1882, Charles Knight (BM!—lectotype, designated here, MBT10001038; H-NYL!, UPS!—isolectotypes) (same specimens as for L. glandulosa, above).
Lecidea dacrydii Müll. Arg., Hedwigia 32, 127 (1893); type: New Zealand, Colenso b349 (BM!—holotype).
Lecidea eucheila Zahlbr., Denkschr. Akad. Wiss. Wien Math.-Naturwiss. Kl. 104, 309 (1941); type: New Zealand, Otago, Mt Cargill, Dunedin, on bark of Dacrydium cupressinum, J. S. Thomson 543 (W—holotype; OTA, CHR!—isotypes).

Fig. 3. A, habit of Australidea canorufescens. B, habit of Malcolmiella interversa. Scales = 1 mm. In colour online.
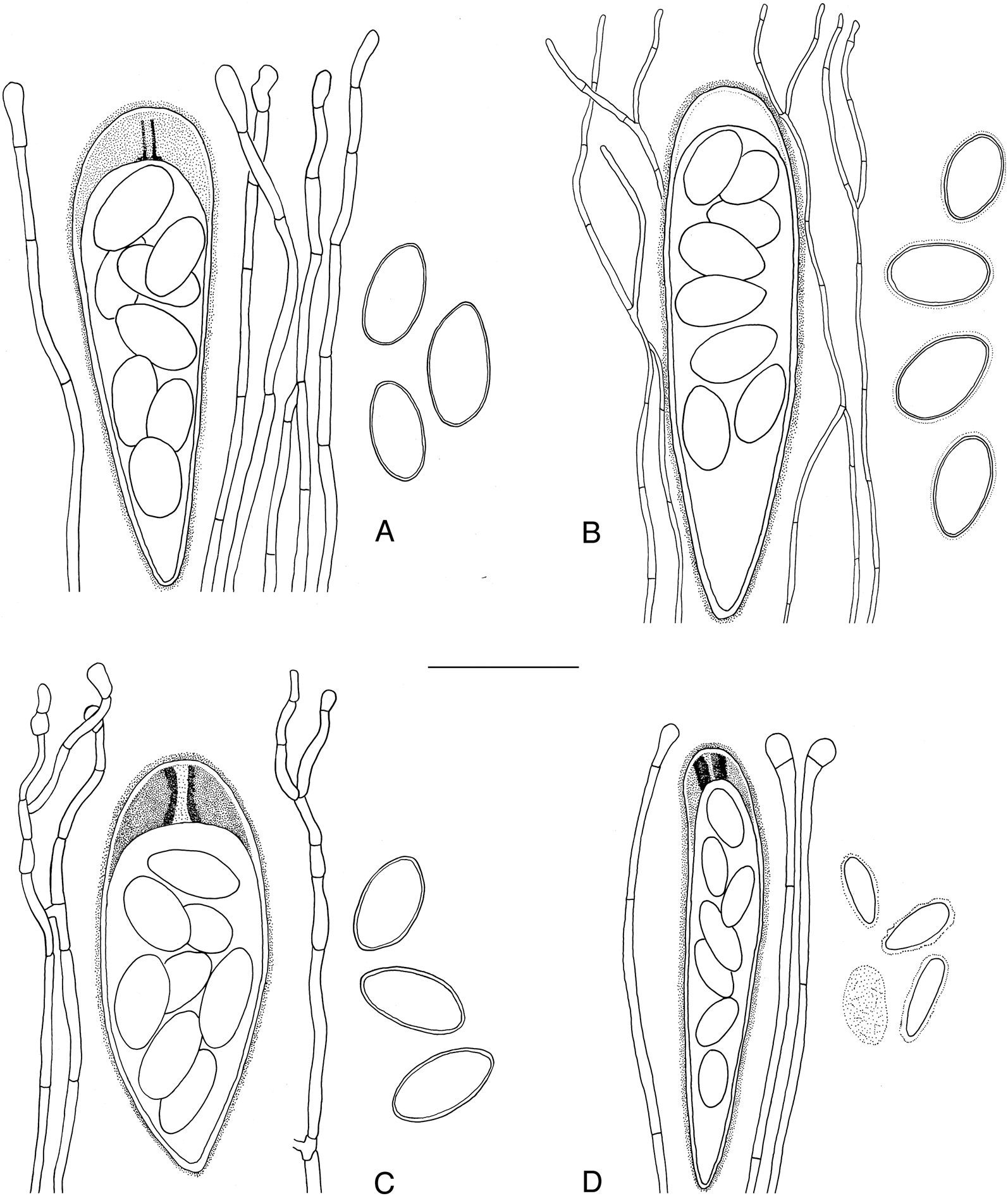
Fig. 4. Comparison of asci (with amyloid parts stippled), paraphyses and ascospores of species of superficially similar corticolous, lecideoid crustose genera. A, Australidea canorufescens. B, Malmidea piperis. C, Myochroidea porphyrospoda. D, Malcolmiella interversa. Scale = 20 μm.
Thallus crustose, pale grey, at first smooth and rimose, soon becoming very uneven, scurfy and abraded, at times almost patchily sorediate, 25–100 μm thick, ecorticate, undelimited although sometimes with a dark grey prothallus at the leading edge; photobiont a unicellular green alga, mostly aggregated in clumps, with individual cells 6–10(–15) μm diam.
Apothecia scattered, biatorine, to 1 mm diam., basally constricted; disc pale brown to reddish brown to brown, frequently a little mottled, rarely flesh-coloured or whitish and ±translucent when very young or overmature, epruinose, plane when well developed but becoming convex and immarginate when old; proper exciple concolorous with, paler or darker than the disc, usually persistent and excluded only in the oldest, most convex apothecia, in section cupulate, usually dilutely reddish brown at the outer, upper edge, hyaline within, 50–120 μm thick laterally, to 125–250 μm thick at the base, composed of a rather loose reticulum of branched and anastomosing hyphae in a gel matrix; excipular hyphae not inspersed and lacking encrusting crystals, 1–1.5 μm wide where unpigmented, up to 3.5–5 μm wide where pigmented but with the terminal cells not markedly enlarged. Hypothecium hyaline, not inspersed, 25–100 μm wide, becoming massive and poorly differentiated from the exciple in old, very convex apothecia. Hymenium 60–100 μm thick, not inspersed, intensely KI+ blue, rather coherent in water and K, mostly hyaline but dilutely reddish brown (as in the exciple) in the uppermost part; pigment ±unchanged or becoming a little duller in K. Paraphyses simple or, very occasionally, sparsely branched, 1.5–2.5 μm thick where unpigmented, in the upper part sometimes internally dilutely reddish brown and gradually expanding to 3–5.5 μm wide but not capitate. Asci clavate, 8-spored, 50–70 × 15–22 μm; tholus prominent in the early stages, becoming compressed as the ascospores develop, amyloid, with a short, markedly darker-staining ring structure with parallel or diverging flanks; ocular chamber not developed. Ascospores hyaline, non-halonate, ovate to ellipsoid, (10–)12–14.5–17(–18) × (6–)7–8.4–10(–11) μm (n = 120); wall not ornamented, uniformly c. 1 μm thick.
Pycnidia not found.
Chemistry
Thallus and apothecia containing no substances detectable by TLC.
Remarks
The distinctiveness of this taxon is illustrated to a large extent by the multiple times it has caught the attention of lichenologists in the past and been described. Five synonyms are listed above, but there could well be additional ones amongst the as-yet-uninvestigated names of crustose lichens described from Australasia in the 19th century. However, the synonyms given by, for example, Galloway (Reference Galloway1985, Reference Galloway2007) should be approached with extreme caution since study of the relevant type specimens revealed that several different, albeit superficially similar, species are involved. The Tasmanian record of Malmidea leptoloma (Müll. Arg.) Kalb & Lumbsch is also based on old herbarium specimens of Australidea canorufescens, dating from a time when the name Lecidea leptoloma was widely misapplied to several corticolous lecideoid species; it should be deleted from the Tasmanian census.
The most similar species to Australidea canorufescens is what has been described from New Zealand as Lecidea fuscoincerta Stirt. (holotype in BM examined). The generic affinities of this lichen are yet to be clarified and must await the collection of fresh material suitable for DNA extraction and amplification. Its brown, biatorine apothecia, scurfy grey thallus, mostly simple paraphyses and ascospores (13−17 × 7−9 μm) are essentially identical to those of A. canorufescens but the asci are different, having a well-developed, weakly amyloid tholus that lacks a ring or any other internal differentiation (cf. the Malmidea-type; Fig. 4B). Furthermore, its exciple is comprised of densely agglomerated prosoplectenchymatous hyphae. So similar is this taxon to A. canorufescens that repeated anatomical observations were required to confirm that these differences were consistent. Lecidea conisalea C. Knight (type specimens in BM and H-NYL studied), also described from New Zealand, appears to be the same species as L. fuscoincerta.
Although Malcolmiella interversa is based on a specimen which was synonymized with Australidea canorufescens (Galloway Reference Galloway1985), these two species are unlikely to be confused, even in the field. Similarly, there are several additional crustose lichens in Australasia with brown, biatorine apothecia, for example Japewiella pruinosula (Müll. Arg.) Kantvilas and species of Bacidia, but all are readily distinguished by their asci and ascospores (Table 3).
Distribution and ecology
Based on herbarium material, Australidea canorufescens occurs in south-eastern Australia, Tasmania and New Zealand. Only in Tasmania has there been an opportunity to study its ecology in greater detail. There it occurs on smooth bark in deep shade in the understorey of wet forests. It appears to be exclusively confined to callidendrous rainforests (terminology after Jarman et al. (Reference Jarman, Kantvilas and Brown1994)) where Atherosperma moschatum is either the dominant or subdominant canopy species, or to old-growth wet eucalypt forests where it colonizes the bark of Pomaderris apetala, an understorey tree typically very richly colonized by cryptogamic epiphytes.
Specimens examined
Australia: Tasmania: Wellard Rivulet, 42°56′S, 147°52′E, 1899, W. A. Weymouth (HO); Guy Fawkes Rivulet, 42°54′S, 147°17′E, 150 m, 1906, W. A. Weymouth 806 (HO); Styx Road, 370 m, 1981, G. Kantvilas 1034/81 (BM, HO); Weldborough, 640 m, 1981, G. Kantvilas 1129/81 (BM, HO); near Lyons River, 340 m, 1982, G. Kantvilas 23/82 (BM, HO); ibid., 280 m, 1982, G. Kantvilas 24/82 (BM, HO); south-eastern slope of MacGregor Peak, 42°59′S, 147°57′E, c. 400 m, 1989, G. Kantvilas 361/89 (HO); Bun Hill, Forestier Peninsula, 42°58′S, 147°56′E, 320 m, 1989, G. Kantvilas 370/89 (HO); Sumac Road, Spur 2, 41°08′S, 145°02′E, 170 m, 1993, G. Kantvilas 310/93 (HO); W of Tahune Bridge, 43°06′S, 146°41′E, 2002, G. Kantvilas 257/02 (HO); Sandspit River, 42°43′S, 147°51′E, 170 m, 2010, G. Kantvilas 106/10 (HO); W of Wielangta Hill, 42°40′S, 147°50′E, 500 m, 2017, G. Kantvilas 102/17 (HO); W of Tahune Bridge, 43°06′S, 146°41′E, 90 m, 2010, G. Kantvilas 241/10 (HO); Sandspit River, Wielangta Forest Walk, 42°42′S, 147°50′E, 200 m, 2017, G. Kantvilas 328/17 & J. Jarman (HO, UPS); Dip Falls, beside car park, 41°02′S, 145°22′E, 210 m, 2019, G. Kantvilas 100/19 (HO, S); Lyell Hwy, c. 2 km beyond Wayatinah turn-off, 42°22′S, 146°30′E, 400 m, 2020, G. Kantvilas 323/20 (HO). Victoria: Tarra Bulga NP, Cyathea Falls, 38°26′47″S, 146°32′19″E, 250 m, 2008, G. Kantvilas 95/08 & J. Elix (HO). New South Wales: c. 1 km W of Mt Banda, 31°10′S, 152°25′E, 1050 m, 1988, G. Kantvilas 625/88 (HO, NSW).
Malcolmiella interversa (Nyl.) Kantvilas, Wedin & M. Svensson comb. nov.
MycoBank No.: MB 839490
Lecidea interversa Nyl., Lich. Nov. Zel., 79 (1888); type: New Zealand, sine loco [probably Wellington], 1867, Charles Knight 87a (H-NYL 20457!, lectotype, selected by Galloway (Reference Galloway1985) [ICN Art. 9.10]).
Malcolmiella cinereovirens Vĕzda, A. Vĕzda: Lich. Rar. Exs., 265 (1997); type: Nova Zelandia [New Zealand], South Island, Nelson, Hackett River, ad confluentem rivulorum Hackett et Miner, 170 m alt., corticola, 25 March 1996, W. Malcolm 2757 (CHR—holotype; HO!, UPS L-89238!—isotypes).
Malcolmiella cinereovirens var. isidiata Vĕzda, A. Vĕzda: Lich. Rar. Exs., 266 (1997); type: Australia, ACT, Blundells Creek Road, 30 km ad occidentem Canberra, 800 m alt., secus rivulum in pluviisilva, ad corticem arborum, 5 September 1995, K. & A. Kalb (HO!, UPS L-89239!—isotypes).
Thallus crustose, pale grey to grey-green, to c. 0.3 mm thick, very scurfy, granular to ±tomentose, undelimited but sometimes with a pale grey, ±byssoid leading edge, ecorticate, sometimes beset with minute, globular, isidioid structures 30–100 μm diam. Photobiont a unicellular green alga with globose to subglobose cells, 7–12 × 5–12 μm, occurring in clusters 16–40 μm wide of up to c. 10 cells wrapped in a gelatinous sheath.
Apothecia biatorine, scattered, basally constricted, generally neatly discoid, 0.25–1 mm wide; disc mostly plane and becoming slightly convex only in the oldest apothecia, pinkish orange, orange to orange-brown, epruinose; proper exciple thin, smooth, pale cream or pale brownish and mostly persistent and becoming inconspicuous only in the most convex apothecia, in section cupulate, hyaline or pale brownish at the edges, 30–60 μm thick, paraplectenchymatous, composed of roundish to irregularly rhomboid cells 6–15 × 3.5–10 μm. Hypothecium pale yellow-brown or hyaline, 60–80 μm thick. Hymenium 55–70 μm, hyaline entirely, or pale orange-brown in the uppermost part, not inspersed, lax in water and KOH. Paraphyses mostly simple, 1.5–2 μm wide, sparsely septate, with apices distinctly capitate, unpigmented, to 3–5 μm wide. Asci 53–65 × 8–11 μm, 8-spored; tholus amyloid, distinctly thickened at least when young, later becoming compressed by developing ascospores, with an intensely amyloid ‘plug’ with parallel or diverging flanks, pierced by a narrow, weakly amyloid channel; ocular chamber not developed. Ascospores ellipsoid, occasionally with slightly attenuated apices, hyaline, halonate, simple but occasionally with the contents divided and appearing pseudoseptate, (9–)10–12.6–15(–16) × (4–)5–5.4–6.5(–7) μm (n = 60); halo c. 1 μm wide, uneven and appearing ornamented.
Pycnidia immersed, very rare (located only by chance); conidia filiform, curved or sigmoid, 16–20 × 1 μm.
Chemistry
Thallus and apothecia containing no substances detectable by TLC.
Remarks
The genus Malcolmiella contains a single species and is easily recognized by the combination of biatorine apothecia, distinctive asci (Fig. 4D) and the halonate, ornamented, simple ascospores. The structure of the asci is not inconsistent with that seen in representatives of the genera grouped with Malcolmiella in our phylogeny. With its orange apothecia and corticolous habit, M. interversa is perhaps most similar to species of Coenogonium, but that genus differs by containing Trentepohlia as the photobiont and by having Gyalecta-type asci with 1-septate ascospores. In contrast, the genera to which Malcolmiella is related (see below) generally have dark-coloured apothecia, occasionally septate ascospores, and occur principally on soil, rock or bryophytes.
Our analysis clearly indicates that Malcolmiella belongs to a group of genera usually assigned to Lecideaceae (e.g. Fryday & Hertel Reference Fryday and Hertel2014; Fryday et al. Reference Fryday, Printzen and Ekman2014; Wijayawardene et al. Reference Wijayawardene, Hyde, Lumbsch, Liu, Maharachchikumbura, Ekanayaka, Tian and Phookamsak2018). However, in earlier phylogenies spanning a broad range of the Lecanoromycetes, the affinities of this group remain unresolved. In these analyses, representatives of this group (Lecidea berengeriana and Romjularia lurida) have ended up on unsupported sister branches to the Collematinae and Peltigerinae (Schmull et al. Reference Schmull, Miadlikowska, Pelzer, Stocker-Wörgötter, Hofstetter, Fraker, Hodkinson, Reeb, Kukwa and Lumbsch2011; Miadlikowska et al. Reference Miadlikowska, Kauff, Högnabba, Oliver, Molnár, Fraker, Gaya, Hafellner, Hofstetter and Gueidan2014), or on a likewise unsupported sister branch to the Teloschistales (Bryobilimbia spp. and Lecidoma demissum; Schmull et al. Reference Schmull, Miadlikowska, Pelzer, Stocker-Wörgötter, Hofstetter, Fraker, Hodkinson, Reeb, Kukwa and Lumbsch2011). The phylogenetic placement of this group in Lecanoromycetidae is unresolved in our analysis but the results indicate that it is not close to the Lecideaceae (Fig. 2).
Two specimens of Lecidea interversa, Charles Knight 87a and 88, are housed in the Nylander Herbarium in H, with the former lectotypified by Galloway (Reference Galloway1985). Galloway (loc. cit.) synonymized it and several other names with Lecidea canorufescens, but that species and at least some of its synonyms belong in Australidea.
Vĕzda (Reference Vĕzda1997) described two varieties of Malcolmiella cinereovirens, his var. isidiata being distinguished by the presence of minute, globose, isidia-like structures scattered on the upper surface. Incipient development of such structures is evident in at least parts of all the specimens examined and they are deemed to have no taxonomic significance. Their anatomy is rather curious. Superficially they resemble apothecial initials but they are very easily detached and contain no apothecial tissue. In section, they are seen to be packages of photobiont and mycobiont cells, wrapped in a fungal sheath c. 5 μm thick from which protrude tapered hyphal spines, 5–6 μm at the base and up to 20 μm long. These presumably anchor the ‘isidia’ to the upper surface of the thallus.
Distribution and ecology
In the original description of Malcolmiella, Vĕzda (Reference Vĕzda1997) states that it occurs on bark, leaves and rock, and records it from South Island, New Zealand, and the ACT, mainland Australia. Malcolmiella is reported here from Tasmania for the first time. It has been collected in Nothofagus cunninghamii-dominated cool temperate rainforest and in moist Melaleuca ericifolia swamp woodland, in both cases as an epiphyte in deep shade where few other lichens were present. It appears to be genuinely rare.
Specimens examined
New Zealand: South Island: Nelson, Brook Waterfalls, 160 m alt., 1997, W. Malcolm & A. Vĕzda (A. Vĕzda: Lich. Rar. Exs., 302) (HO).—Australia: Tasmania: Denium Hill, at end of Robbins Island Track, 40°45′S, 144°53′E, 5 m alt., 1993, G. Kantvilas 154/93 & J. A. Elix (HO); Sumac Road, Spur 2, south of Arthur River, 41°08′S, 145°02′E, 170 m alt., 1993, G. Kantvilas 312/93 (HO, PRA).
Acknowledgements
For skilful laboratory work, we thank the staff at the Department of Bioinformatics and Genetics at the Swedish Museum of Natural History, in particular Bodil Cronholm. Funding was gratefully received from the Swedish Research Council (VR), grant 2016-03589 to MW. Research by MS on the taxonomy of lecideoid lichens is financially supported by the Swedish Taxonomy Initiative (grant no. 2016-206 4.3). For their hospitality and provision of working space, GK acknowledges with thanks the herbarium staff of the Swedish Museum of Natural History (during a visit in 2017) and the Finnish Museum of Natural History (2016, 2018). We thank Jean Jarman for the photographs and preparing the line drawings for publication.
Author ORCIDs
Gintaras Kantvilas, 0000-0002-3788-4562; Mats Wedin, 0000-0002-8295-5198.










