Introduction
The species of the phylum Kinorhyncha (mud dragons) are exclusively meiobenthic, occur worldwide from tropical to polar regions and inhabit various marine environments such as sandy beaches, muddy sea floor, seamounts, deep sea and submarine caves (Sørensen & Pardos, Reference Sørensen and Pardos2008; Neuhaus, Reference Neuhaus and Schmidt-Rhaesa2013; Yamasaki, Reference Yamasaki2016; Sørensen et al., Reference Sørensen, Rohal and Thistle2018; Yamasaki et al., Reference Yamasaki, Neuhaus and George2019). After the rearrangement of the classification system based on the recent molecular and morphological phylogenetic analyses, 11 families are recognized in the phylum (Sørensen et al., Reference Sørensen, Dal Zotto, Rho, Herranz, Sánchez, Pardos and Yamasaki2015). Among the families, Echinoderidae represents the most speciose family, comprised of five genera and about 150 species (Sørensen et al., Reference Sørensen, Dal Zotto, Rho, Herranz, Sánchez, Pardos and Yamasaki2015; Sánchez et al., Reference Sánchez, Pardos and Martínez Arbizu2019; Varney et al., Reference Varney, Funch, Kocot and Sørensen2019; Yamasaki et al., Reference Yamasaki, Neuhaus and George2019; Cepeda et al., Reference Cepeda, Álvarez-Castillo, Hermoso-Salazar, Sánchez, Gómez and Pardos2019a, Reference Cepeda, Pardos and Sánchez2019b, Reference Cepeda, Sánchez and Pardos2019c). Kinorhynch families, genera and some species-groups comprised of closely related species are distributed worldwide (Neuhaus, Reference Neuhaus and Schmidt-Rhaesa2013; Randsø et al., Reference Randsø, Yamasaki, Bownes, Herranz, Di Domenico, Qi and Sørensen2019). On the other hand, species are mostly reported from single localities and only a few species have broad distribution patterns, i.e. Echinoderes pterus Yamasaki et al., Reference Yamasaki, Grzelak, Sørensen, Neuhaus and George2018a recorded from the Arctic Sea, Atlantic Ocean and Mediterranean Sea and Echinoderes unispinosus Yamasaki et al., Reference Yamasaki, Neuhaus and George2018b from the Atlantic Ocean, Mediterranean Sea and East Pacific Ocean (Sørensen et al., Reference Sørensen, Rohal and Thistle2018; Yamasaki et al., Reference Yamasaki, Grzelak, Sørensen, Neuhaus and George2018a, Reference Yamasaki, Neuhaus and George2018b, Reference Yamasaki, Neuhaus and George2019). This general pattern of species being restricted to a confined geographic area is probably due to their low dispersal ability, or preference for a specific habitat (Artois et al., Reference Artois, Fontaneto, Hummon, McInnes, Todaro, Sørensen, Zullini and Fontaneto2011; Sánchez et al., Reference Sánchez, Pardos, Herranz and Benito2011, Reference Sánchez, Herranz, Benito and Pardos2012); it should be said, however, that the scarcity of investigations could have prevented an unveiling of an ampler distribution for some species.
Although kinorhynchs inhabit various marine environments, investigations of cave species have been scarcely carried out. Until now, kinorhynchs were found from 12 caves in the north Caribbean Sea, Canary Islands (Atlantic Ocean), western to middle Mediterranean Sea, Atolls of the Maldives (Indian Ocean), southern Japan (Pacific Ocean) and eastern Australia (Coral Sea) (Sánchez & Martínez, Reference Sánchez and Martínez2019). Of the specimens found in these caves, some were identified as putative taxa and others reported as belonging to the following eight nominal species: Centroderes barbanigra Neuhaus et al., Reference Neuhaus, Pardos, Sørensen and Higgins2014; Echinoderes cavernus Sørensen et al., Reference Sørensen, Jørgensen and Boesgaard2000; Echinoderes dujardinii Claparède, Reference Claparède1863; Leiocanthus bretti Sánchez & Martínez, Reference Sánchez and Martínez2019; Meristoderes macracanthus Herranz et al., Reference Herranz, Thormar, Benito, Sánchez and Pardos2012; Pycnophyes kukulkani Sánchez & Martínez, Reference Sánchez and Martínez2019; Pycnophyes cf. zelinkaei Southern, Reference Southern1914; and Ryuguderes iejimaensis Yamasaki, Reference Yamasaki2016 (summarized in Sánchez & Martínez, Reference Sánchez and Martínez2019).
Daidokutsu is one of the most investigated submarine caves in the world. The cave is located on the east coast of Iejima Island, Ryukyu Islands, Japan. The cave entrance opens at ~19 m depth on the reef slope, and the cave inside is about 40 m long, gradually deepening to ~30 m depth at its maximum (Kitamura et al., Reference Kitamura, Hiramoto, Kase, Yamamoto, Mariko and Ohashi2007; Omori et al., Reference Omori, Kitamura, Fujita, Honda and Yamamoto2010; Yamamoto et al., Reference Yamamoto, Kitamura, Irino, Kase and Ohashi2010). According to Omori et al. (Reference Omori, Kitamura, Fujita, Honda and Yamamoto2010), the cave has been completely submerged for 8000 years, under the influence of the sea-level rising after the last glacial period. The floor of the cave is covered by a layer of muddy sediment and many animals of various taxa have been found from the cave, for example, Tardigrada (Fujimoto, Reference Fujimoto2015; Fujimoto et al., Reference Fujimoto, Jørgensen and Hansen2017; Fujimoto & Jimi, Reference Fujimoto and Jimi2020), Ostracoda (Tabuki & Hanai, Reference Tabuki and Hanai1999; Chiu et al., Reference Chiu, Yasuhara, Iwatani, Kitamura and Fujita2017), Mysida (Hanamura & Kase, Reference Hanamura and Kase2001) and Mollusca (Kase & Hayami, Reference Kase and Hayami1992). The kinorhynch Ryuguderes iejimaensis has recently been described from this cave (Yamasaki, Reference Yamasaki2016).
In this study, three new Echinoderes species from Daidokutsu, as the second to fourth species of Kinorhyncha from this cave, are described. They also represent the ninth to eleventh record of kinorhynch species from the cave environment. We furthermore discuss the distribution patterns of two species groups in Echinoderes established in this study, and the origin and endemism of echinoderid kinorhynchs in the cave environment.
Materials and methods
An 8-litre mud sediment sample was collected on 25 April 2015 by scuba at 28–30 m depth in the Daidokutsu submarine cave, Iejima Island, Okinawa, Japan (26°43′29″N 127°49′52″E). Meiofaunal organisms including kinorhynchs were extracted from the sample using the decantation method and the flotation method with Ludox® HS-40 (Higgins & Thiel, Reference Higgins and Thiel1988; Giere, Reference Giere2009) and preserved in 10% buffered formalin. The preserved organisms were subsequently transferred to 70% ethanol and sorted under a stereomicroscope.
Kinorhynch specimens for light microscopy (LM) were dehydrated in glycerol and mounted individually in Fluoromount G® between two cover slips (a 22 mm × 24 mm square cover slip and a circle cover slip 15 mm in diameter) attached to a plastic H-S slide (Shirayama et al., Reference Shirayama, Kaku and Higgins1993). The LM specimens were observed with either an Olympus BX51 microscope or a Zeiss Axioskop 50 microscope. A camera lucida equipped on the latter microscope was used to measure morphometrics of all the LM specimens and to make drafts for line art illustrations. The lengths of penile spines were not measured because the spines are so transparent that the correct lengths of them are difficult to measure, and also these lengths are not used as a taxonomic character in Echinoderes with the same reason. Final line art illustrations were drawn with Adobe Illustrator CS6 based on the drafts. Specimens were photographed with a Zeiss AxioCam MRc5 mounted on a Zeiss Axioplan 2 MOT and an Axio Vs40 v.4.8.2.0 software.
Specimens for scanning electron microscopy (SEM) were transferred from ethanol to distilled water through a graded series of ethanol, post-fixed with 1% OsO4 in 0.05 M phosphate buffer (pH = 7.3) with 0.3 M sodium chloride and 0.05% sodium azide for 2.5 h, dehydrated through a graded series of ethanol, critical-point dried with a BalTec CPD 030, mounted on aluminium stubs, sputter-coated with gold-palladium with a Polaron SC 7640, and observed with a Zeiss EVO LS 10 scanning electron microscope.
The map was drawn with the Generic Mapping Tools (GMT) (Wessel et al., Reference Wessel, Smith, Scharroo, Luis and Wobbe2013), and subsequently modified with Adobe Illustrator CS6.
The terminology follows Neuhaus (Reference Neuhaus and Schmidt-Rhaesa2013). The classification system follows Sørensen et al. (Reference Sørensen, Dal Zotto, Rho, Herranz, Sánchez, Pardos and Yamasaki2015). All specimens have been deposited in the invertebrate collection of the Hokkaido University Museum, Hokkaido University, Sapporo, Japan (catalogue numbers ICHUM 5976–5992).
Results
Systematics
Class CYCLORHAGIDA Zelinka, Reference Zelinka1896
Order ECHINORHAGATA Sørensen et al., Reference Sørensen, Dal Zotto, Rho, Herranz, Sánchez, Pardos and Yamasaki2015
Family ECHINODERIDAE Zelinka, Reference Zelinka1894
Genus Echinoderes Claparède, Reference Claparède1863
Echinoderes gama sp. nov.
(Figures 1–4, Tables 1 & 2)
http://zoobank.org/426C630B-1C0E-4CFF-8095-8C3AE3F0835D

Fig. 1. Echinoderes gama sp. nov., camera lucida drawings. (A, B) holotype, female (ICHUM 5976), whole animal, dorsal and ventral view, respectively; (C, D) paratype male (ICHUM 5977), segments 9–11, dorsal and ventral view, respectively. Abbreviations: gco1/2, type-1/2 gland cell outlet; ldt, laterodorsal tube; ltas, lateral terminal accessory spine; lts, lateral terminal spine; lvs, lateroventral acicular spine; lvt, lateroventral tube; mds, middorsal acicular spine; pe, penile spine; si, sieve plate; slt, sublateral tube; ss, sensory spot; trp, trichoscalid plate.
Table 1. Measurements for adult Echinoderes gama sp. nov. (in micrometres or as percentage)

(ac), acicular spine; LD, laterodorsal tube; LTAS, lateral terminal accessory spine; LTS, lateral terminal spine; LV, lateroventral spine/tube; MD, middorsal spine; MSW, maximum sternal width; S, segment length; SL, sublateral tube; SW, standard width; TL, trunk length; (tu), tube.
Table 2. Summary of locations of cuticular structures and appendages in Echinoderes gama sp. nov.
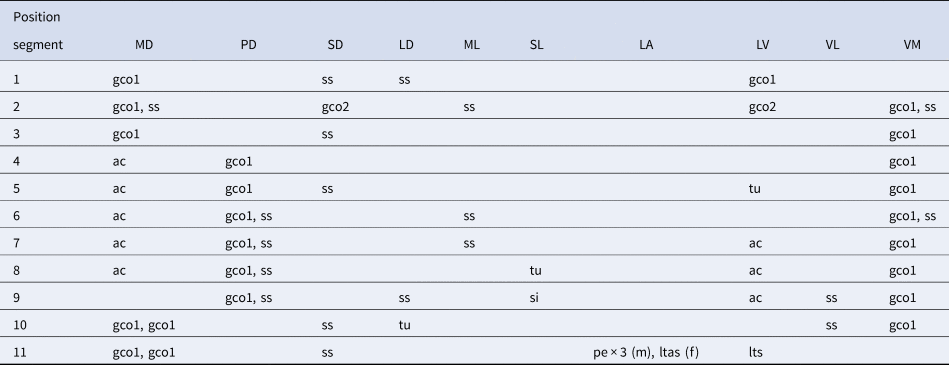
ac, acicular spine; (f), female condition of sexually dimorphic character; gco1/2, type-1/2 gland cell outlet; LA, lateral accessory; LD, laterodorsal; ltas, lateral terminal accessory spine; lts, lateral terminal spine; LV, lateroventral; (m), male condition of sexually dimorphic character; MD, middorsal; ML, midlateral; pe, penile spine; PD, paradorsal; SD, subdorsal; si, sieve plate; SL, sublateral; ss, sensory spot; tu, tube; VL, ventrolateral; VM, ventromedial.
Type material
Holotype: female, mounted in Fluoromount G® (ICHUM 5976), collected by HY and SF, at 28–30 m depth in Daidokutsu submarine cave, Iejima Island, Okinawa, Japan (26°43′29″N 127°49′52″E), on 25 April 2015. Paratypes: four males and two females, mounted in Fluoromount G® (ICHUM 5977–5982), collection data as holotype.
Additional material examined
Three males mounted on aluminium stubs for SEM observations, collection data as holotype.
Diagnosis
Echinoderes with middorsal acicular spines on segments 4–8; lateroventral acicular spines on segments 7–9; lateroventral tubes on segment 5; sublateral tubes on segment 8; laterodorsal tubes on segment 10; type-2 gland cell outlets in subdorsal and lateroventral position on segment 2.
Etymology
The species name comes from gama (‘cave’), a word from the Okinawan local language, referring to the species' type locality.
Description
Adult with head, neck and 11 trunk segments (Figures 1A, B, 2A, B & 3A). Measurements and positions of cuticular structures (sensory spots, gland cell outlets, spines, tubes and sieve plates) summarized in Tables 1 & 2, respectively.

Fig. 2. Echinoderes gama sp. nov., Nomarski photomicrographs. (A, C–F) holotype, female (ICHUM 5976); (B, G) paratype, male (ICHUM 5977). (A) head, neck and segments 1–11, dorsal view; (B) head, neck and segments 1–11, ventral view; (C), neck and segments 1–8, dorsal view; (D), neck and segments 1–8, ventral view; (E) segments 7–11, dorsal view; (F) segments 7–11, ventral view; (G) segments 7–11, ventral view. Black arrows, white arrowheads and black arrowheads indicate sensory spots, type-1 gland cell outlets and type-2 gland cell outlets, respectively. Numbers after abbreviations indicate corresponding segment number. Abbreviations: he, head; ldt, laterodorsal tube; ltas, lateral terminal accessory spine; lts, lateral terminal spine; lvs, lateroventral acicular spine; lvt, lateroventral tube; mds, middorsal acicular spine; ne, neck; pe, penile spine; si, sieve plate; slt, sublateral tube; tr, trunk.

Fig. 3. Echinoderes gama sp. nov., males, scanning electron micrographs. (A) segments 1–11, lateral view (left side); (B) head, ventral view; (C), segments 1–3, lateral view (left side); (D), segments 1–3, ventral view; (E) segments 5–7, lateral view (right side); (F) segments 5–7, ventral view; (G) segments 3–7, lateral view (left side); (H) segments 8 and 9 (left side); (I) segments 9–11, ventral view; (J) segments 10 and 11, lateral view (left side). Black arrows point to sensory spots and white/black arrowheads mark type-1/2 gland cell outlets. Numbers after abbreviations indicate corresponding sector/ring of head part or segment number of trunk part. Abbreviations: ldt, laterodorsal tube; lts, lateral terminal spine; lvs, lateroventral acicular spine; lvt, lateroventral tube; mds, middorsal acicular spine; mvp, midventral placid; pe, penile spine; psp, primary spinoscalid; sec, sector; si, sieve plate; slt, sublateral tube; sp, spinoscalid; trp, trichoscalid plate; trs, trichoscalid.

Fig. 4. Echinoderes gama sp. nov. and Echinoderes kajiharai sp. nov., polar diagram of mouth cone, introvert and placids. Grey shaded area shows mouth cone and bold bent bars symbolize placids. The table lists the scalid arrangement by sector.
Head consisting of retractable mouth cone and introvert. Arrangement of oral styles, scalids and placids summarized in Figure 4. Mouth cone with inner oral styles and one ring of nine outer oral styles. Detailed arrangement of inner oral styles not examined. Each outer oral style consists of triangular distal and rectangular basal parts. Introvert composed of six rings of spinoscalids and one ring of trichoscalids (Figures 3B & 4). Ring 01 with 10 primary spinoscalids, rings 02 and 04 with 10 spinoscalids, and rings 03 and 05 with 20 spinoscalids. Each spinoscalid of rings 01–05 with basal sheath and end piece. Rings 06 not examined in detail, but with at least six spinoscalids. Six trichoscalids attached with trichoscalid plate in sectors 2, 4, 5, 7, 8 and 10.
Neck with 16 placids (Figures 1A, B, 2A–D, 3B & 4). Midventral placid broadest. Remaining placids similar in size. Two ventral and four dorsal trichoscalid plates present.
Segment 1 consisting of complete cuticular ring (Figure 1A, B). This and following 10 segments with pachycyclus at anterior margin of each segment. Sensory spots in subdorsal and laterodorsal position (Figures 1A, B, 2C, D & 3C). Each sensory spot composed of one central pore and numerous micropapillae. Type-1 gland cell outlets present in middorsal and lateroventral position (Figures 1A, B & 2C, D). Cuticular hairs arising from perforation sites: most hairs located around sensory spots, and others sporadically on remaining dorsal area (Figures 1A, 2C & 3C). No cuticular hairs on ventral side (Figures 1B, 2D & 3D). Posterior part of this and following nine segments with primary pectinate fringe (Figures 1A, B & 3C, D). Pectinate fringe teeth on this segment long and slender.
Segment 2 with complete cuticular ring as segment 1 (Figure 1A, B). Middorsal, midlateral, ventromedial sensory spots present (Figures 1A, B, 2C, D & 3C, D). Each sensory spot of this and following segments consisting of one central pore and fewer micropapillae than those on segment 1. Type-1 gland cell outlets present in middorsal and ventromedial positions (Figures 1A, B & 2D). Type-2 gland cell outlets in subdorsal and lateroventral positions (Figures 1A, B, 2C, D & 3C, D). Primary pectinate fringe as on preceding segment.
Segment 3 and following eight segments consisting of one tergal and two sternal plates (Figure 1A, B). Sensory spots present in subdorsal position (Figures 1A, 2C & 3C). Type-1 gland cell outlets in middorsal and ventromedial position (Figures 1A, B & 2D). Pectinate fringe teeth of primary pectinate fringe on this and following six segments as those on preceding segments, except for ventromedial area with conspicuously shorter teeth (Figures 1B & 3F).
Segment 4 with middorsal acicular spine (Figures 1A & 2C). Type-1 gland cell outlets present in paradorsal and ventromedial positions (Figures 1A, B & 2C, D).
Segment 5 with middorsal acicular spine and lateroventral tubes (Figures 1A, B, 2B–D & 3E–G). Sensory spots in subdorsal position (Figures 1A, & 2C). Type-1 gland cell outlets in paradorsal and ventromedial positions (Figures 1A, B & 2C, D).
Segment 6 with middorsal acicular spine (Figures 1A & 2C). Sensory spots present in paradorsal, midlateral and ventromedial positions (Figures 1A, B, 2C, D & 3F, G). Type-1 gland cell outlets in paradorsal and ventromedial positions (Figures 1A, B & 2D).
Segment 7 with middorsal and lateroventral acicular spines (Figures 1A, B, 2C–G & 3E–H). Sensory spots present in paradorsal and midlateral positions (Figures 1A, B, 2C, D & 3G). Type-1 gland cell outlets in paradorsal and ventromedial positions (Figures 1A, B & 2D).
Segment 8 with middorsal and lateroventral acicular spines, and sublateral tubes (Figures 1A, B, 2A, C–G & 3H). Sensory spots present in paradorsal position (Figures 1A & 2E). Type-1 gland cell outlets in paradorsal and ventromedial positions (Figures 1A, B & 2D, F).
Segment 9 with lateroventral acicular spines (Figures 1, 2B, F, G & 3H, I). Paradorsal, laterodorsal and ventrolateral sensory spots present (Figures 1, 2E–G & 3H, I). Type-1 gland cell outlets in paradorsal and ventromedial positions (Figures 1 & 2F). Small sieve plates present in sublateral position (Figures 1, 2E–G & 3H).
Segment 10 with laterodorsal tubes (Figures 1A, C, 2E & 3J). Laterodorsal tubes in males longer than those in females (Table 1). Subdorsal and ventrolateral sensory spots present (Figures 1, 2E–G & 3I). Two middorsal type-1 gland cell outlets aligned in tandem (Figure 1A, C). Additional pair of type-1 gland cell outlets present in ventromedial position (Figures 1B & 2F). Pectinate fringe teeth of primary pectinate fringe thinner and shorter than those on preceding segment (Figures 1 & 3I).
Segment 11 with lateral terminal spines (Figures 1, 2A, B, E–G & 3I, J). Three pairs of penile spines present in males: dorsal and ventral pairs long and tube-like, whereas middle one stout and triangular-shaped (Figures 1C, 2G & 3J). Lateral terminal accessory spines present in females (Figures 1A & 2A, E). Subdorsal sensory spots present (Figure 1A, C). Type-1 gland cell outlet present middorsally (Figures 1A, C & 2E). Posterior edge of tergal plate protruding subdorsally, forming short pointed tergal extensions (Figure 1). Posterior edges of sternal plates rounded (Figures 1B, D, 2F & 3I).
Remarks
Although the presence of the middorsal acicular spines on segments 4–8 is the most common middorsal-spine pattern in the genus shared by 59 of 127 species, E. gama sp. nov. differs from them in the arrangement of the lateroventral acicular spines. Most of the 59 species have lateroventral acicular spines on segments 6–9, whereas only three species lack lateroventral acicular spines on segment 6, namely Echinoderes eximus Higgins & Kristensen, Reference Higgins and Kristensen1988, Echinoderes lusitanicus Neves et al., Reference Neves, Sørensen and Herranz2016, and Echinoderes tchefouensis Lou, Reference Lou1934 (Higgins & Kristensen, Reference Higgins and Kristensen1988; Sørensen et al., Reference Sørensen, Rho, Min, Kim and Chang2012; Neves et al., Reference Neves, Sørensen and Herranz2016; Grzelak & Sørensen, Reference Grzelak and Sørensen2018). Nevertheless, E. gama sp. nov. can be easily distinguished from them in the presence of the lateroventral acicular spines on segment 7, which are absent in the three species.
Echinoderes kajiharai sp. nov.
(Figures 5–8, Tables 3 & 4)
http://zoobank.org/565F2D87-B7D9-4DE2-9AA8-DAAD41ABD575

Fig. 5. Echinoderes kajiharai sp. nov., camera lucida drawings. (A, B) holotype, male (ICHUM 5983), whole animal, ventral and dorsal view, respectively. Abbreviations: gco1/2, type-1/2 gland cell outlet; las, lateral accessory acicular spine; lts, lateral terminal spine; lvs, lateroventral acicular spine; lvt, lateroventral tube; mds, middorsal acicular spine; mlt, midlateral tube; pe, penile spine; si, sieve plate; ss, sensory spot; trp, trichoscalid plate.

Fig. 6. Echinoderes kajiharai sp. nov., Nomarski photomicrographs, holotype, male (ICHUM 5983). (A) head, neck and segments 1–11, dorsal view; (B) neck and segments 1–5, dorsal view; (C) neck and segments 1–5, ventral view; (D) segments 4–8, dorsal view; (E) segments 7–11, dorsal view; (F) segments 7–11, ventral view. Black arrows, white arrowheads and black arrowheads indicate sensory spots, type-1 gland cell outlets, and type-2 gland cell outlets, respectively. Numbers after abbreviations indicate corresponding segment number. Abbreviations: he, head; las, lateral accessory acicular spine; lts, lateral terminal spine; lvs, lateroventral acicular spine; lvt, lateroventral tube; mdp, middorsal placid; mds, middorsal acicular spine; mlt, midlateral tube; mvp, midventral placid; ne, neck; pe, penile spine; si, sieve plate; te, tergal extension; tr, trunk.
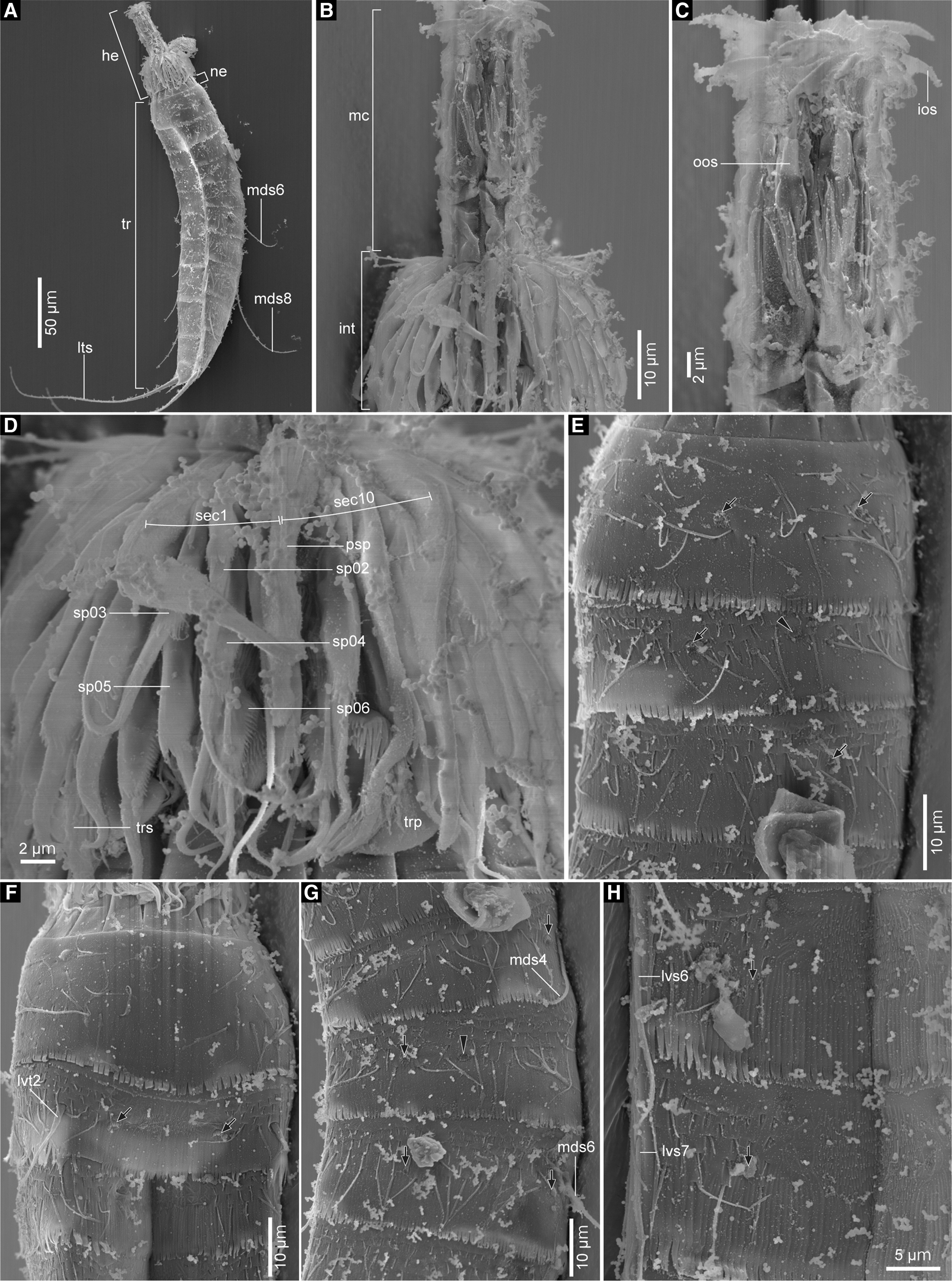
Fig. 7. Echinoderes kajiharai sp. nov., males, scanning electron micrographs. (A) head, neck and segments 1–11, lateroventral view (left side); (B) head, lateral view (left side); (C) mouth cone, lateral view (left side); (D) introvert, lateroventral view (left side); (E) segments 1–3, laterodorsal view (left side); (F) segments 1–3, ventral view; (G) segments 4–6, laterodorsal view (left side); (H) left sternal plates on segments 6 and 7. Black arrows point to sensory spots and black arrowheads mark type-2 gland cell outlets. Numbers after abbreviations indicate corresponding sector/ring of head part or segment number of trunk part. Abbreviations: he, head; int, introvert; ios, inner oral style; lts, lateral terminal spine; lvs, lateroventral acicular spine; lvt, lateroventral tube; mc, mouth cone; mds, middorsal acicular spine; ne, neck; oos, outer oral style; psp, primary spinoscalid; sec, sector; sp, spinoscalid; tr, trunk; trp, trichoscalid plate; trs, trichoscalid.

Fig. 8. Echinoderes kajiharai sp. nov., males (A, C, D, F) and female (B, E), scanning electron micrographs. (A) segments 6 and 7, ventral view; (B) segments 8 and 9, lateral view (left side); (C) segments 8 and 9, lateral view (left side); (D) segments 10 and 11, lateral view (left side); (E) segments 10 and 11, lateral view (left side); (F) segments 10 and 11, ventral view. Black arrows point to sensory spots and black arrowheads mark type-2 gland cell outlets. Numbers after abbreviations indicate segment number. Abbreviations: ltas, lateral terminal accessory spine; lts, lateral terminal spine; las, lateral accessory acicular spine; lvs, lateroventral acicular spine; mds, middorsal acicular spine; mlt, midlateral tube; pe, penile spine; te, tergal extension.
Table 3. Measurements for adult Echinoderes kajiharai sp. nov. (in micrometres or as percentage)

ac, acicular spine; LA, lateral accessory spine; LTS, lateral terminal spine; LV, lateroventral spine/tube; MD, middorsal spine; ML, midlateral tube; MSW, maximum sternal width; S, segment length; SW, standard width; TL, trunk length; tu, tube.
Table 4. Summary of locations of cuticular structures and appendages in Echinoderes kajiharai sp. nov.

ac, acicular spine; (f), female condition of sexually dimorphic character; gco1/2, type-1/2 gland cell outlet; LA, lateral accessory; LD, laterodorsal; ltas, lateral terminal accessory spine; lts, lateral terminal spine; LV, lateroventral; (m), male condition of sexually dimorphic character; MD, middorsal; ML, midlateral; pe, penile spine; PD, paradorsal; SD, subdorsal; si, sieve plate; SL, sublateral; ss, sensory spot; tu, tube; VL, ventrolateral; VM, ventromedial.
Type material
Holotype: Male, mounted in Fluoromount G® (ICHUM 5983), collected by HY and SF, at 28–30 m depth in Daidokutsu submarine cave, Iejima Island, Okinawa, Japan (26°43′29″N 127°49′52″E), on 25 April 2015. Paratypes: four males, mounted in Fluoromount G® (ICHUM 5984–5987), collection data as holotype.
Additional material examined
Two males and one female mounted on aluminium stubs for SEM observations, collection data as holotype.
Diagnosis
Echinoderes with middorsal acicular spines on segments 4, 6 and 8; lateral accessory acicular spines on segment 9; lateroventral acicular spines on segments 6–8; lateroventral tubes on segments 2 and 5; midlateral tubes on segment 10; type-2 gland cell outlets in laterodorsal position on segments 2 and 5, and subdorsal position on segments 8 and 9.
Etymology
The species epithet is named after Dr Hiroshi Kajihara, a Japanese taxonomist, for his great contributions to taxonomy, as well as for always supporting and encouraging the first author's research.
Description
Adult with head, neck and 11 trunk segments (Figures 5, 6A & 7A). Measurements presented in Table 3. Positions of cuticular structures (sensory spots, gland cell outlets, spines, tubes and sieve plates) summarized in Table 4.
Head consisting of retractable mouth cone and introvert (Figure 7B). Arrangement of oral styles, scalids and placids shown in Figure 4. Mouth cone with inner oral styles and one ring of nine outer oral styles (Figure 7C). Detailed arrangement of inner oral styles not examined. Each outer oral style consists of triangular distal and rectangular basal parts (Figure 7B, C). Introvert composed of six rings of spinoscalids and one ring of trichoscalids (Figure 7D). Ring 01 with 10 primary spinoscalids, rings 02 and 04 with 10 spinoscalids, and rings 03 and 05 with 20 spinoscalids. Each spinoscalid of rings 01–05 with basal sheath and end piece. Rings 06 not examined in detail, but with at least six spinoscalids. Six trichoscalids attached with trichoscalid plate in sectors 2, 4, 5, 7, 8 and 10.
Neck with 16 placids (Figures 5 & 6B, C), of which midventral one broadest, and remaining ones of similar size. Two ventral and four dorsal trichoscalid plates (Figure 5).
Segment 1 consisting of complete cuticular ring (Figures 5 & 6B, C). This and following 10 segments with thick pachycyclus at anterior margin of each segment. Sensory spots in subdorsal and midlateral positions (Figures 5B, 6B & 7E). Each sensory spot composed of one central pore and numerous micropapillae. Type-1 gland cell outlets present in middorsal and lateral accessory positions (Figures 5 & 6B, C). Cuticular hairs arising from perforation sites covering entire segment except for ventromedial area on this and following nine segments (Figures 5, 6B, C & 7E, F). Posterior part of this segment and following nine segments with primary pectinate fringe (Figures 5 & 7E, F).
Segment 2 with complete cuticular ring as segment 1 (Figures 5 & 6B, C). Lateroventral tubes present (Figures 5A, 6C & 7F). Sensory spots in middorsal, midlateral and ventromedial positions (Figures 5, 6B, C & 7E, F). Type-1 gland cell outlets present in middorsal and ventromedial positions (Figures 5 & 6C). Type-2 gland cell outlets located laterodorsally (Figures 5B, 6B & 7E).
Segment 3 and following eight segments consisting of one tergal and two sternal plates (Figure 5). Sensory spots present in subdorsal position (Figures 5B, 6B & 7E). Type-1 gland cell outlets as on preceding segment.
Segment 4 with middorsal acicular spine (Figures 5B, 6A, B, D & 7G). Paradorsal sensory spots beside base of middorsal acicular spine (Figures 5B, 6B, D & 7G). Type-1 gland cell outlets present in subdorsal and ventromedial positions (Figures 5 & 6B–D).
Segment 5 with lateroventral tubes (Figures 5A & 6C). Sensory spots situated midlaterally (Figures 5, 6B, D & 7G). Type-1 gland cell outlets as on preceding segment. Type-2 gland cell outlet present on laterodorsal position (Figures 5B, 6B, D & 7G).
Segment 6 with middorsal and lateroventral acicular spines (Figures 5, 6A, D, 7A, G, H & 8A). Sensory spots present in paradorsal, midlateral and ventromedial positions (Figures 5, 6D, 7G, H & 8A). Type-1 gland cell outlets present as on segment 4.
Segment 7 with lateroventral acicular spines (Figures 5A, 6F, 7H & 8A). Sensory spots in ventromedial position (Figures 5A, 6F, 7H & 8A). Type-1 gland cell outlets as on segment 4.
Segment 8 with middorsal and lateroventral acicular spines (Figures 5, 6A, D–F, 7A & 8B, C). Sensory spots in subdorsal and laterodorsal positions (Figures 5, 6D–F & 8B, C). Type-1 gland cell outlets as on segment 4. Subdorsal type-2 gland cell outlet present (Figures 5B, 6D, E & 8B, C).
Segment 9 with lateral accessory acicular spines (Figures 5, 6F & 8B, C, E), of which position may not be fully clear when specimen observed in unproper orientation. Paradorsal, laterodorsal and ventrolateral sensory spots present (Figures 5, 6E, F & 8B, C). Type-1 gland cell outlets as on segment 4. Type-2 gland cell outlets present subdorsally (Figures 5B, 6E & 8B, C). Small sieve plates present in sublateral position (Figures 5 & 6F).
Segment 10 with midlateral tubes (Figures 5, 6E, F & 8D, E). Sensory spots in subdorsal and ventrolateral positions (Figures 5, 6E, F & 8E, F). Two type-1 gland cell outlets in middorsal position (Figure 5B). Additional pair of type-1 gland cell outlets present ventromedially (Figures 5A & 6F).
Segment 11 with lateral terminal spines (Figures 5, 6A, E, F, 7A & 8D–F). Two pairs of penile spines present in males, and pair of lateral terminal accessory spines in females (Figures 5A, 6F & 8D–F). Subdorsal sensory spots present (Figures 5B & 8E). Posterior edge of tergal plate protruding subdorsally, forming long pointed tergal extensions (Figures 5, 6F & 8E, F). Posterior edges of sternal plates rounded (Figures 5A, 6F & 8F).
Remarks
Among the congeners, E. kajiharai sp. nov. is the most similar to Echinoderes multiporus Yamasaki et al., Reference Yamasaki, Neuhaus and George2018b and Echinoderes schwieringae Yamasaki et al., Reference Yamasaki, Neuhaus and George2019 by sharing the presence of (1) middorsal acicular spines on segments 4, 6, 8, (2) lateroventral/ventrolateral tubes on segments 2 and 5, (3) lateral accessory/lateroventral acicular spines on segments 6–9, (4) midlateral tubes on segment 10, and (5) subdorsal/laterodorsal type-2 gland cell outlets at least on segments 2, 5, 8 and 9. The combination of these similarities suggests that they might be closely related to each other, representing the Echinoderes multiporus species group, as with the Echinoderes coulli species group and the Echinoderes spinifurca species group (see e.g. Sørensen et al., Reference Sørensen, Rohal and Thistle2018; Randsø et al., Reference Randsø, Yamasaki, Bownes, Herranz, Di Domenico, Qi and Sørensen2019; Varney et al., Reference Varney, Funch, Kocot and Sørensen2019). The presence/absence of female-specific ventrolateral/ventromedial papillae in E. kajiharai sp. nov. could not be confirmed due to the lack of female specimens for LM observation. However, the presence of the papillae both in E. multiporus and E. schwieringae suggests that the female papillae are probably present in E. kajiharai sp. nov. as well, making it another diagnostic feature for the E. multiporus species group.
The members of the E. multiporus species group can be distinguished from each other by the position and the number of the type-2 gland cell outlets. Echinoderes multiporus has the highest number of the outlets, namely in the subdorsal position on segment 2 and the laterodorsal position on segments 4–9 (Yamasaki et al., Reference Yamasaki, Neuhaus and George2018b). Echinoderes schwieringae possesses the subdorsal outlets on segments 2 and 4, and the laterodorsal outlets on segments 5 and 7–9 (Yamasaki et al., Reference Yamasaki, Neuhaus and George2019). Echinoderes kajiharai sp. nov., on the other hand, has the lowest number of the outlets, i.e. laterodorsally on segments 2 and 5, and subdorsally on segments 8 and 9.
Echinoderes kajiharai sp. nov., further differs from the other two species of the E. multiporus species group in the position of acicular spines on segment 9. The spines on segment 9 in E. kajiharai sp. nov. are positioned more laterally than those on the preceding segments, locating them in lateral accessory position, whereas in E. multiporus and E. schwieringae, the spines on segment 9 are in the same lateroventral position as those on the preceding segments.
Echinoderes uozumii sp. nov.
(Figures 9–13, Tables 5 & 6)
http://zoobank.org/3FAC9678-E067-45CB-AB98-ADF634367E97

Fig. 9. Echinoderes uozumii sp. nov., camera lucida drawings. (A, B) holotype, female (ICHUM 5988), whole animal, ventral and dorsal view, respectively; (C, D) paratype male (ICHUM 5989), segments 9–11, ventral and dorsal view, respectively. Abbreviations: gco1/2, type-1/2 gland cell outlet; ldt, laterodorsal tube; ltas, lateral terminal accessory spine; lts, lateral terminal spine; lvs, lateroventral acicular spine; lvt, lateroventral tube; mds, middorsal acicular spine; pe, penile spine; si, sieve plate; slt, sublateral tube; ss, sensory spot; trp, trichoscalid plate.

Fig. 10. Echinoderes uozumii sp. nov., Nomarski photomicrographs. (A–F) holotype, female (ICHUM 5988); (G) paratype, male (ICHUM 5989). (A) head, neck and segments 1–11, dorsal view; (B) neck and segments 1–6, dorsal view; (C) neck and segments 1–6, ventral view; (D) segments 5–10, ventral view; (E) segments 7–10, dorsal view; (F) segments 10 and 11, ventral view; (G) segments 10 and 11, ventral view. Black arrows, white arrowheads, and black arrowheads indicate sensory spots, type-1 gland cell outlets, and type-2 gland cell outlets, respectively. Numbers after abbreviations indicate corresponding segment number. Abbreviations: ltas, lateral terminal accessory spine; lts, lateral terminal spine; lvs, lateroventral acicular spine; lvt, lateroventral tube; mds, middorsal acicular spine; pe, penile spine; slt, sublateral tube.
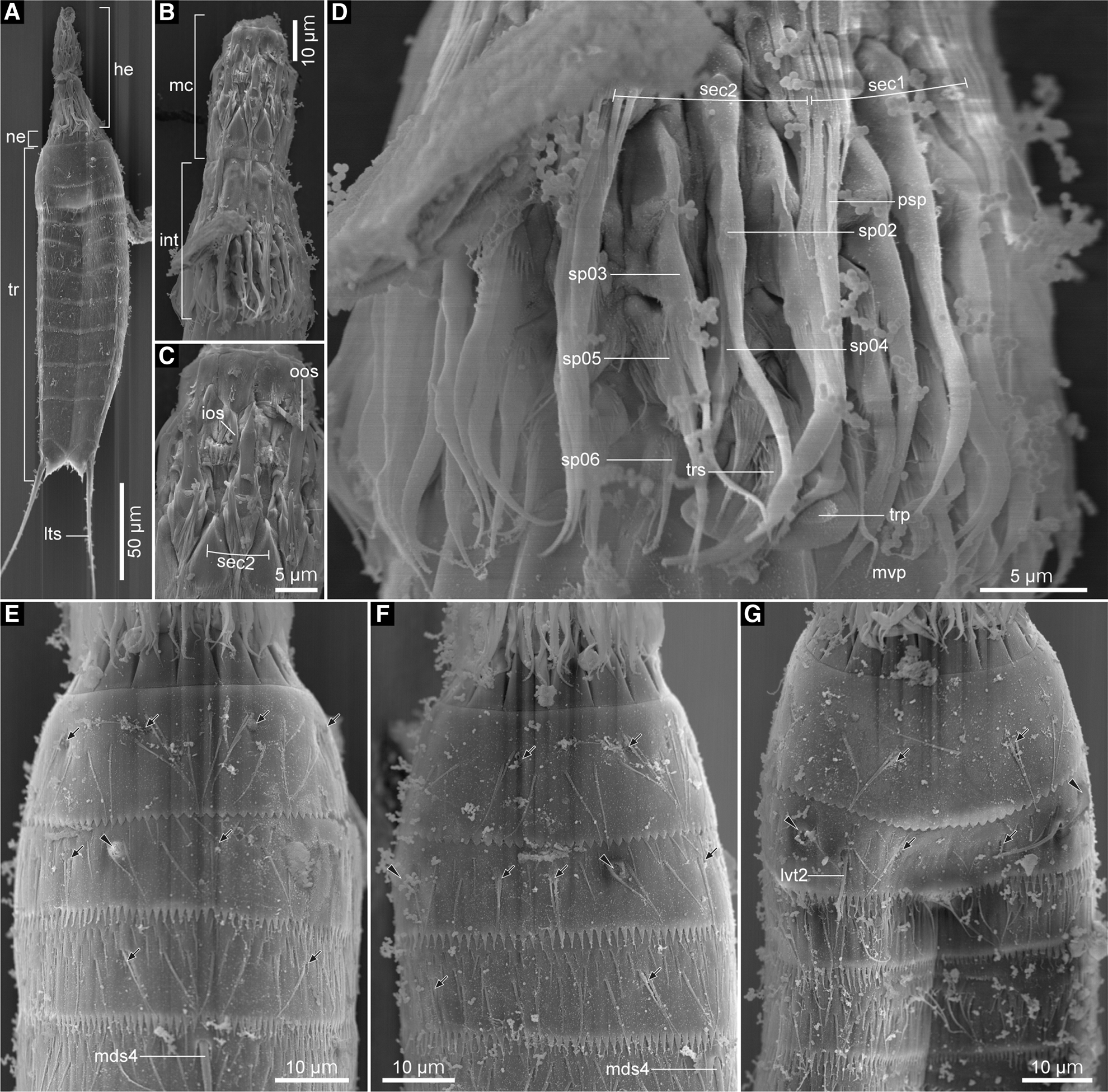
Fig. 11. Echinoderes uozumii sp. nov., male (B–D) and females (A, E–G), scanning electron micrographs. (A) head, neck and segments 1–11, ventral view; (B) head, ventral view; (C) mouth cone, ventral view; (D) introvert, ventral view; (E) neck and segments 1–4, dorsal view; (F) neck and segments 1–4, laterodorsal view (left side); (G) neck and segments 1–4, ventral view. Black arrows and arrowheads mark sensory spots and type-2 gland cell outlets, respectively. Numbers after abbreviations indicate corresponding sector/ring of head part or segment number of trunk part. Abbreviations: he, head; int, introvert; ios, inner oral style; lts, lateral terminal spine; lvt, lateroventral tube; mc, mouth cone; mds, middorsal acicular spine; mvp, midventral placid; ne, neck; oos, outer oral style; psp, primary spinoscalid; sec, sector; sp, spinoscalid; tr, trunk; trp, trichoscalid plate; trs, trichoscalid.

Fig. 12. Polar diagram of mouth cone, introvert and placids in Echinoderes uozumii sp. nov. Grey shaded area shows mouth cone and bold bent bars symbolize placids. The table lists the scalid arrangement by sector.

Fig. 13. Echinoderes uozumii sp. nov., male (J) and females (A–I), scanning electron micrographs. (A) segments 3–5, dorsal view; (B) segments 35–, laterodorsal view (left side); (C) segments 5–7, ventral view; (D) segments 6–8, dorsal view; (E) segments 6–8, laterodorsal view (left side); (F) segments 7–9, lateroventral view (right side); (G) segments 8–10, ventral view; (H) segments 10 and 11, dorsal view; (I) segment 11, ventral view; (J) segment 11, ventral view. Black arrows point to sensory spots. Numbers after abbreviations indicate corresponding segment number. Abbreviations: ldt, laterodorsal tube; ltas, lateral terminal accessory spine; lts, lateral terminal spine; lvs, lateroventral acicular spine; lvt, lateroventral tube; mds, middorsal acicular spine; pe, penile spine; si, sieve plate; slt, sublateral tube.
Table 5. Measurements for adult Echinoderes uozumii sp. nov. (in micrometres or as percentage)
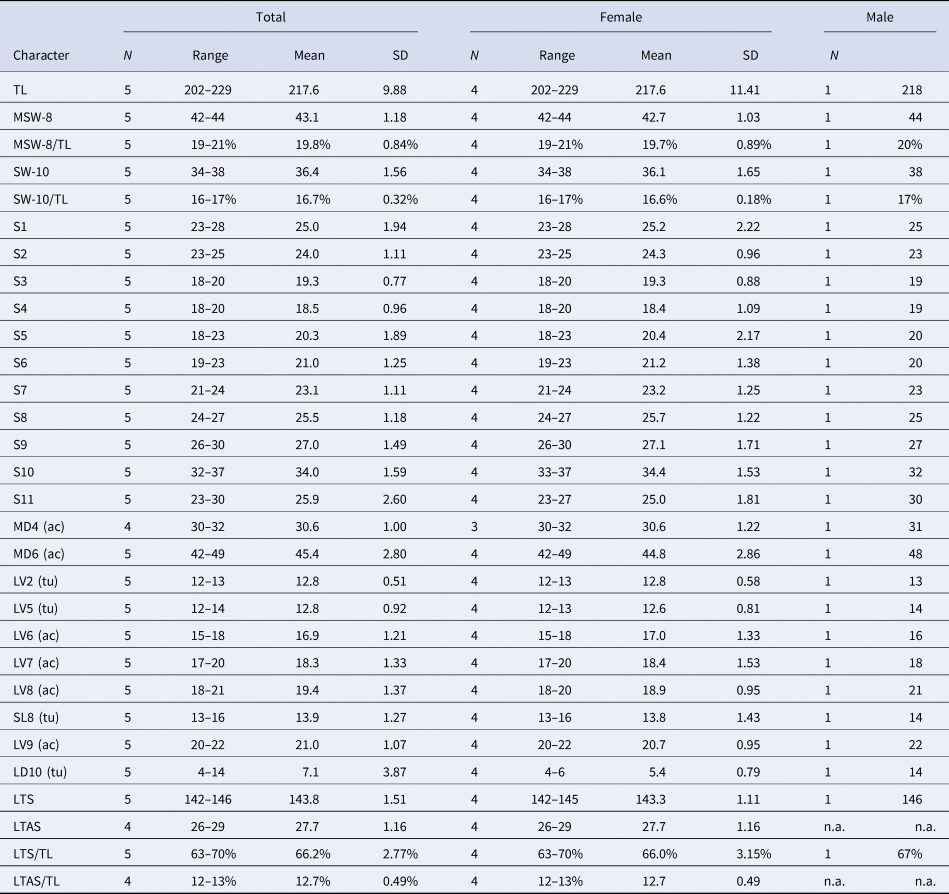
ac, acicular spine; LD, laterodorsal; LTAS, lateral terminal accessory spine; LTS, lateral terminal spine; LV, lateroventral spine/tube; MD, middorsal spine; MSW, maximum sternal width; S, segment length; SL, sublateral tube; SW, standard width; TL, trunk length; tu, tube.
Table 6. Summary of locations of cuticular structures and appendages in Echinoderes uozumii sp. nov.

ac, acicular spine; (f), female condition of sexually dimorphic character; gco1/2, type-1/2 gland cell outlet; LA, lateral accessory; LD, laterodorsal; ltas, lateral terminal accessory spine; lts, lateral terminal spine; LV, lateroventral; (m), male condition of sexually dimorphic character; MD, middorsal; ML, midlateral; pe, penile spine; PD, paradorsal; SD, subdorsal; si, sieve plate; SL, sublateral; ss, sensory spot; tu, tube; VL, ventrolateral; VM, ventromedial.
Type material
Holotype: Female, mounted in Fluoromount G® (ICHUM 5988), collected by HY and SF, at 28–30 m depth in Daidokutsu submarine cave, Iejima Island, Okinawa, Japan (26°43′29″N 127°49′52″E), on 25 April 2015. Paratypes: one male and three females, mounted in Fluoromount G® (ICHUM 5989–5992), collection data as holotype.
Additional material examined
One male and seven females mounted on aluminium stubs for SEM observations, collection data as holotype.
Diagnosis
Echinoderes with middorsal acicular spines on segments 4 and 6; lateroventral acicular spines on segments 6–9; lateroventral tubes on segments 2 and 5; sublateral tubes on segment 8; laterodorsal tubes on segment 10; type-2 gland cell outlets in subdorsal and lateral accessory positions on segment 2; pectinate fringe teeth of primary pectinate fringe on segment 1 with blunt tip, and conspicuously shorter than those on following segments.
Etymology
The species is named after Ryosuke Uozumi for his contribution to the ecological study of kinorhynchs in Uozumi et al. (Reference Uozumi, Yamasaki and Hirose2018).
Description
Adult with head, neck and 11 trunk segments (Figures 9A, B, 10A & 11A). Measurements shown in Table 5. Positions of cuticular structures (sensory spots, gland cell outlets, spines, tubes and sieve plates) summarized in Table 6.
Head consisting of retractable mouth cone and introvert (Figure 11A–D). Arrangement of oral styles and scalids summarized in Figure 12. Mouth cone with inner and outer oral styles (Figure 11B, C). Detail of inner oral styles not observed. Nine outer oral styles present in one ring. Each outer oral style consists of triangular distal and rectangular basal part (Figure 11C). Introvert composed of six rings of spinoscalids and one ring of trichoscalids (Figure 11D). Each spinoscalid of ring 01–05 consisting of basal sheath and distal end piece. Ring 01 with 10 primary spinoscalids, with longest distal end pieces among all spinoscalids. Rings 02 and 04 with 10 spinoscalids; rings 03 and 05 with 20 spinoscalids. Rings 06 with at least eight shortest spinoscalids. Spinoscalids of ring 06 in sectors 5 and 7 not examined. Six trichoscalids attached with trichoscalid plate in sectors 2, 4, 5, 7, 8 and 10. Each trichoscalid covered with hairs.
Neck with 16 placids (Figures 9A, B & 10B, C). Midventral placid broadest. Remaining placids similar in size. Two trichoscalid plates present ventrally and four dorsally.
Segment 1 consisting of complete cuticular ring (Figures 9A, B & 10B, C). This and following 10 segments with thick pachycyclus at anterior margin of each segment. Sensory spots in subdorsal, laterodorsal and ventromedial positions (Figures 9A, B, 10B, C & 11E–G). Each sensory spot on this and following nine segments composed of one central pore and numerous micropapillae, and surrounded by one to three cuticular hairs. Type-1 gland cell outlets present in middorsal and lateroventral positions (Figures 9A, B & 10B, C). Cuticular hairs arising from perforation sites sporadically covering this segment (Figures 9A, B, 10B, C & 11E–G). Posterior part of this segment with primary pectinate fringe, with short and blunt pectinate fringe teeth (Figures 9A, B & 11E–G).
Segment 2 with complete cuticular ring as segment 1 (Figures 9A, B & 10B, C). Lateroventral tubes present (Figures 9A & 11G). Sensory spots present: one in middorsal, two pairs in laterodorsal, and one pair in ventromedial positions (Figures 9A, B, 10B, C & 11E–G). Type-1 gland cell outlets present in middorsal and ventromedial positions (Figures 9A, B & 1C). Type-2 gland cell outlets in subdorsal and lateral accessory positions (Figures 9A, B, 10A–C & 11E–G). Cuticular hairs as in segment 1. Primary pectinate fringe on this and following eight with medium-length pectinate fringe teeth (Figures 9A, B & 11E–G).
Segment 3 and following eight segments consisting of one tergal and two sternal plates (Figures 9A, B & 10B, C). Sensory spots present in subdorsal and midlateral positions (Figures 9A, B, 10B, C, 11E–G & 13A, B). Type-1 gland cell outlets as on preceding segment. Cuticular hairs on this and following seven segments more densely aligned than those on preceding segments (Figures 9A, B, 10A, B, 11E–G & 13A, B).
Segment 4 with middorsal acicular spine (Figures 9B, 10A, B, 11E, F, 13A, B). Type-1 gland cell outlets present in paradorsal and ventromedial positions (Figures 9A, B & 10C).
Segment 5 with lateroventral tubes (Figures 9A, 10C, D & 13C). Sensory spots in subdorsal, midlateral and ventromedial positions (Figures 9A, B, 10B–D & 13A–C). Type-1 gland cell outlets as in segment 2.
Segment 6 with middorsal and lateroventral acicular spines (Figures 9A, B, 10A–E & 13C–E). Sensory spots present in paradorsal, midlateral and ventromedial positions (Figures 9A, B, 10B–D & 13C–E). Type-1 gland cell outlets present as on segment 4.
Segment 7 with lateroventral acicular spines (Figures 9A, 10D & 13C, F). Sensory spots in subdorsal, midlateral and ventromedial positions (Figures 9A, B, 10D, E & 13C–F). Type-1 gland cell outlets as on segment 2.
Segment 8 with sublateral tubes and lateroventral acicular spines (Figures 9A, 10D & 13E–G). Sensory spots in paradorsal position (Figures 9B, 10E & 13D, E). Type-1 gland cell outlets as on segment 4.
Segment 9 with lateroventral acicular spines (Figures 9A, C, 10D & 13F, G). Paradorsal, subdorsal, midlateral and ventrolateral sensory spots present (Figures 9, 10D, E & 13F, G). Type-1 gland cell outlets as on segment 4. Small sieve plates present in sublateral position (Figures 9 & 13F).
Segment 10 with laterodorsal tubes (Figures 9B, D & 13H). Length of laterodorsal tubes in males longer than those in females (Table 5). Subdorsal and ventrolateral sensory spots present (Figures 9, 10D, E & 13G, H). Two middorsal type-1 gland cell outlets aligned in tandem (Figures 9B, D & 10E). Additional pair of type-1 gland cell outlets present in ventromedial position (Figures 9A, C & 10D).
Segment 11 with lateral terminal spines (Figures 9, 10A, F, G, 11A & 13H–J). Three pairs of penile spines present in males (Figures 9C, D, 10G & 13J). Dorsal and ventral penile spines long and tube-like, whereas middle ones stout and triangular-shaped. One pair of lateral terminal accessory spines present in females (Figures 9A, B, 10F & 13H, I). Paradorsal sensory spots present (Figures 9B, D & 13H). Two type-1 gland cell outlets present middorsally (Figure 9B, D). Posterior edge of tergal plate forming triangular tergal extensions (Figures 9 & 13H–J). Posterior edges of sternal plates rounded.
Remarks
The presence of the middorsal acicular spines only on segments 4 and 6 is an uncommon feature within Echinoderes. This trait is shared only by E. uozumii sp. nov., Echinoderes astridae Sørensen, Reference Sørensen2014, and Echinoderes bispinosus Higgins, Reference Higgins1982 (Higgins, Reference Higgins1982; Sørensen, Reference Sørensen2014). Among the three species, E. uozumii sp. nov. and E. astridae differ from E. bispinosus by the presence of sublateral tubes on segment 8. However, E. uozumii sp. nov. and E. astridae are identical in the spine/tube pattern, except for the presence/absence of laterodorsal tubes on segment 10. The arrangement of the type-2 gland cell outlets is also identical for the two species. The most prominent difference between the two species is the primary pectinate fringe on segment 1. In E. uozumii sp. nov., the pectinate fringe teeth of primary pectinate fringe on segment 1 are short and blunt, obviously differing in shape in comparison with those on following segments. While in Echinoderes astridae, the pectinate fringe teeth on segment 1 are medium-length and sharp-angled, which are similar in shape to those on following segments (Sørensen, Reference Sørensen2014). Additional differences are (1) the presence/absence of some sensory spots (E. uozumii sp. nov. has one or two more pairs of sensory spots on segments 2, 3, 5, 9–11 than E. astridae), (2) trunk length (202–229 μm in E. uozumii sp. nov., whereas 276–312 μm in E. astridae) and (3) relative length of lateral terminal spine (142–146 μm representing 63–70% of trunk length in E. uozumii sp. nov., whereas 127–132 μm representing 42–46% of trunk length in E. astridae) (Sørensen, Reference Sørensen2014).
Two additional species resemble E. uozumii sp. nov. in having the middorsal acicular spines on segments 4 and 6: Echinoderes aff. bispinosus reported from the coast of Turkey by Sönmez et al. (Reference Sönmez, Köroğul and Karaytuğ2016) and Echinoderes sp. 1 from Singapore by Sørensen et al. (Reference Sørensen, Gąsiorowski, Randsø, Sánchez and Neves2016). Although the former was identified as ‘E. aff. bispinosus’ in the original record by Sönmez et al. (Reference Sönmez, Köroğul and Karaytuğ2016), Sørensen et al. (Reference Sørensen, Gąsiorowski, Randsø, Sánchez and Neves2016) suggested ‘E. aff. bispinosus’ is similar to E. bispinosus but would represent an undescribed species. In comparison with the brief descriptions provided for the two species, E. uozumii sp. nov. differs from the two at least in the presence of the type-2 gland cell outlets in subdorsal and lateral accessory positions on segment 2 (Sönmez et al., Reference Sönmez, Köroğul and Karaytuğ2016; Sørensen et al., Reference Sørensen, Gąsiorowski, Randsø, Sánchez and Neves2016). In addition, E. uozumii sp. nov. differs from Echinoderes sp. 1 in Sørensen et al. (Reference Sørensen, Gąsiorowski, Randsø, Sánchez and Neves2016) in having lateroventral acicular spines on segment 7 (Sørensen et al., Reference Sørensen, Gąsiorowski, Randsø, Sánchez and Neves2016).
All the above-mentioned species, including three known, one new and two undescribed species, share several morphological features: the presence of (1) middorsal acicular spines on segments 4 and 6, (2) lateroventral/ventrolateral tubes on segments 2 and 5 and (3) lateroventral acicular spines at least on segments 6, 8 and 9. These similarities suggest the close relationship of the three species, composing the Echinoderes bispinosus species group.
Discussion
Wide geographic distributions of E. multiporus and E. bispinosus species groups
Despite their low motility and lack of a planktonic larval stage, many meiofauna are distributed quite widely and this phenomenon is referred as the ‘meiofauna paradox’ (Higgins & Thiel, Reference Higgins and Thiel1988; Giere, Reference Giere2009; Cerca et al., Reference Cerca, Purschke and Struck2018). Recent investigations have revealed that the meiofauna paradox is present in Kinorhyncha at species-group or species level (Neuhaus & Sørensen, Reference Neuhaus and Sørensen2013; Herranz & Leander, Reference Herranz and Leander2016; Yamasaki et al., Reference Yamasaki, Grzelak, Sørensen, Neuhaus and George2018a, Reference Yamasaki, Neuhaus and George2019; Randsø et al., Reference Randsø, Yamasaki, Bownes, Herranz, Di Domenico, Qi and Sørensen2019). This is also the case for the E. multiporus species group and the E. bispinosus species group (Figure 14).

Fig. 14. Map of locations of all records of the E. multiporus species group (circles) (Yamasaki et al., Reference Yamasaki, Neuhaus and George2018b, Reference Yamasaki, Neuhaus and George2019; this study) and the E. bispinosus species group (triangles) (Higgins, Reference Higgins1982; Sørensen, Reference Sørensen2014; Sönmez et al., Reference Sönmez, Köroğul and Karaytuğ2016; Sørensen et al., Reference Sørensen, Gąsiorowski, Randsø, Sánchez and Neves2016; Cepeda et al., Reference Cepeda, Sánchez and Pardos2019c; this study).
With regard to the E. multiporus species group, E. multiporus and E. schwieringae have been recorded from seamounts: the former was from the summit to slope of Eratosthenes Seamount in the east Mediterranean Sea and from the slope of Senghor Seamount in the North-east Atlantic Ocean (Yamasaki et al., Reference Yamasaki, Neuhaus and George2018b, Reference Yamasaki, Neuhaus and George2019); the latter was found on the summit of Senghor Seamount in the North-east Atlantic Ocean (Yamasaki et al., Reference Yamasaki, Neuhaus and George2019). Echinoderes kajiharai sp. nov., on the other hand, was from the Daidokutsu submarine cave in the North-west Pacific Ocean (this study), so the distribution of the species group seems to be even wider than was previously supposed.
Concerning the E. bispinosus species group, all the congeners inhabit shallow waters in low-latitude regions: E. bispinosus was described from Bermuda, North Atlantic Ocean (Higgins, Reference Higgins1982); E. astridae was described from the Brazilian coast, South-west Atlantic Ocean (Sørensen, Reference Sørensen2014), and more recently reported in the Hispaniola Island in the Caribbean Sea (Cepeda et al., Reference Cepeda, Sánchez and Pardos2019c); ‘Echinoderes aff. bispinosusi’ was reported from a Turkish beach, east Mediterranean Sea (Sönmez et al., Reference Sönmez, Köroğul and Karaytuğ2016); Echinoderes sp. 1 in Sørensen et al. (Reference Sørensen, Gąsiorowski, Randsø, Sánchez and Neves2016) was from the Singapore coast, South China Sea (Sørensen et al., Reference Sørensen, Gąsiorowski, Randsø, Sánchez and Neves2016); and E. uozumii sp. nov. from the Daidokutsu submarine cave, North-west Pacific Ocean (this study). The last finding expands the species group's distribution range to the East Asian region.
Recent findings of widely distributed species/species groups of Kinorhyncha have drawn interest to how these wide distribution patterns were formed. Until now, three mechanisms have been proposed. The first mechanism is the dispersal by oceanic currents (Neuhaus & Sørensen, Reference Neuhaus and Sørensen2013; Yamasaki et al., Reference Yamasaki, Hiruta, Kajihara and Dick2014; Ishii et al., Reference Ishii, Yamasaki, Uozumi and Hirose2016). This would occur for kinorhynchs in association with suspension in the water column (Yamasaki et al., Reference Yamasaki, Hiruta, Kajihara and Dick2014), drifting by regulating its hydrophilic mucus secretion (Ishii et al., Reference Ishii, Yamasaki, Uozumi and Hirose2016), and rafting on other drifting organisms and substrates (Yamasaki et al., Reference Yamasaki, Hiruta, Kajihara and Dick2014). The second mechanism is dispersal effected by human activity (Herranz & Leander, Reference Herranz and Leander2016). An intertidal kinorhynch, Echinoderes ohtsukai Yamasaki & Kajihara, Reference Yamasaki and Kajihara2012, was supposedly introduced to British Columbia, Canada, associated with oyster cultivation (Herranz & Leander, Reference Herranz and Leander2016). The third mechanism suggests that past tectonic drifting produced the present-day wide distribution of kinorhynchs (Randsø et al., Reference Randsø, Yamasaki, Bownes, Herranz, Di Domenico, Qi and Sørensen2019). The wide distribution pattern of Echinoderes coulli species group is considered to have formed under the effect of plate tectonics since the Devonian period (Randsø et al., Reference Randsø, Yamasaki, Bownes, Herranz, Di Domenico, Qi and Sørensen2019). In the case of the E. multiporus species group, at least the second mechanism of artificial invasion can be rejected, because there has been no fishery cultivation in their habitats, i.e. seamounts and a submarine cave, as well as the considerably small impact of human activity. Whereas for the E. bispinosus species group, none of the three mechanisms can be ruled out. Further analyses of their phylogenetic relationships within the group are required to support or reject the remaining mechanisms.
Origin and endemism of echinoderid fauna in Daidokutsu
Animals living in submarine caves often show high endemism and unique features in fauna, morphology and phylogenetic relationship (Moldovan et al., Reference Moldovan, Kováč and Stuart2018). This endemism and uniqueness have been caused by an isolation of the cave environment from open waters, and attracted researchers to study their origin, adaptation and evolution in the cave environment (Moldovan et al., Reference Moldovan, Kováč and Stuart2018). Nevertheless, investigations of kinorhynchs in caves have been scarcely conducted. So far, only eight species are known from the cave environment, three of which represent the species of Echinoderidae, i.e. E. cavernus, E. dujardinii and M. macracanthus (Riedl, Reference Riedl1966; Sørensen et al., Reference Sørensen, Jørgensen and Boesgaard2000; Dal Zotto & Todaro, Reference Dal Zotto and Todaro2016). Among the three species, only E. cavernus is exclusively known from the cave environment, whereas the other two have been reported both inside and outside caves (Sánchez & Martínez, Reference Sánchez and Martínez2019). Based on these echinoderid records from caves made so far, it would be rather plausible to regard most cave-dwelling representatives of Echinoderes as not endemic to the cave environment.
The three species described in this study, E. gama sp. nov., E. kajiharai sp. nov. and E. uozumii sp. nov., have been found only from Daidokutsu. However, the scarce knowledge of kinorhynch fauna outside the cave does not allow us to reject the possibility that they inhabit other neighbouring environments outside of Daidokutsu. Because Daidokutsu is a young submarine cave, which submerged ~8000 years ago (Omori et al., Reference Omori, Kitamura, Fujita, Honda and Yamamoto2010), the three kinorhynchs arguably invaded the cave relatively recently from the neighbouring waters, for instance from muddy bottom (similar sediment type to the cave) of open waters, under overhangs, or even other caves in the Ryukyu Islands. Considering their recent invasion to Daidokutsu, they may still inhabit such environments outside Daidokutsu also. It also can be assumed that E. kajiharai sp. nov. and E. uozumii sp. nov. can survive in non-cave environments, because all the other congeners of the E. multiporus and the E. bispinosus species groups inhabit non-cave environments. For now, we cannot conclude whether the three new species exclusively inhabit caves. Further investigations in the neighbouring environment of Daidokutsu, and phylogenetic analyses of the E. multiporus and the E. bispinosus species groups will shed light on their endemism and migration process into the cave.
Acknowledgements
We greatly appreciate Mr K. Yasumura for his help collecting sediment samples; and Dr M. V. Sørensen and an anonymous reviewer for reviewing the manuscript and providing helpful comments.
Financial support
This study was supported by a grant from the Fujiwara Natural History Public Interest Incorporated Foundation to HY; a KAKENHI Grant (15K18598) from the Japan Society for the Promotion of Science to HY; and Japan Society for the Promotion of Science Grant-in-Aid for JSPS fellows (Grant No. 25987) to SF.






















