INTRODUCTION
Epibiosis is defined as a non-symbiotic spatial association between a substratum organism (basibiont) and a sessile organism (epibiont), with the latter growing attached to the basibiont's outer surface and, if present, trophic exchange with the substratum organism is facultative (Wahl, Reference Wahl1989, Reference Wahl, Dürr and Thomasson2010). Epibiosis is predominantly found in the aquatic environment, with water providing an effective food vector for many sessile organisms (Harder, Reference Harder, Flemming, Murthy, Venkatesan and Cooksey2008). In fact, there are many described examples of marine epibiosis (Morton, Reference Morton1988; Whal & Mark, Reference Wahl and Mark1999) and in some habitats, for example, soft-bottoms, biogenic or living surfaces may actually comprise the largest proportion of hard stable substrata available for colonization (e.g. Creed, Reference Creed2000; Harder, Reference Harder, Flemming, Murthy, Venkatesan and Cooksey2008; Munguia & Miller, Reference Munguia and Miller2008). The list of marine basibionts is long but generally includes slow-moving or sessile, non-burrowing, long-lived and large organisms (Wahl, Reference Wahl1989; Wahl & Mark, Reference Wahl and Mark1999). Most epibionts are facultative, occurring on both living and non-living substrata (Wahl & Mark, Reference Wahl and Mark1999).
Ecologically, epibiosis can entail a range of benefits and disadvantages for both the epi- and basibiont, which are summarized in Wahl (Reference Wahl1989). According to Wahl & Mark (Reference Wahl and Mark1999), some groups of species, including macro- and microalgae, and sessile filter-feeders, such as bryozoans, cnidarians and porifera, are more frequently found as epibionts. In contrast, molluscs and crustaceans are more frequently found as basibionts (see figure 2 in Wahl & Mark, Reference Wahl and Mark1999).
Patellid limpets are considered key organisms capable of structuring rocky habitats and in areas where grazing is intense few species but the most grazing-resistant forms of algae (i.e. crustose algae) are able to grow (Hawkins & Hartnoll, Reference Hawkins and Hartnoll1983; Jenkins et al., Reference Jenkins, Moore, Burrows, Garbat, Hawkins, Ingolfsson, Sebens, Snelgrove, Wethey and Woodin2008; Martins et al., Reference Martins, Thompson, Neto, Hawkins and Jenkins2010). In contrast, the shells of limpets can support a dense cover of algae (including those that are grazed off of the rock) and animals.
Patellid limpets are traditionally an important food species in the archipelagos of Macaronesia (north-east Atlantic), where they are collected and consumed, especially during summer months (Martins et al., Reference Martins, Jenkins, Hawkins, Neto and Thompson2011). As a consequence of intense harvesting, populations of these species are considered overexploited, at least at some islands (Hawkins et al., Reference Hawkins, Côrte-Real, Pannacciulli, Weber and Biship2000; Navarro et al., Reference Navarro, Ramírez, Tuya, Fernandez-Gil, Sanchez-Jerez and Haroun2005; Martins et al., Reference Martins, Jenkins, Hawkins, Neto and Thompson2008a). Patella aspera Röding (1798) occurs predominantly in the shallow subtidal of Macaronesia and often supports a dense cover of algal turf on the shells. In a previous account of the epibiota found on the shells of P. aspera collected from two sites of the Azores, Fralick et al. (Reference Fralick, Hehre and Mathieson1985) identified a total of 20 species of macroalgae. In this study we add to the previous work by examining the epibiota on a much larger sample covering the entire Azores archipelago. In addition, we explore the data for evidence of the processes responsible for structuring these epibiotic communities.
MATERIALS AND METHODS
Sampling
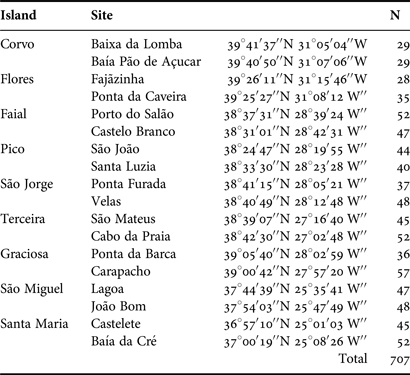
A total of 784 individuals of Patella aspera were sampled on two different populations in each of the nine islands of the Azores (Table 1). The soft part of each individual was removed for molecular studies (in preparation), and the shells were frozen or preserved in ethanol. Upon inspection, shell integrity was verified and broken shells were discarded from the analysis. Those that were in good condition were retained (N = 707, see Table 1 for details on sampling sites and numbers), hydrated in water, and all the epibiota present on the shells were identified to the lowest possible taxonomic level using a dissecting and optical microscope. Total cover of sessile epibiota on each shell was estimated using the semi-quantitative scale DAFOR, while mobile animals (i.e. limpets) were enumerated. In addition, shell length was measured using a Vernier calliper with a precision of 0.1 mm.
Table 1. Summary information about sampling sites and the numbers of individuals examined.

Data analysis
The relationship between basibiont shell length and epibiota richness was examined using linear regression. Spatial variation in shell size and epibiota richness was examined using a two-way fully nested design with the following factors: island (nine levels, random) and site (two levels, random and nested within island). The analyses were run on PERMANOVA (Anderson, Reference Anderson2001, Reference Anderson2005) based on Euclidean distances, with 999 permutations which produce estimates of variation analogous to the classical ANOVA (Anderson, Reference Anderson2001). For the analysis of the spatial variation in richness, shell length was included as a covariate to standardize for spatial variation in shell size.
RESULTS
Variation in basibiont size
Basibiont shell length ranged between 16.4 and 74.3 mm, with a mean shell length of 40.5 mm (±0.4 standard error (SE), N = 707). Of the 707 individuals selected, only 74 (~10%) were larger than the minimum legal catch size (50.0 mm), an indication of the high level of exploitation of this species. In addition, there was significant spatial variation in limpet shell length at the scale of the islands and sites (Table 2).
Table 2. A two-way hierarchical PERMANOVA examining the spatial variability in mean basibiont size (shell length) among islands and sites.

Variation in epibiont richness
A total of 190 taxa were identified of which 97% were algae with only five animal taxa recorded. Only five shells out of the 707 (0.7%) examined were completely devoid of epibionts. A total of 17 new records for the Azorean algal flora were found on the epibiota species identified (four Chlorophyta, two Heterokontophyta (Phaeophyceae) and 11 Rhodophyta, Table 3). Encrusting coralline algae (i.e. Lithothamnion spp.) was the most frequent taxa, occurring on 54% of all shells (Figure 1). Small turf-forming algal species were the dominant erect taxa, amongst which Gelidium sp., Ceramium rubrum, Jania sp. and Polysiphionia denudata were the most common, being recorded on at least 30% of the shells (Figure 1). The large majority of the taxa identified (>85% of the species) was recorded in less than 10% of the shells (Figure 1).

Fig. 1. Epibiont percentage occupancy on shells of Patella aspera. Taxa that were not present on at least 5% of the shells are not included for clarity.
Table 3. New algal additions to the Azorean flora found as epibiota on shells of Patella aspera.

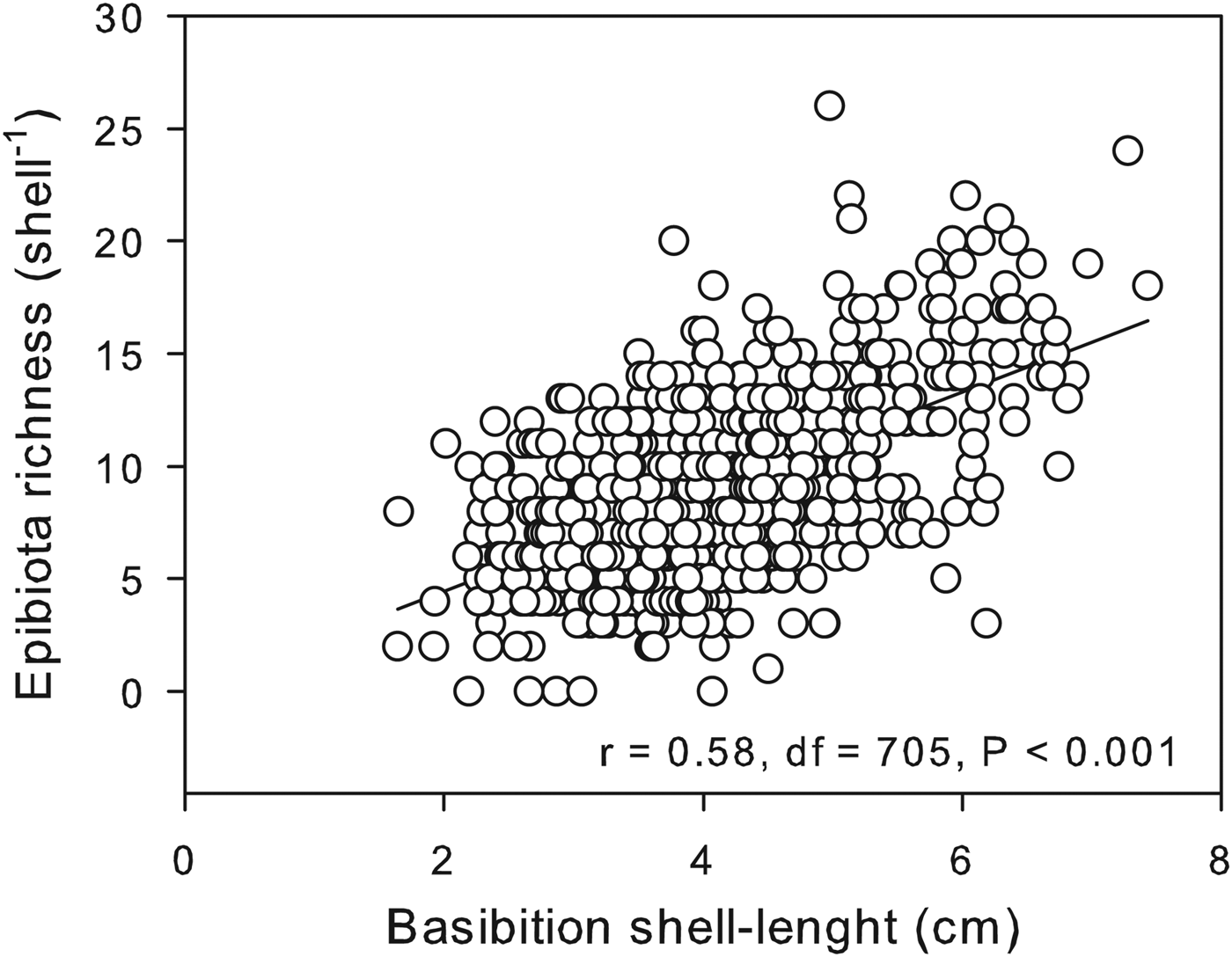
The mean number of epibionts found on shells of Patella aspera was 9.00 ± 0.15 (mean ± SE) and ranged between 0 and 26 taxa. A significant and positive linear regression was found between basibiont shell length and epibiont richness (Figure 2) indicating that larger (and older) limpets support a richer epibiota.

Fig. 2. Relationship between basibiont size (limpet shell length) and epibiota richness.
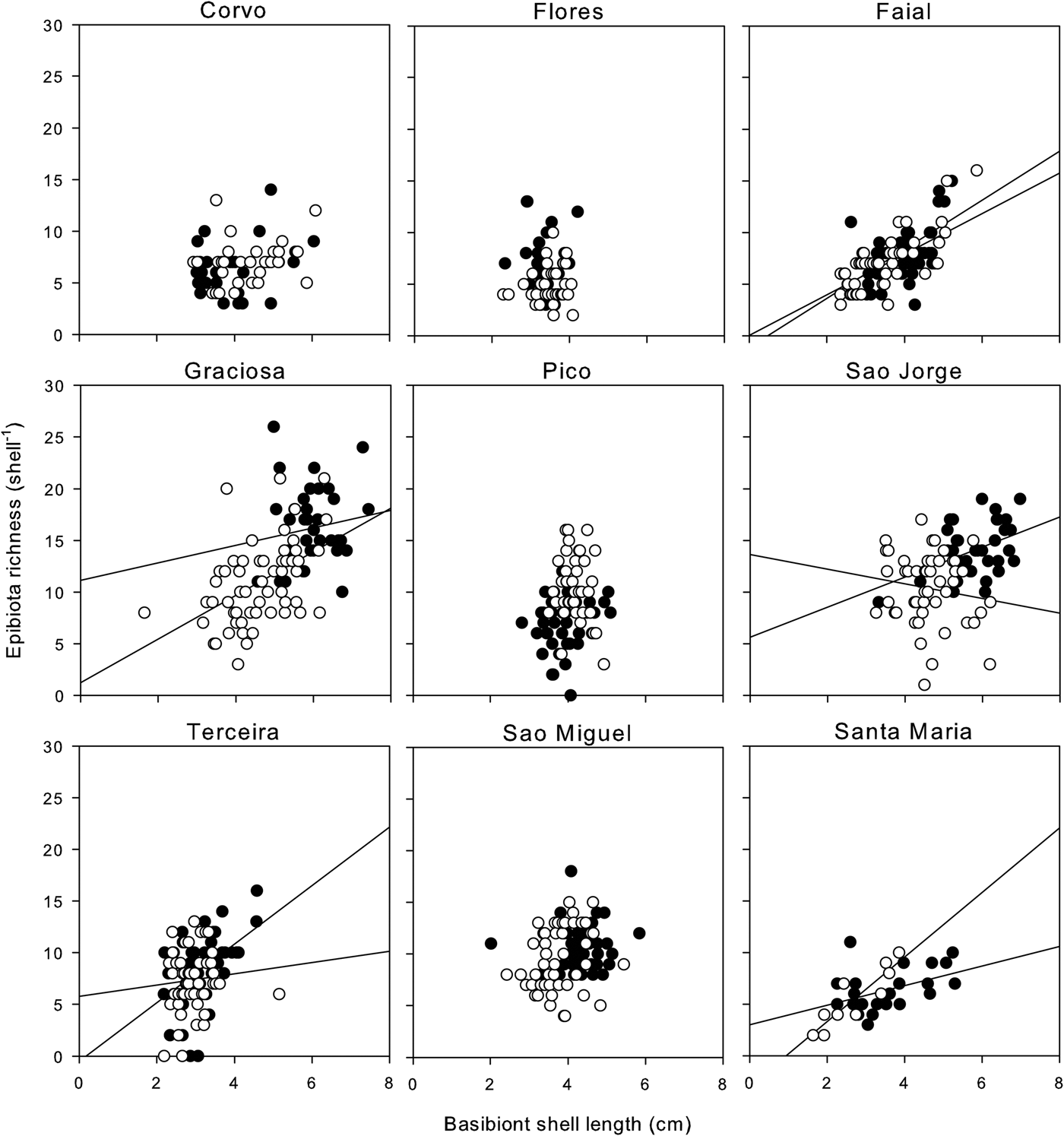
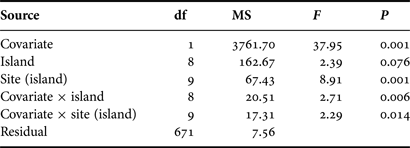
Analysis of the spatial variation in epibiota richness showed significant interactions between the covariate (shell length), island and site (Table 4). Analysis of Figure 3 shows that a positive and significant relationship between basibiont size and epibiont richness was spatially inconsistent, being present at some islands, but not at others. Similarly, the relationship was variable between sites within islands. These results suggest that the relationship between the basibiont and epibiota was influenced by processes other than the basibiont size alone, including processes operating at large- (island) and intermediate- (site) spatial scales.

Fig. 3. Relationship between basibiont size (limpet shell length) and epibiota richness broken down between islands and sites. Filled and open circles represent each of the two sites sampled on each island, respectively. Significant correlation lines are indicated and extended to the axes for clarity.
Table 4. A two-way PERMANOVA examining differences in epibiota richness on shells of Patella aspera among islands and sites. Individual shell length was used as the covariate.

DISCUSSION
An overwhelmingly large number of epibiota was found on shells of Patella aspera, unlike the earlier record by Fralick et al. (Reference Fralick, Hehre and Mathieson1985), which is not surprising given the restricted sample examined by the latter. This large diversity found on the shells highlights the idea that P. aspera, despite being a key herbivore, also plays an important habitat for many of the species found and on which it feeds. Apart from the new records (see below), for which there is no information regarding their distribution in the Azores, all taxa found on shells have also been recorded on a natural rocky substratum (authors, personal observation). This suggests that: (1) shells of limpets do not provide a ‘unique’ habitat; and (2) no obligatory epibiont exists among the taxa here found. Nevertheless, the shells of P. aspera may provide a key microhabitat that increases the survivorship of colonizing algae, especially where they occur in areas devoid of conspicuous macroalgae (barrens) created by the sea urchin Arbacia lixula.
The new additions to the Azorean algal flora reported in this study correspond to species that have probably gone unnoticed until now due to their small size and/or obscure life cycle, although a northern extension of such taxa due to climate changes in the last decades (i.e. increase in sea temperature) cannot be excluded. In fact, all the new additions here reported are known from southern locations in the Macaronesian region (Guiry & Guiry, Reference Guiry and Guiry2013), with their presence in the Azores representing a hypothetical recent northern extension in their distribution. Distribution shifts in marine species associated with recent increases in sea temperature have been referred to before for the north-east Atlantic, with several examples showing that rising sea temperatures or climate related changes can result in southern species shifting northward (Hawkins et al., Reference Hawkins, Southward and Genner2003; Perry et al., Reference Perry, Low, Ellis and Reynolds2005).
A substantially lower number of completely unfouled shells was generally found (0.74%) compared to other mollusc basibionts, such as turbo snails (Wernberg et al., Reference Wernberg, Tuya and Thomsen2010) and fan mussels (Addis et al., Reference Addis, Secci, Brundu, Manunza, Corrias and Cau2009). Whal & Sönnichsen (Reference Wahl and Sönnichsen1992) found that epibiosis on the shells of Littorina littorea was spatially variable. In this case, intertidal populations from Helgoland rarely carried any epibiota, whereas subtidal populations from the Kiel Bight were frequently fouled. They found that the extent of shell fouling was primarily driven by littorinid density (probably associated with mucus secretion, bulldozing or mutual grazing), whereas differences in habitat conditions played a secondary role. The overall low density of P. aspera (as a consequence of exploitation), and the fact that herbivore epibionts were rarely found, may thus explain the insignificant proportion of unfouled shells. There are, however, other possibilities. Many studies have shown that epibiosis can influence predator–prey interactions, either by facilitating or hampering the success of predators (Wahl et al., Reference Wahl, Hay and Enderlein1997; Laudien & Wahl, Reference Laudien and Wahl1999; Enderlein et al., Reference Enderlein, Moorthi, Rohrscheidt and Wahl2003). For instance, Silva (Reference Silva2008) and Silva et al. (Reference Silva, Hawkins, Boaventura and Thompson2008) found that limpets can generally be important prey for crabs. A strong and selective preference of crabs for unfouled shells (Wahl et al., Reference Wahl, Hay and Enderlein1997) could thus also lead to a generally low number of unfouled shells. Moreover, selective harvesting by man (e.g. unfouled shells are more easily detected) could also add to the effects of predation. Without rigorous experimental work, however, it is impossible to determine the reason why so few shells remain free of epibionts.
As in most studies examined (e.g. Wernberg et al., Reference Wernberg, Tuya and Thomsen2010), there was a positive relationship between basibiont size and epibiota richness. This is not surprising, given the ubiquitous species–area relationship (see Connor & McCoy, Reference Connor and McCoy1979; McGuinness, Reference McGuinness1984; Lomolino, Reference Lomolino2000 for reviews). However, in the case of living substrata, this positive relationship could also be the result of temporal accumulation of species, as larger shells are also generally associated with older individuals.
Results also indicated that the relationship between basiont size and epibiota richness was variable between islands and between locations within islands. Not only did slopes differ, but also there were islands and sites where the relationship was non-existent. Wernberg et al. (Reference Wernberg, Tuya and Thomsen2010) also found spatially variable results between basibiont size and epiflora richness, and attributed these differences to spatial variation in the density of epizooic herbivores. In our case, epizooic herbivores were rare and thus unlikely to produce such results. However, these results do indicate that epibiota richness is not only driven by basibiont size, but also that other processes (e.g. regional and local species pool, local environmental and biotic conditions) have the ability to influence these assemblages. In this regard, epibiont assemblages are no different from the generality of marine communities (e.g. Fraschetti et al., Reference Fraschetti, Terlizzi and Benedetti-Cecchi2005; Martins et al., Reference Martins, Thompson, Hawkins, Neto and Jenkins2008b).
In conclusion, we found a highly diverse assemblage living on the shells of P. aspera. Epibiota richness was positively influenced by basibiont size, but there was evidence that larger-scale processes operating at the scales of island and location also played a role in structuring these assemblages. The basibiont–epibiont relationship resembles, in many ways, the studies of island biogeography (e.g. MacArthur & Wilson, Reference MacArthur and Wilson1963; Whittaker et al., Reference Whittaker, Triantis and Ladle2008) suggesting that it might be a good model system in which to experimentally test hypotheses about ecosystems that are not so amenable to experimentation (e.g. islands and lakes).
FINANCIAL SUPPORT
G.M.M. was supported by a post-doctoral grant awarded by Fundação para a Ciência e Tecnologia (FCT), Portugal (SFRH/BDP/63040/2009). J.F. was supported by a PhD grant awarded by FRCT, Azores (M3.1.2/F/021/2011). This research was funded by the project Patelgene (PTDC/BIA-BIC/115837/2009, FCT) and by the European Regional Development Fund (ERDF) through the COMPETE—Operational Competitiveness Programme and national funds through FCT—Foundation for Science and Technology, under the project ‘PEst-/MAR/LA0015/2011’.