Cephalocarida is a class of small benthic crustaceans distributed from the intertidal zone to approximately 1550 m depth. To date, only eleven species belonging to five genera have been described. Lightiella magdalenina Carcupino, Floris, Addis, Castelli, Curini-Galletti, Reference Carcupino, Floris, Addis, Castelli and Curini-Galletti2006, the most recently discovered species from La Maddalena Archipelago (Sardinia, Italy) (41°13′N 9°25′E), is characterized by a high degree of endemism. No other species have been reported in Europe, and the discovery of L. magdalenina in the Mediterranean Sea (Carcupino et al., Reference Carcupino, Floris, Addis, Castelli and Curini-Galletti2006) fills a gap in the worldwide distribution of the entire class. Its type locality is characterized by a muddy sand bottom very rich in organic matter with little seagrass beds. Since its first description (Sanders, Reference Sanders1955), Cephalocarida was considered the most primitive living crustacean class. However, it remains a poorly known taxon, and its phylogenetic position is controversial. Molecular data are only available for one species, Hutchinsoniella macracantha Sanders, Reference Sanders1955, and refer to a complete mitochondrial genome (Lavrov et al., Reference Lavrov, Brown and Boore2004), to two mitochondrial genes (Giribet et al., Reference Giribet, Edgecombe and Wheeler2001) and to six nuclear genes (Spears & Abele, Reference Spears, Abele, Fortey and Thomas1997; Colgan et al., Reference Colgan, McLauchlan, Wilson, Livingston, Edgecombe, Macaranas, Cassis and Gray1998; Regier & Shultz, Reference Regier and Shultz1998; Reference Regier and Shultz2001; Shultz & Regier, Reference Shultz and Regier2000; Giribet et al., Reference Giribet, Edgecombe and Wheeler2001; Richter et al., Reference Richter, Olesen and Wheeler2007). Nevertheless, the molecular analyses have not provided unequivocal results in terms of phylogenetic relationships: even when the survey was limited to mtDNA, Cephalocarida has been tentatively related with Remipedia (Giribet et al., Reference Giribet, Edgecombe and Wheeler2001), Maxillopoda and Pentastomida (Lavrov et al., Reference Lavrov, Brown and Boore2004).
The aim of this short note is to provide molecular data for this rare and poorly known crustacean class, based on the sequencing of two mitochondrial genes of L. magdalenina. These genes, selected for their value in phylogenetic analysis, are Cytochrome c Oxidase subunit I (COI) and Cytochrome b (Cyt-b). The former has been proposed by Hebert et al. (Reference Hebert, Cywinska, Ball and deWaard2003) as a sort of genetic ‘barcode’, which can serve as the core of a global bioidentification system for animals. The latter, widely used in vertebrate evolutionary studies, is particularly effective for the reconstruction of molecular phylogeny in invertebrates (Simmons & Weller, Reference Simmons and Weller2001).
For PCR amplifications of a partial region of both genes, we used primers designed by Folmer et al. (Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994) for COI, and Boore & Brown (Reference Boore and Brown2000) for Cyt-b. Standard DNA procedures were used to extract from the whole body of a specimen about 2 mm long. The PCR amplification mix contained: 0.4 µM of each primer; 2.5 U of Taq DNA Polymerase; 2.5 mM of MgCl2; 200 µM of dNTPs. The PCR profile consisted of 35 cycles (denaturation: 1′ at 94°C; annealing: 1′ at 52°C; extension: 1′30″ at 72°C).
The PCR amplifications yielded a product of 618 bp for the COI gene, and 345 bp for the Cyt-b gene (Genbank accession numbers: EU530536 and EU530537). Comparison of the two sequences with those of H. macracantha revealed 143 changes (66 transitions and 77 transversions) for COI and 126 changes (44 transitions, 79 transversions, and 3 deletions belonging to the same codon) for Cyt-b. These mutations led to 38 amino acid changes over 206 for the COI enzyme, and 50 amino acid changes and the deletion of one aspartate over 116 for the Cyt-b enzyme. For further analysis, the two genes were combined, excluding the third base of each codon, for a total of 644 bp.
A median joining network analysis was carried out with Network 4.5.0.0 software (http://www.fluxus-engineering.com) (Bandelt et al., Reference Bandelt, Forster and Rohl1999), using the sequences of L. magdalenina and those of 8 representative species of Pancrustacea, whose complete genomes were reported in Lavrov et al. (Reference Lavrov, Brown and Boore2004) (Figure 1). As shown by the short length of the torso, the network analysis, based on a neighbour joining (NJ) approach, is not appropriate for deep level phylogeny. However, it allows discrimination of the phylogenetic status of specific residues. The cephalocarid clade is separated by the basal median vector by 49 changes, which represent the plesiomorphic status for the class. Lightiella magdalenina and H. macracantha are separated by 114 nucleotide differences: 62 of them are apomorphic for L. magdalenina, 32 for H. macracantha, while 20 cannot be univocally assigned (resulting in a triangular reticulation of the network). The prevalence of apomorphisms over plesiomorphisms suggests a very ancient separation of the two species from a common ancestor. Equality of the evolutionary rate of the two species was tested for both genes with the method proposed by Tajima (Reference Tajima1993) using the MEGA4 software (Tamura et al., Reference Tamura, Dudley, Nei and Kumar2007). In spite of the observed differences in the number of apomorphic nucleotides, the Tajima relative rate test of neutrality was non-significant for both genes, whatever the other sequence used as outgroup. A maximum likelihood (ML) analysis was carried out with Treefinder (http://www.treefinder.de) (Jobb, Reference Jobb2008), applying edge support (LR-ELW) (Strimmer & Rambaut, Reference Strimmer and Rambaut2002) (Figure 2). Lightiella magdalenina was analysed together with another 30 Pancrustacea species, using as outgroup 5 Myriapoda and one Chelicerata species. The general topology of ML analyses appears to be consistent with the network structure, showing a deep divergence of the cephalocarid clade and an ancient separation of the two species. As previously observed by Hassanin (Reference Hassanin2006), the affinity between Cephalocarida and Copepoda suggested by ML analysis, could be interpreted as a consequence of a long branch attraction phenomenon due to reverse strand bias (Felsenstein, Reference Felsenstein1978).
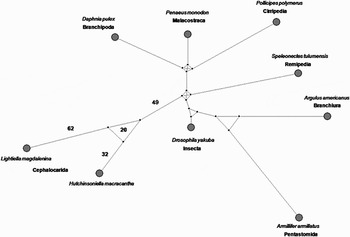
Fig. 1. Median joining network of the combined Cytochrome c Oxidase I and Cytochrome b partial genes from Lightiella magdalenina and 8 Pancrustacea species.

Fig. 2. Maximum likelihood unrooted tree of combined Cytochrome b and Cytochrome c Oxidase I partial genes from Lightiella magdalenina and 36 arthropod species (above). Numbers refer to the LR-ELW values (10,000 replicates) for the Cephalocarida branch.
A discussion of arthropod evolution is far beyond the scope of this short note. Nevertheless, our contribution of molecular data for a newly discovered species increases the knowledge on the genetic variation within Cephalocarida, which can be useful for future research in this strongly debated field.
ACKNOWLEDGEMENT
We are indebted to an anonymous referee for sharing additional arthropod sequences and for useful help in phylogenetic analysis.




