Introduction
Zooplankton are of great importance in energy transfer and structuring of marine food webs. Thus, detailed descriptions of zooplankton variability and its main drivers are necessary to broaden knowledge of how marine ecosystems function. Chaetognaths are an important planktonic group, commonly present in high densities in marine pelagic environments (Bone et al., Reference Bone, Kapp and Pierrot-Bults1991; Liang, Reference Liang2002). They are active predators, grabbing their prey with rigid hooks (Feigenbaum, Reference Feigenbaum, Bone, Kapp and Pierrot-Bults1991; Casanova, Reference Casanova and Boltovskoy1999). Due to their position in the pelagic food web as an intermediate link in the energy flow to higher trophic levels such as fish and ubiquitous occurrence in marine environments, chaetognaths have a key role structuring both coastal and oceanic pelagic communities (Bone et al., Reference Bone, Kapp and Pierrot-Bults1991; Casanova, Reference Casanova and Boltovskoy1999).
As typical for most zooplankton taxa (e.g. Boltovskoy, Reference Boltovskoy1999), chaetognaths may be sensitive to changes in the physical structure of the water column, and different hydrographic conditions may lead to different feeding, growth, reproduction and survival rates, influencing their abundance levels and population dynamics (e.g. Ramírez & Viñas, Reference Ramírez and Viñas1982; Daponte et al., Reference Daponte, Pertierra, Palmieri and Nuñez2008; Wu et al., Reference Wu, Li, Huanh, Yin and Tan2014). Apart from the physical environment, biotic factors such as food availability and quality also have an important role influencing chaetognath distribution and population dynamics (Gibbons & Stuart, Reference Gibbons and Stuart1994; Liang et al., Reference Liang, Ara, Miranda, Bérgamo and Bernardes2003; Sato et al., Reference Sato, Hernández and Viñas2011; Noblezada & Campos, Reference Noblezada and Campos2012). The influence of physical and biological environment on chaetognath populations and their spatial and temporal dynamics from shelf and oceanic ecosystems is relatively well-known, with many available studies on the South-western Atlantic (e.g. Crelier & Daponte, Reference Crelier and Daponte2004; Araújo & Ribeiro, Reference Araújo and Ribeiro2005; Souza et al., Reference Souza, Luz and Mafalda2014) and in most other main regions worldwide (e.g. Gibbons & Stuart, Reference Gibbons and Stuart1994; Nair et al., Reference Nair, Terazaki and Jayalakshmy2002; Ruiz-Boijseauneau et al., Reference Ruiz-Boijseauneau, Sanvicente-Añorve and Fernández Álamo2004; Noblezada & Campos, Reference Noblezada and Campos2012; Wu et al., Reference Wu, Li, Huanh, Yin and Tan2014).
In contrast, estuarine chaetognath populations have been poorly studied. Detailed data on abundance, population structure and/or reproduction are available only from a few scattered estuaries and species worldwide (Reeve, Reference Reeve1964; Mulkana & McIlwain, Reference Mulkana and McIlwain1973; Grant, Reference Grant1977; Srinivasan, Reference Srinivasan1980; Nair & Sankarankutty, Reference Nair and Sankarankutty1988; Liang et al., Reference Liang, Ara, Miranda, Bérgamo and Bernardes2003), with even fewer data formally analysing the influence of food availability and hydrographic parameters. Estuaries are typically characterized by a haline horizontal gradient which has a paramount influence on the structure and functioning of communities (Whitfield & Elliott, Reference Whitfield, Elliott, Wolanski and McLusky2011), including zooplankton abundance, diversity and assemblage structure (Xu et al., Reference Xu, Liu, Xu, Sun and Chen2014; Miyashita & Calliari, Reference Miyashita and Calliari2016). This may be particularly true for chaetognaths since they are exclusively marine and most species are more common over the continental shelf and offshore (Bone et al., Reference Bone, Kapp and Pierrot-Bults1991; Casanova, Reference Casanova and Boltovskoy1999). General latitudinal climatic patterns, reflected mostly by the temperature and rainfall, are expected to influence the seasonal dynamics of estuarine plankton, which typically attain lower abundances during winter in subtropical to temperate latitudes (Reeve, Reference Reeve1964; Marques et al., Reference Marques, Azeiteiro, Martinho and Viegas2009; Nogueira Júnior et al., Reference Nogueira, Nascimento, Maciel, Tilbert, Oliveira, Hoffmeyer, Sabatini, Brandini, Calliari and Santinelli2018), and tend to follow the rainfall regime in tropical regions (Nair, Reference Nair1974; Hernández et al., Reference Hernández, Suárez-Morales and Gasca2005). However, the general absence of baseline detailed data hampers broad generalizations and understanding the responses of different species. In the present study we test the role of physical (temperature and salinity) and biological (food availability) factors as drivers of spatial and temporal dynamics of chaetognath population structure through an annual cycle in the subtropical Babitonga Bay estuary, South Brazil.
Materials and methods
Study area
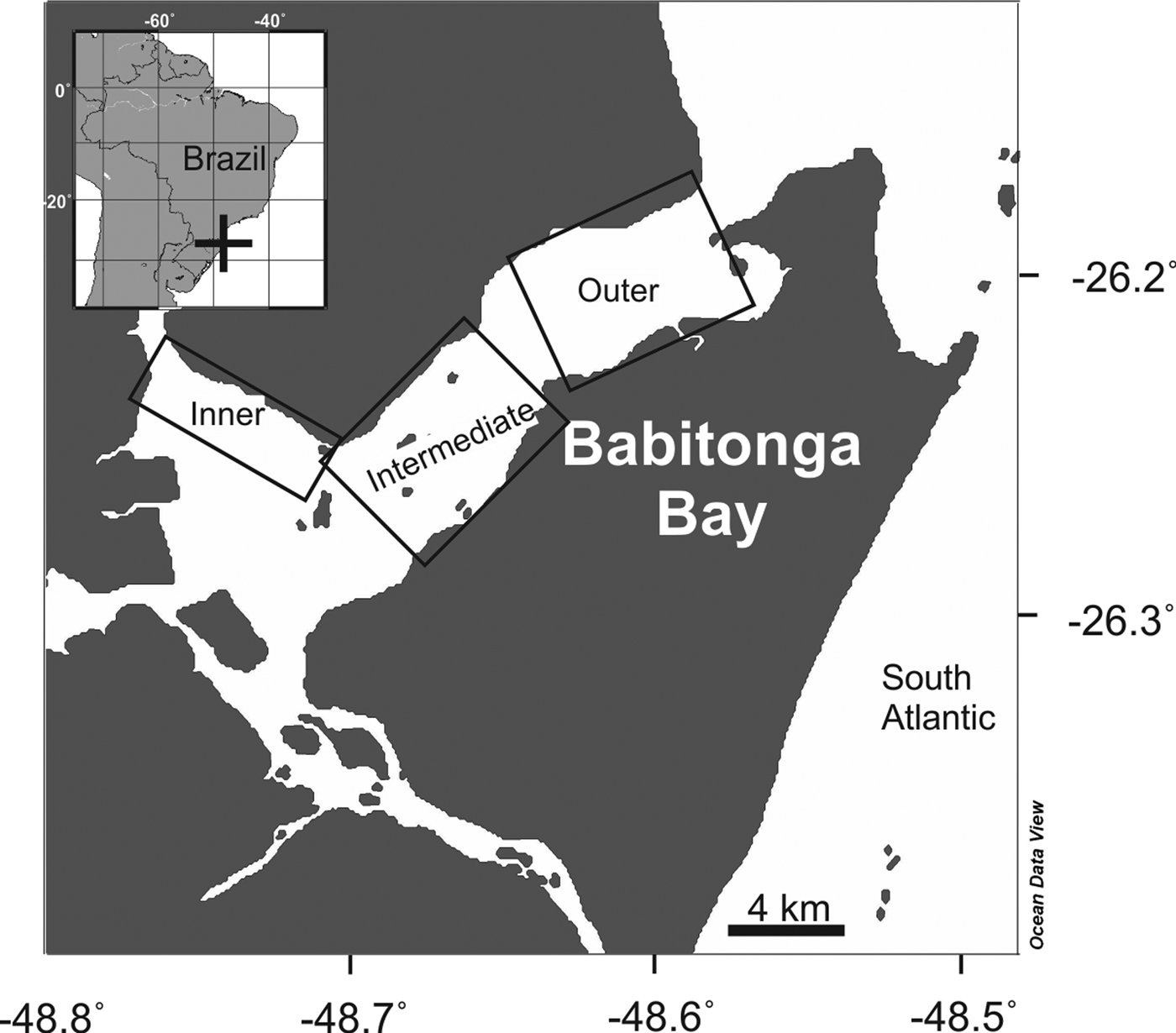
Babitonga Bay estuary is located in the state of Santa Catarina, south Brazil (Figure 1), with an area of ~130 km2 and average depth of 6 m, and up to 28 m depth in the main canal. The region is characterized by a wet subtropical climate, with mean rainfall of ~2000 mm year−1, a rainy season between spring and late summer/early autumn and a dry season during autumn and winter (DNIT-IME, 2004; Cremer et al., Reference Cremer, Morales and Oliveira2006). It has a high biological productivity, sustaining high abundance and diversity of aquatic fauna and flora (Brandini et al., Reference Brandini, Alquini, Pereira, Leite, Cremer, Morales and Oliveira2006; Vilar et al., Reference Vilar, Spach and Santos2011; Nogueira Júnior, Reference Nogueira2012). Babitonga Bay estuary has a tidal range of ~1.30 m (DNIT-IME, 2004; Cremer et al., Reference Cremer, Morales and Oliveira2006). The tide is mixed with semidiurnal dominance and diurnal inequalities with a natural oscillation period of ~3.6 h (Truccolo & Schettini, Reference Truccolo and Schettini1999).
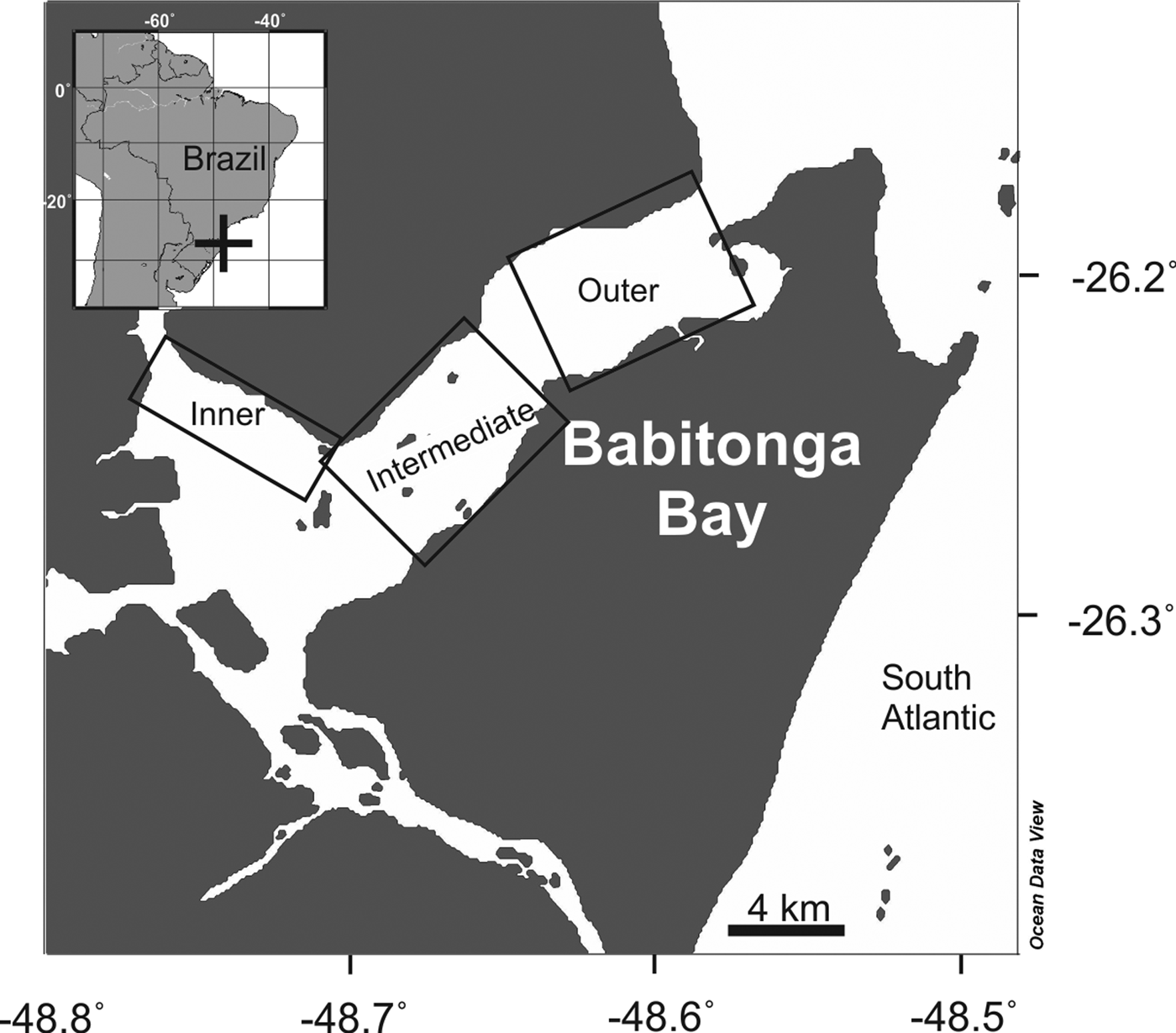
Fig. 1. Map of Babitonga Bay, South Brazil, showing the three sectors sampled between October 2007 and August 2008. Generated using Ocean Data View software (Schlitzer, Reference Schlitzer2017).
Sampling
Eight seasonal surveys, two in each season, were performed at Babitonga Bay estuary between October 2007 and August 2008. During each field campaign three sectors of the estuary were sampled (Figure 1). In each sector three replicate zooplankton samples were performed with a 40 cm mouth diameter and 200 µm mesh WP-2 plankton net (N = 72), and six replicate samples with a 50 cm mouth diameter and 500 µm mesh WP-2 plankton net (N = 144). Nets were slowly (≤1.5 knots), obliquely hauled for 2–5 min covering most of the water column. Nets were coupled with a mechanical flowmeter (Hydrobios). All hauls (N = 216) were performed during daylight, between 9 a.m. and 3 p.m. Samples were fixed in 4% formaldehyde filtered (<30 µm) seawater solution directly after retrieval of the nets.
Subsurface and near-bottom temperature and salinity were measured with a multi-probe (Horiba U-10) and water transparency with a Secchi disk. Chlorophyll-a was estimated based on subsurface samples filtered with Whatman filters (25 mm diameter, GF/F), with pigments extracted in the laboratory (Parsons et al., Reference Parsons, Mata and Lalli1984) and measured on a calibrated fluorometer (Turner Designs – Trilogy).
Whole samples taken with the 200 µm mesh net (N = 72) were analysed under a stereomicroscope (Zeiss, Stemi, 2000) and the chaetognaths were separated, identified (mainly following Casanova, Reference Casanova and Boltovskoy1999) and quantified. The dominant Parasagitta friderici was further classified as either juvenile or adult following standard classification of the species (Boltovskoy, Reference Boltovskoy1975; Mendes et al., Reference Mendes, Figueiredo and Valentin2012). Gelatinous organisms were also manually removed from the samples (Nogueira Júnior, Reference Nogueira2012) and the rest of the 200 µm samples were used to estimate zooplankton wet weight (mostly copepods, cladocerans and other crustaceans) by gravimetry after removing the water excess with blotted paper (Omori & Ikeda, Reference Omori and Ikeda1994), using a digital analytical balance (Lab Genius DL-224) with precision of 0.1 mg. Samples taken with the 500 µm mesh (N = 144) were analysed in their entirety under the stereomicroscope and fish larvae counted. Zooplankton biomass and fish larvae abundance were used to characterize different types of food items and their availability for the chaetognaths.
Data analysis
Two-way analysis of variance (ANOVA) was used to test if the abundance of adult or juvenile P. friderici changes significantly (P < 0.05) considering temporal (months sampled; eight levels) and spatial (sectors within the estuary; three levels) factors and their interaction (Zar, Reference Zar2010). To test the effect of different hydrographic and biological variables on juvenile and adult P. friderici abundances, we used Generalized Additive Models (GAMs). GAMs are able to deal with non-linear relationships between a dependent variable and multiple predictors in the same model through non-parametric generalizations of multiple linear regressions that are less restrictive about the underlying distribution of data (Hastie & Tibshirani, Reference Hastie and Tibshirani1990). Among predictors used, we included physical parameters, such as temperature, salinity and water transparency, and biological parameters such as zooplankton biomass and fish larvae abundance, considered as food source availability for chaetognaths, and chlorophyll-a as an indirect measure of biological productivity. Statistical analyses were performed in STATISTICA 13.0.
Results
Abiotic environment
Salinity was always >26 in the outer sector, varying between 26 and 31.5 and between 27.3 and 33.6 in the sub-surface and near the bottom respectively without a clear temporal pattern. In the intermediate and inner sector salinities were lower, ranging in the inner sector from 10.1 to 28.4, and from 16 to 28.8 in the sub-surface and near bottom respectively (Figure 2). While in the outer sector no clear temporal pattern could be discerned, in the intermediate and inner sectors salinity was considerably lower in February (Figure 2), associated with higher rainfall. In February, April, July and August, salinities in the inner and intermediate sectors were similar, while the outer sector salinity was always distinct from the other two sectors (Figure 2). Temperature remained between 25–27°C throughout the summer, decreasing to 19.2–19.9°C in August; no spatial differences in the temperature were observed, either horizontally or vertically (Figure 2).
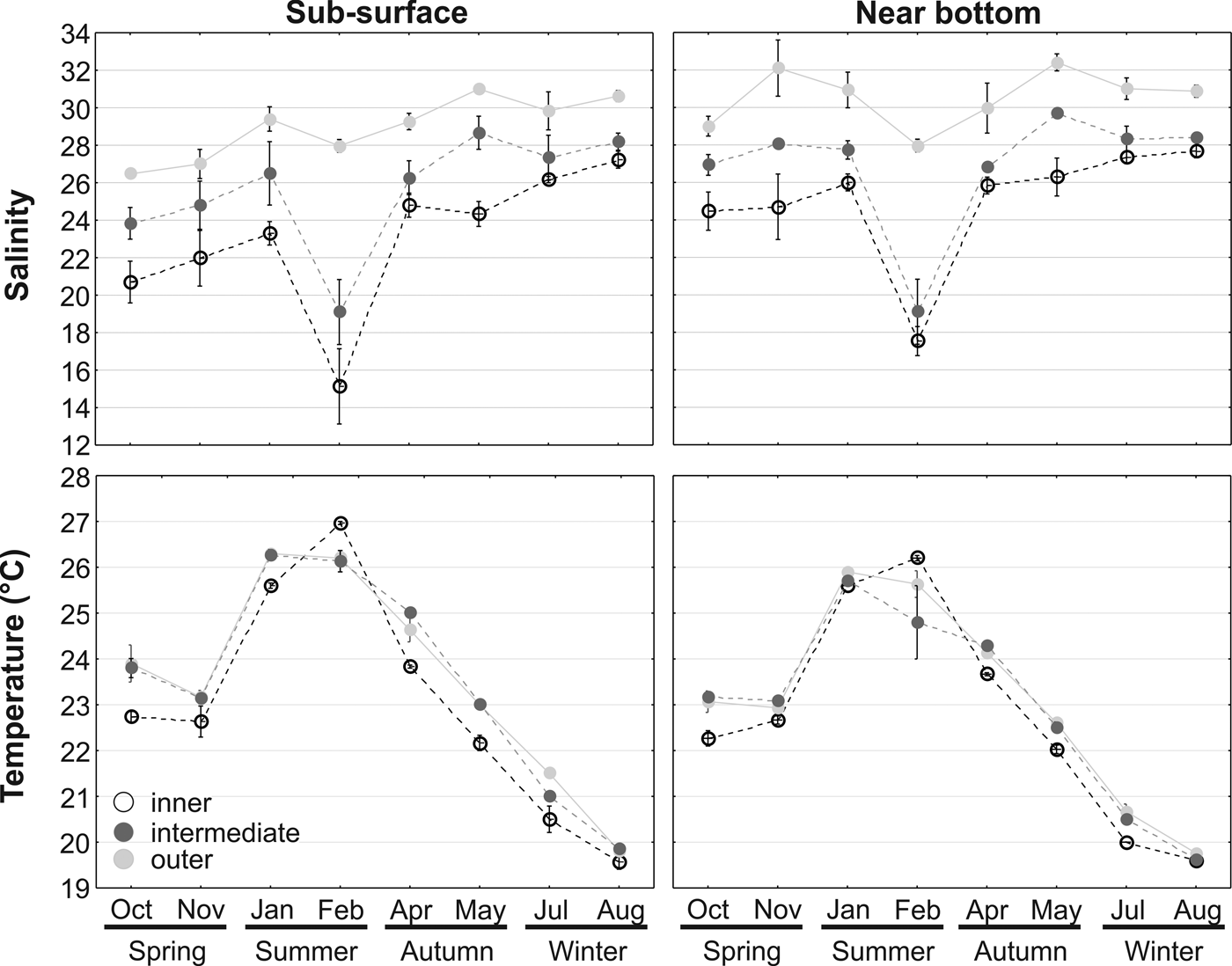
Fig. 2. Abiotic environment. Seasonal variation of sub-surface and near bottom salinity and temperature in the three sectors of Babitonga Bay, South Brazil, between October 2007 and August 2008. Symbols represent the means and vertical bars the standard deviation.
Biotic environment
Two seasonal peaks of chlorophyll-a concentration were observed during summer and early winter, tending to be lower in the outer sector particularly during summer (Figure 3A). Zooplankton biomass increased through spring, peaking in January and later decreasing, with a second smaller peak in August in the inner sector (Figure 3C). Fish larvae peaked in November in the outer sector and in January in the intermediate sector. In the inner sector, a peak of fish larvae also occurred in November–January, however, abundances were considerably lower (Figure 3C).
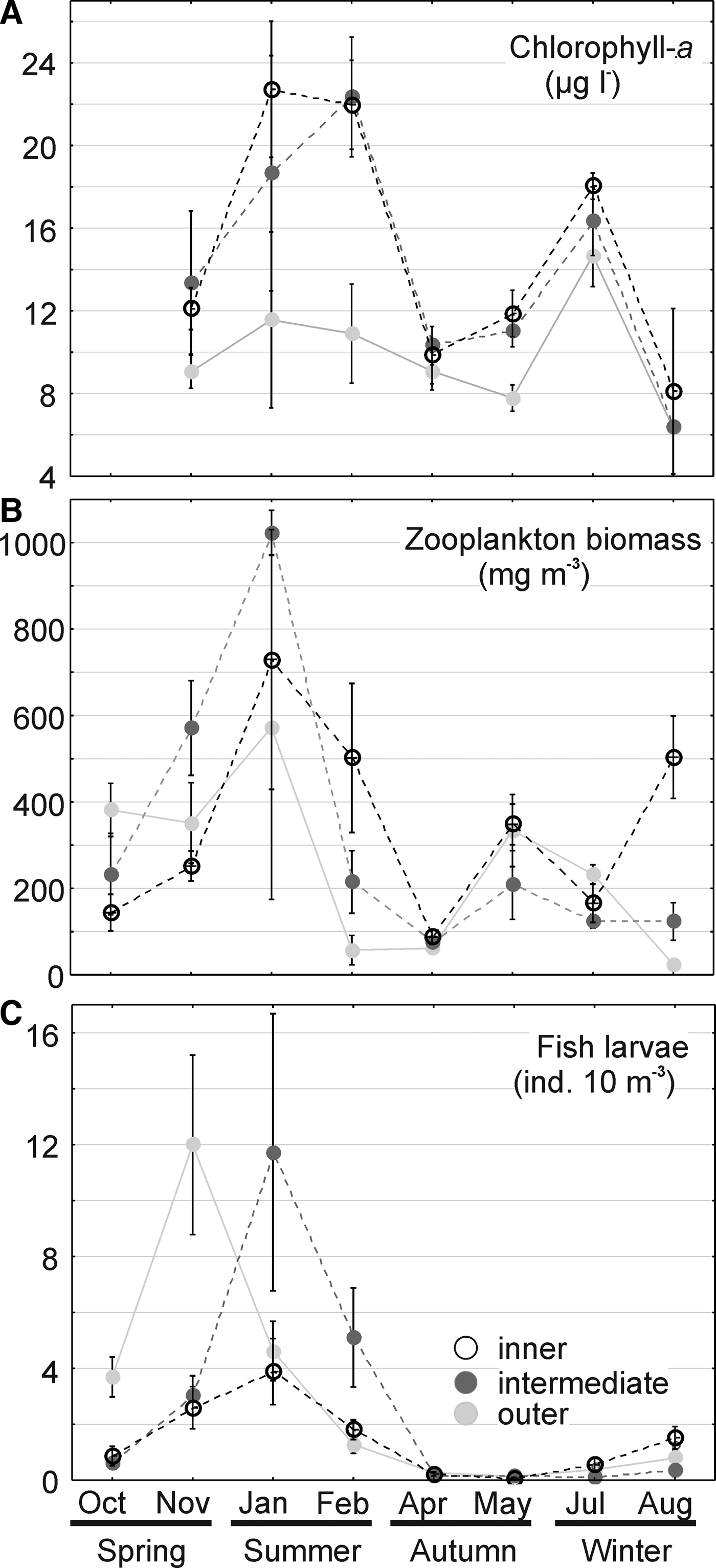
Fig. 3. Biotic environment. Seasonal variations of chlorophyll-a (a), zooplankton biomass (b) and fish larvae abundance (c) in the three sectors of Babitonga Bay estuary, South Brazil, between October 2007 and August 2008. Symbols represent the means and vertical bars the standard deviation.
Chaetognaths
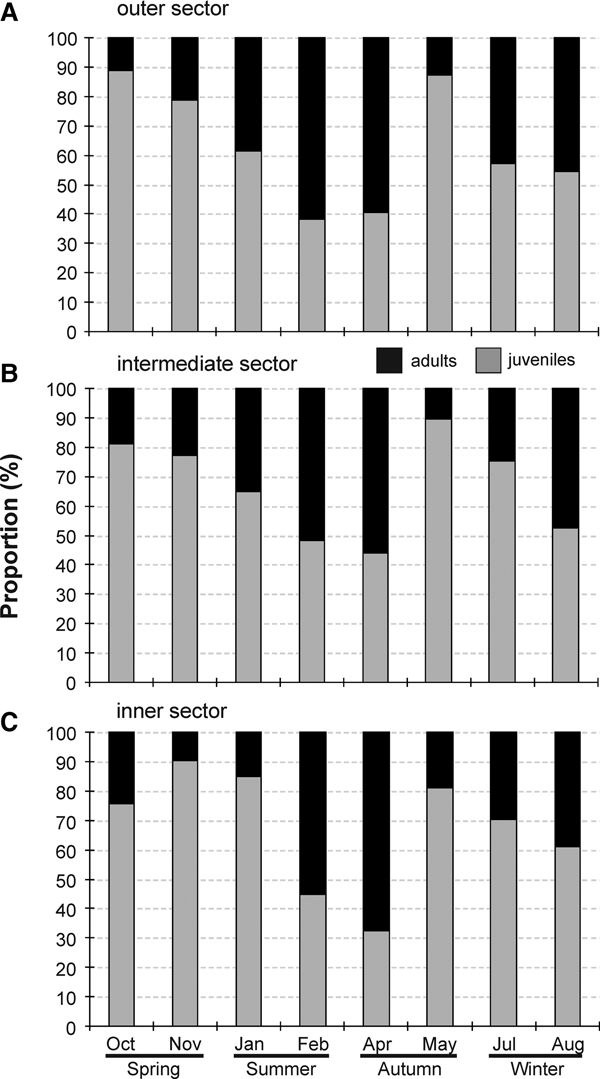
Parasagitta friderici, originally described from Cape Verde (Ritter-Záhony, Reference Ritter-Záhony1911), was always the dominant species in the present study, representing from 93–100% of the chaetognaths from all the samples analysed. Other species found included Parasagitta tenuis (Conant, 1896) and Flaccisagitta enflata (Grassi, 1881), always in low, intermittent abundances, and exclusively in the outer sector in the case of the latter. Parasagitta friderici occurred in all 72 zooplankton samples taken and its abundance ranged from <1 to 250 ind. m−3, averaging (±SD) 36 ± 52 ind. m−3. Juveniles dominated the population throughout most of the year (Figure 4) and represented ~80% of the total annual population, reaching densities of up to 233 ind. m−3. Adults (up to 24 ind. m−3) exceeded juveniles in February and April in all three sectors, representing between 55 and 68% of total population. In August, adult contribution also was relatively high, particularly in the outer sector, where they represented 49% of the population (Figure 4).

Fig. 4. Seasonal changes in the proportion (% of ind. m−3) of juveniles (grey bars) and adults (black bars) in the three sectors of Babitonga Bay estuary between October 2007 and August 2008.
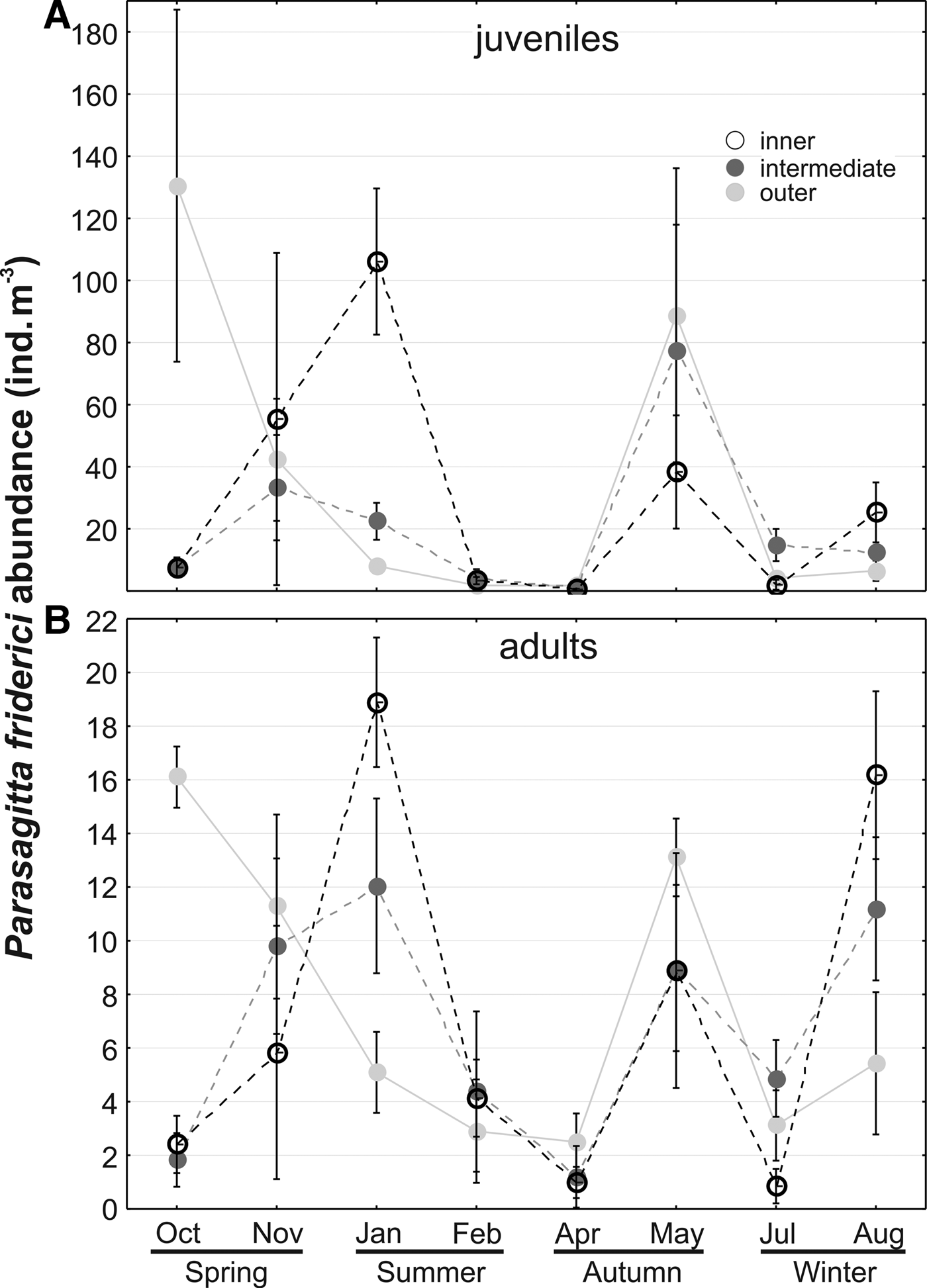
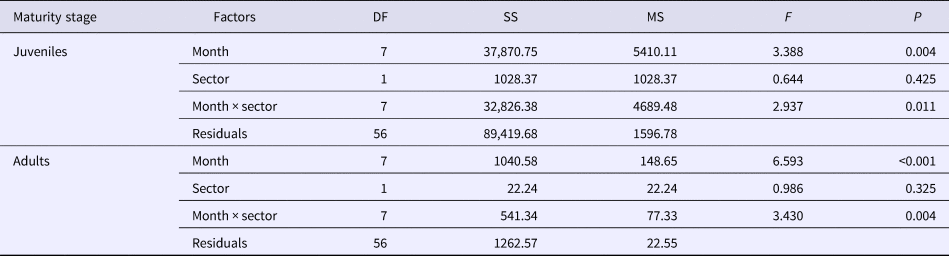
Abundance of both juveniles and adults did not differ between different sectors but differed significantly (P < 0.05) through time, considering months, and the interaction between months and sectors (Table 1). This indicates that the spatial distribution was not constant throughout the year. For instance, in October both juveniles and adults were more abundant in the outer sector, while in January and August both tended to be less abundant in this sector (Figure 5A, B). In the inner sector, abundance of juveniles increased through the spring and peaked in early summer (January), when they averaged ~105 ind. m−3. Lower abundances of both juveniles and adults occurred between February and April in all sectors (Figure 5). A second peak of juveniles was observed in May, mostly in the intermediate and outer sectors. In contrast, adult abundances also peaked in August particularly in the inner sector (Figure 5).
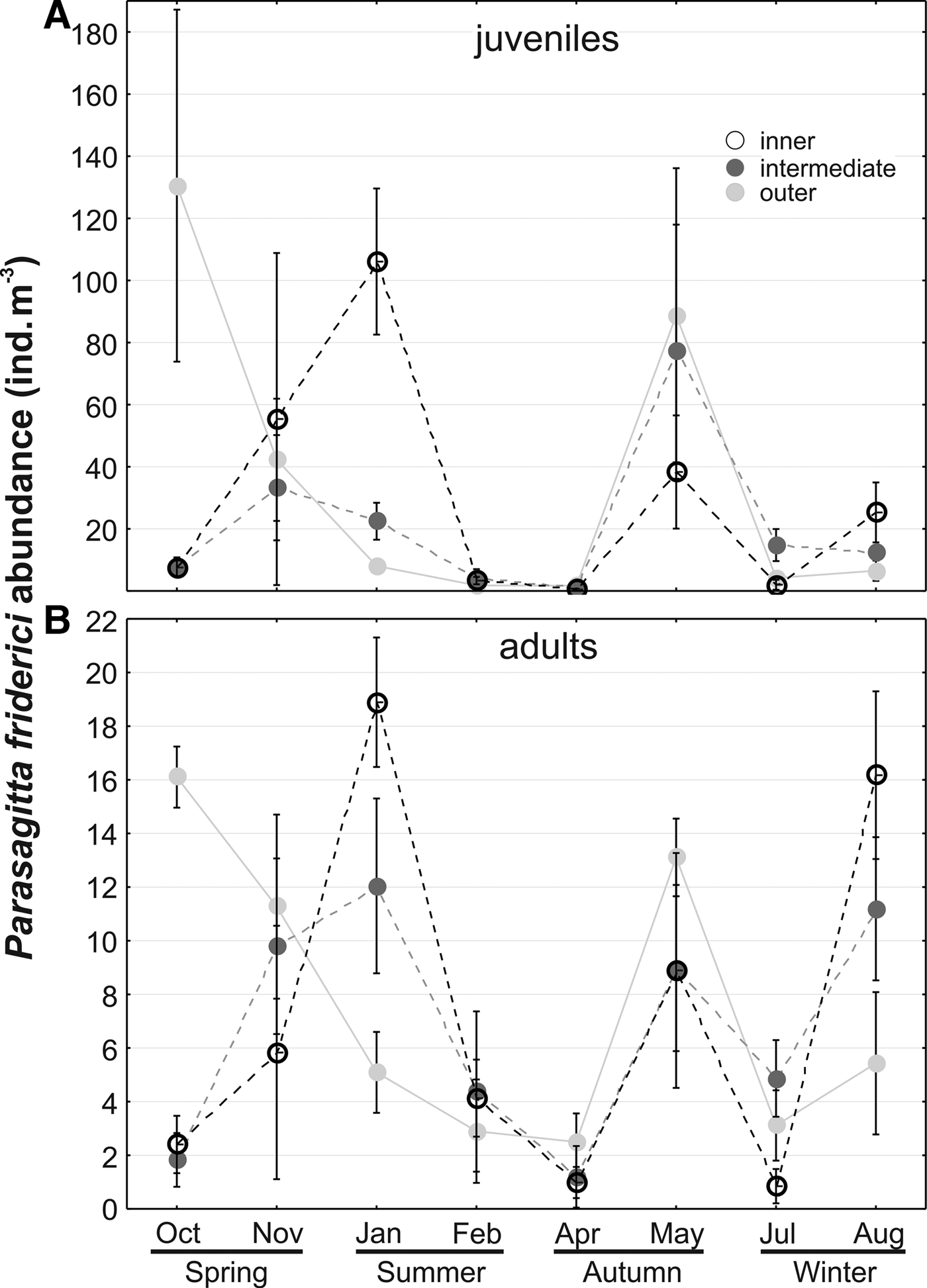
Fig. 5. Seasonal variation of Parasagitta friderici juvenile (a) and adult (b) abundance in the three sectors of Babitonga Bay estuary, South Brazil, between October 2007 and August 2008. Symbols represent the means and vertical bars the standard deviation. Notice different scales.
Table 1. Summary of the ANOVA testing for differences in abundance of Parasagitta friderici juveniles and adults considering months and sectors as factors and their interaction
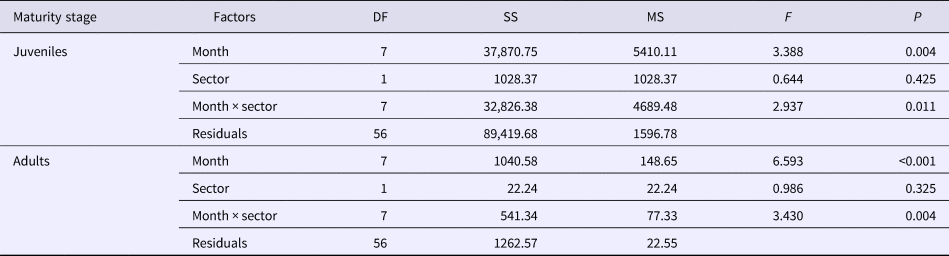
DF, degree of freedom; SS, sum of squares; MS, mean squares; F, parameter of the ANOVA; P, probability associated to the test.
Differences are considered significant if P < 0.05.
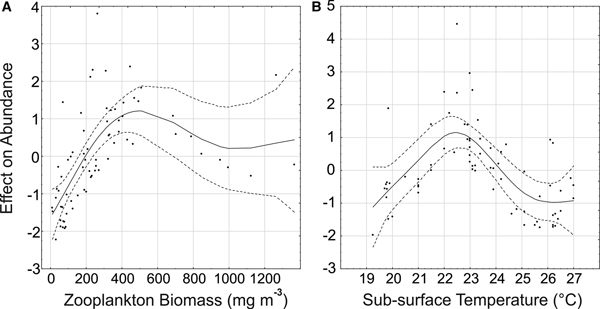
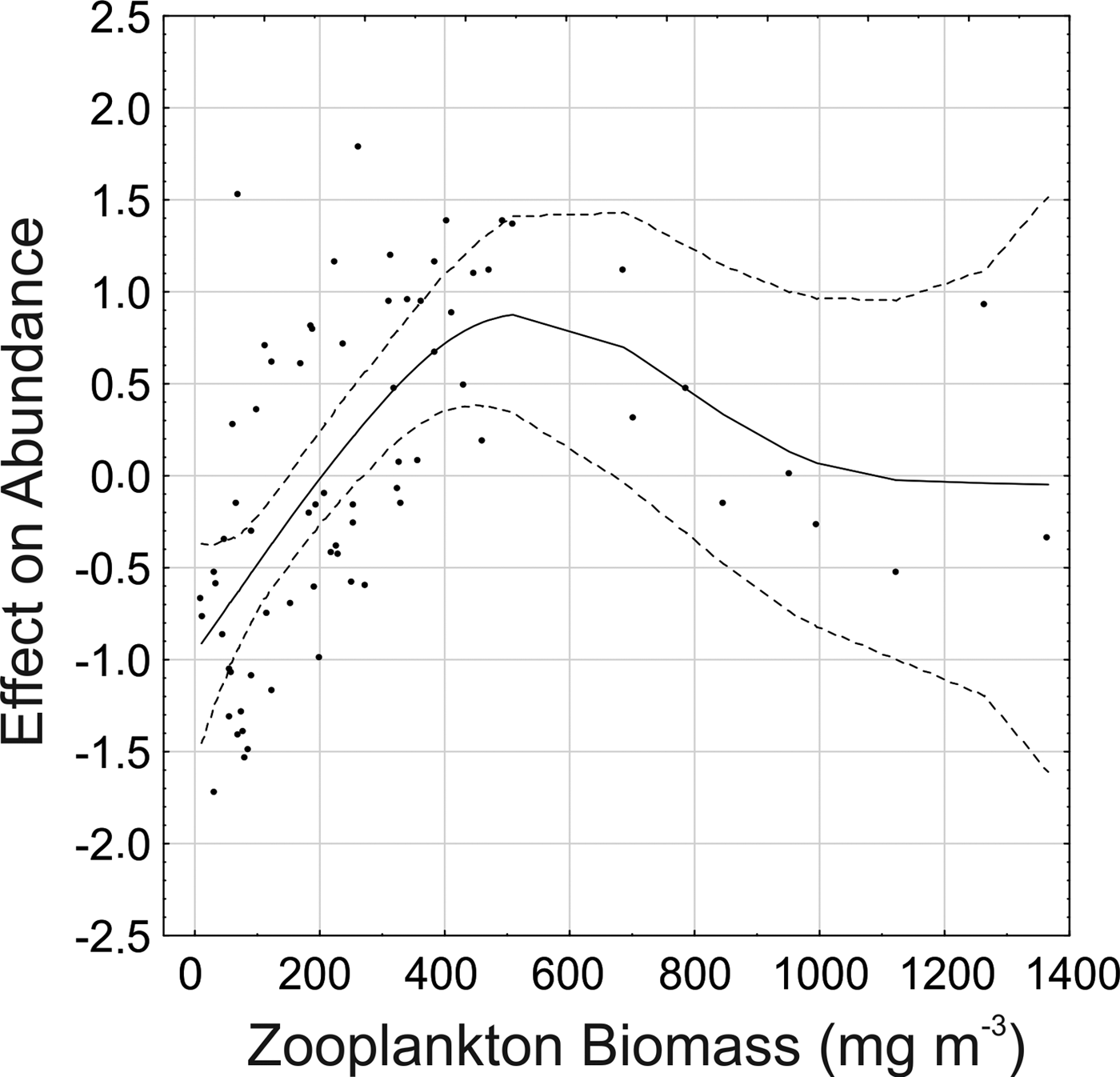
The Generalized Additive Model (GAM) explained 69.2% of the deviance in P. friderici juvenile abundance. From the six variables considered (temperature, salinity, water transparency, chlorophyll-a, zooplankton biomass and fish larvae), the model indicated significant (P < 0.001) relationships with zooplankton biomass and sub-surface temperature. For adults, the model explained a lower proportion (45.3%), and suggested a significant (P < 0.001) relationship only with zooplankton biomass.
There is a clear tendency of increasing abundance of both juvenile (Figure 6A) and adults (Figure 7) with increasing zooplankton biomass up to around 450 mg m−3. Biomass values above 800 mg m−3 were associated mainly with mid-low adult and juvenile densities (Figures 6A & 7) at the inner sector in January (Figure 5A, B). Abundance of juveniles also tended to increase with water temperature, up to 22–23°C, where most of the high abundances were found (Figure 6B). In warmer waters juvenile abundance tended to decrease, but on a few occasions in January, high juvenile density also occurred with temperatures above 26°C.
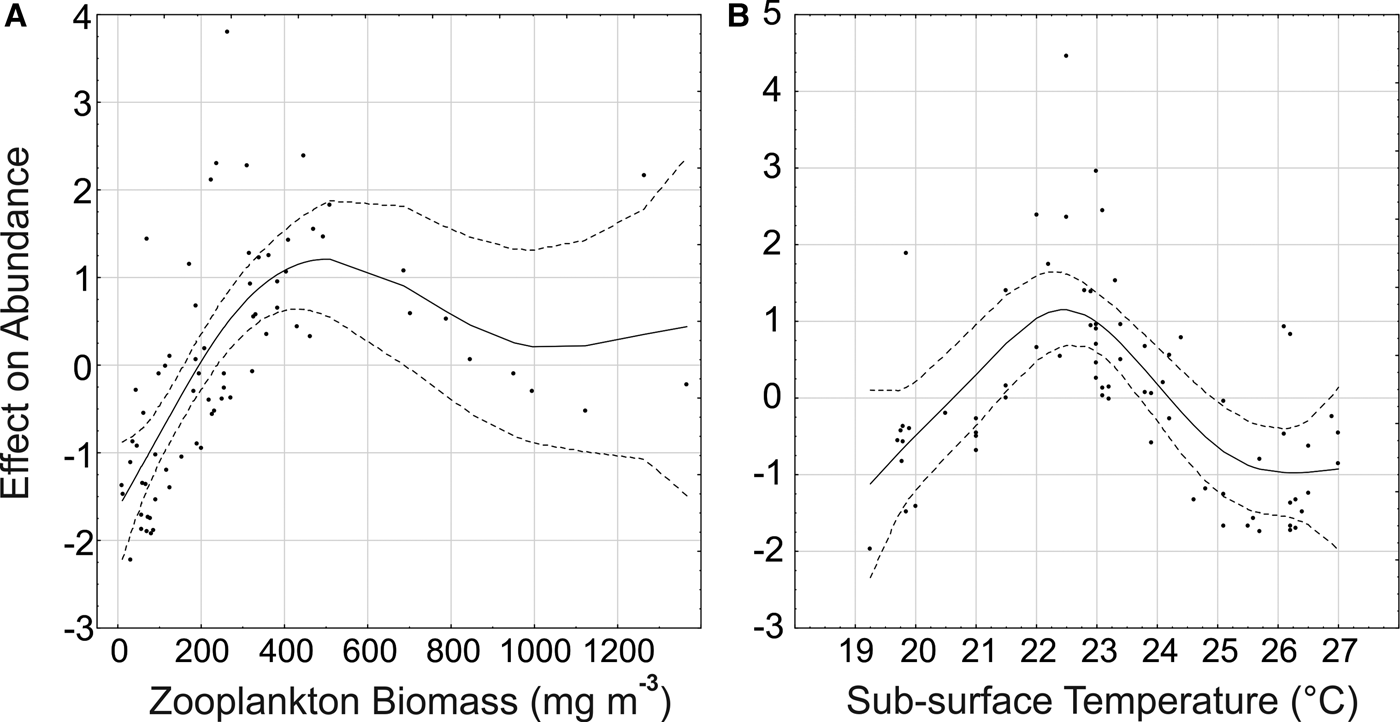
Fig. 6. Results of the Generalized Additive Model for Parasagitta friderici juveniles showing the abundance trends and the effect modelled of zooplankton biomass (a) and sub-surface temperature (b). Solid lines show the smoothing function and dotted lines the 95% confidence interval.
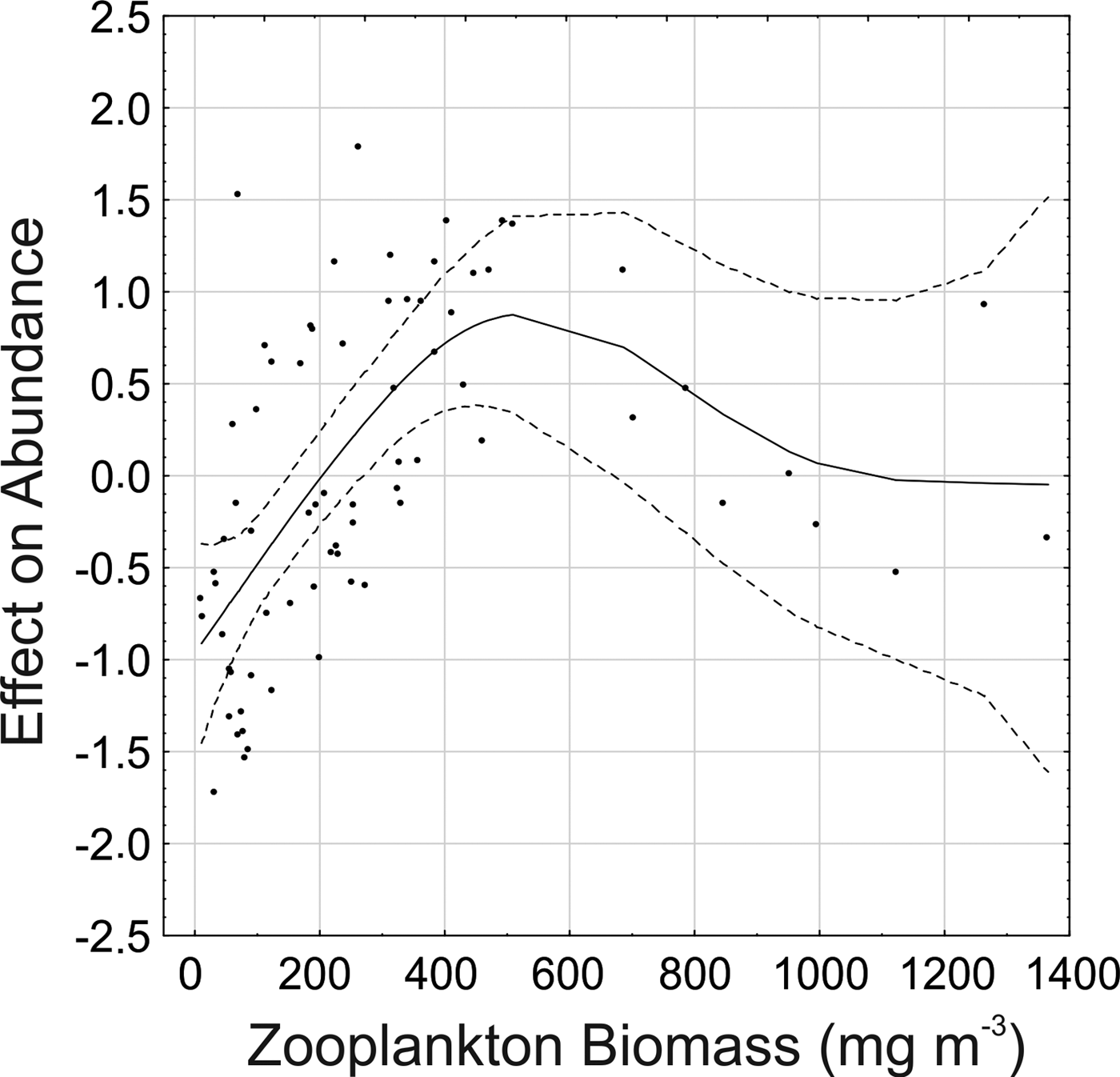
Fig. 7. Result of the Generalized Additive Model for Parasagitta friderici adults showing the abundance trends and the effect modelled of zooplankton biomass. Solid lines show the smoothing function and dotted lines the 95% confidence interval.
Discussion
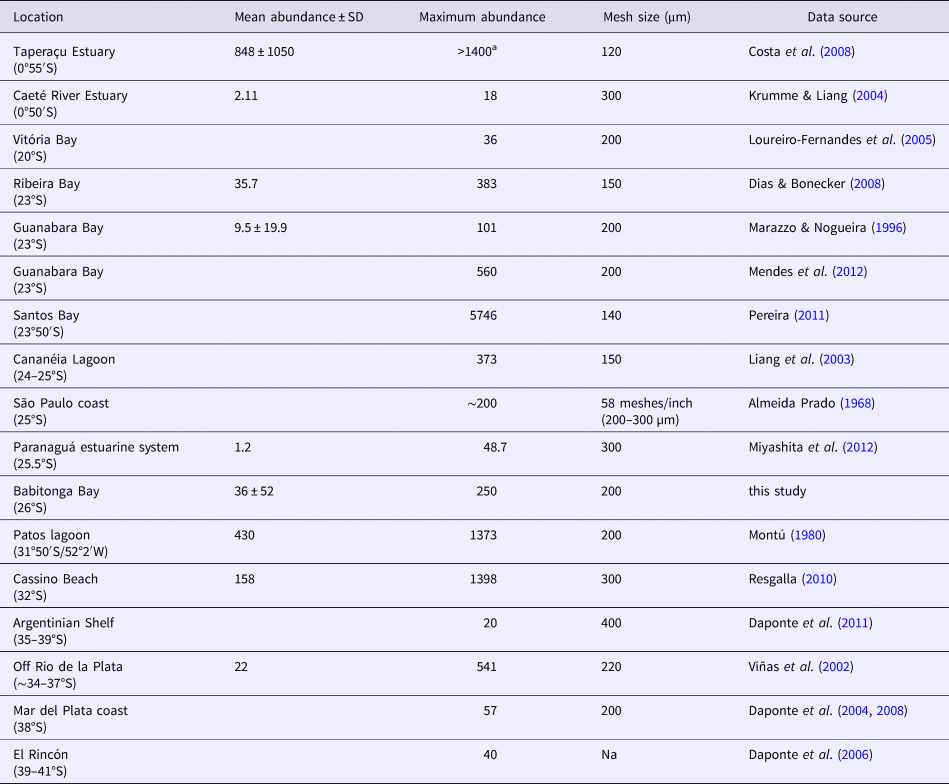
The high dominance of P. friderici observed here is common in South-western Atlantic coastal and estuarine ecosystems, including tropical (0–2°S; Krumme & Liang, Reference Krumme and Liang2004; Costa et al., Reference Costa, Pereira and Costa2008) and subtropical to temperate latitudes (~20–40°S; Boltovskoy, Reference Boltovskoy1975; Loureiro Fernandes et al., Reference Loureiro Fernandes, Sterza and Neves2005; see also references from Table 2). Indeed, this species is amongst the few chaetognaths abundant in and restricted to coastal and neritic waters (Pierrot-Bults & Nair, Reference Pierrot-Bults, Nair, Bone, Kapp and Pierrot-Bults1991). It is the dominant species in many coastal and estuarine ecosystems throughout its distribution, such as both sides of the North (Furnestin, Reference Furnestin1957; Fraser, Reference Fraser1960; McLelland, Reference McLelland1980; Blanco-Bercial et al., Reference Blanco-Bercial, Alvarez-Marqués and Cabal2006; Champalbert et al., Reference Champalbert, Pagano, Sene and Corbin2007; Marques et al., Reference Marques, Azeiteiro, Martinho and Viegas2009) and South (Stuart & Verheye, Reference Stuart and Verheye1991; Gibbons & Stuart, Reference Gibbons and Stuart1994) Atlantic up to latitude of ~40°, in the eastern Pacific (Hossfeld, Reference Hossfeld1996) and the south Mediterranean (Aziz, Reference Aziz2005; Zakaria, Reference Zakaria2006).
Table 2. Comparison of mean and maximum abundance (ind. m−3) of Parasagitta friderici from several South-western Atlantic estuarine and open shallow shelf waters
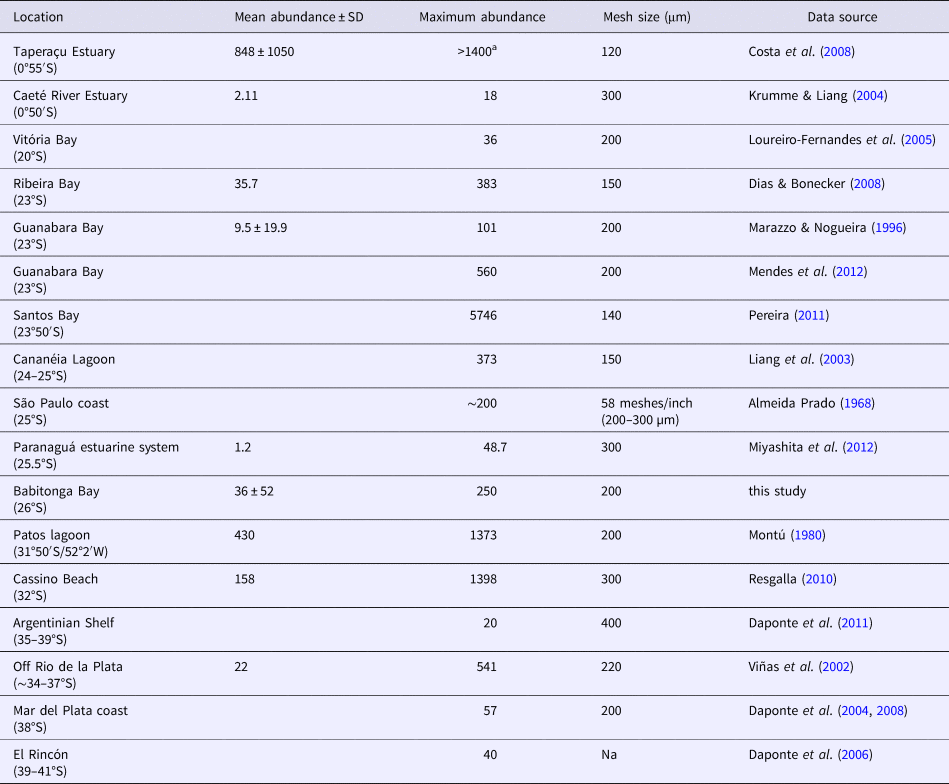
SD, standard deviation.
a The authors only mention mean abundance and thus we provided a conservative estimation of maximum abundances of >1400 ind. m−3.
Densities of P. friderici populations found in the South-western Atlantic vary a lot, from <20 ind. m−3 in the tropical Caeté River estuary and on the temperate Argentinian Shelf to up to 5746 ind. m−3 in the subtropical Santos Bay (Table 2). There is no latitudinal trend in P. friderici abundance throughout the South-western Atlantic, with very high densities both in tropical (0.5°S), subtropical (23°50′S) and nearly temperate (31–32°S) latitudes (Table 2). However, near its southernmost limit (>35°S) P. friderici abundances are typically low, never exceeding 60 ind. m−3 (Table 2), perhaps due to temperature constraints of this warm water species in higher latitudes. The values found here, up to 250 ind. m−3 (annual mean of total population = 36 ± 52 ind. m−3) are within this range and, in comparison to other known South-western Atlantic populations (Table 2), cannot be considered either particularly abundant or scarce. Populations of this species elsewhere throughout its distribution also are within this abundance range (Stuart & Verheye, Reference Stuart and Verheye1991; Gibbons & Stuart, Reference Gibbons and Stuart1994; Hossfeld, Reference Hossfeld1996; Champalbert et al., Reference Champalbert, Pagano, Sene and Corbin2007; Marques et al., Reference Marques, Azeiteiro, Martinho and Viegas2009), as are other estuarine and coastal chaetognaths worldwide (Reeve, Reference Reeve1964; Srinivasan, Reference Srinivasan1971; Nair, Reference Nair1974; Grant, Reference Grant1977; Nair et al., Reference Nair, Gajnhiye and Desai1981; Besiktepe & Unsal, Reference Besiktepe and Unsal2000; Hernández et al., Reference Hernández, Suárez-Morales and Gasca2005; Modéran et al., Reference Modéran, Bouvais, David, Noc, Simon-Bouhet, Niquil, Miramand and Fichet2010; Noblezada & Campos, Reference Noblezada and Campos2012; Wu et al., Reference Wu, Li, Huanh, Yin and Tan2014).
The constant and abundant presence of juveniles throughout the year indicates continuous reproduction and recruitment. Although we did not evaluate in detail reproduction in the present study, and not necessarily all adult individuals are reproducing continuously, the year-round occurrence of many juveniles clearly suggests the population is reproducing throughout the year, which is to be expected for tropical and subtropical chaetognaths in general (Ghirardelli, Reference Ghirardelli1968; Ramírez & Viñas, Reference Ramírez and Viñas1982). Even though continuous, reproduction of P. friderici was not uniform. Reproductive peaks, as suggested by juvenile peaks, occurred between October and January and in May, as indicated by higher abundances and proportion of juveniles (Figures 4 & 5). In addition, between February and April, and in August, densities were considerably lower and adults represented a larger proportion of the population, suggestive of less intense recruitment in these periods. Although existing data on P. friderici are scarce and scattered, they suggest a general trend of continuous reproduction and recruitment with sporadic peaks commonly during spring, as well as higher proportion of adults in late summer and/or early autumn which is probably food-related according to the present data. This pattern includes populations from tropical (Mendes et al., Reference Mendes, Figueiredo and Valentin2012), subtropical (Furnestin, Reference Furnestin1957; Liang et al., Reference Liang, Ara, Miranda, Bérgamo and Bernardes2003; this study) and temperate (~38°S; Ramírez & Viñas, Reference Ramírez and Viñas1982; Daponte et al., Reference Daponte, Capitanio, Nahabedian, Vinãs and Negri2004) latitudes.
The few other studied estuarine chaetognaths worldwide up to ~40° of latitude also have similar patterns of high juvenile dominance suggesting year-round reproduction with intermittent peaks, as is the case for tropical populations of Zonosagitta bedoti (Béraneck, 1895), F. enflata and P. tenuis (Srinivasan, Reference Srinivasan1980; Nair & Sankarankutty, Reference Nair and Sankarankutty1988; Ramaiah & Nair, Reference Ramaiah and Nair1993), subtropical Ferosagitta hispida (Conant, 1895) (Reeve, Reference Reeve1964) and temperate Aidanosagitta regularis (Aida, 1897) (36°S; Webb & Sewell, Reference Webb and Sewell2015). Populations from colder regions such as Parasagitta setosa (J. Müller, 1847) from Black Sea (Besiktepe & Unsal, Reference Besiktepe and Unsal2000), Parasagitta elegans (Verril, 1873) from Charente estuary, France (Modéran et al., Reference Modéran, Bouvais, David, Noc, Simon-Bouhet, Niquil, Miramand and Fichet2010) and P. tenuis and F. hispida from Chesapeake Bay, USA (Grant, Reference Grant1977) tend to have marked seasonal cycles typically with abundance peaks and high dominance of juveniles mostly during summer, or occasionally winter such as P. elegans from Chesapeake Bay (Grant, Reference Grant1977).
The presence of P. friderici throughout the salinity range sampled in the present study, from 10.1 to 33.6, is not surprising considering that this chaetognath is known to commonly dwell in both lower and higher salinities (Furnestin, Reference Furnestin1957; Liang et al., Reference Liang, Ara, Miranda, Bérgamo and Bernardes2003; Blanco-Bercial et al., Reference Blanco-Bercial, Alvarez-Marqués and Cabal2006; Cervetto et al., Reference Cervetto, Calliari, Rodriguez-Graña, Lacerot, Castiglioni, Menafra, Rodríguez-Gallego, Scarabino and Conde2006). Consequently, salinity did not significantly explain part of either adult or juvenile abundance variations in the present study according to the GAM, supporting the hypothesis that salinity variations are not of great importance for this species (Fraser, Reference Fraser1960). This high tolerance of P. friderici to salinity variations is an important adaptation to thrive within estuarine systems and is different to most other chaetognath species worldwide which are commonly highly influenced by this factor (e.g. Srinivasan, Reference Srinivasan1971; Nair, Reference Nair1974; Grant, Reference Grant1977; Blanco-Bercial et al., Reference Blanco-Bercial, Alvarez-Marqués and Cabal2006). Moreover, since salinity commonly is the main factor affecting spatial structure of estuarine zooplankton in general (e.g. Xu et al., Reference Xu, Liu, Xu, Sun and Chen2014; Miyashita & Calliari, Reference Miyashita and Calliari2016) and of chaetognaths in particular (Srinivasan, Reference Srinivasan1971, Reference Srinivasan1980; Nair, Reference Nair1974; Loureiro Fernandes et al., Reference Loureiro Fernandes, Sterza and Neves2005), this high tolerance of P. friderici probably accounts for the similar densities of both juvenile and adults in all three sectors sampled. Hitherto, laboratory survival experiments have not been performed and are necessary to determine the extent of P. friderici resistance and the effects of reduced salinity on its biological parameters such as feeding, growth and reproductive output.
Most environmental variables tested in the present study were not significantly related to either juvenile or adult abundance according to the GAM analyses. Zooplankton biomass was the best predictor of both adults and juveniles, along with temperature for the latter category. Accordingly, P. friderici is known to occur in a wide range of hydrographic conditions, wider than the ones found here for both salinity (see above) and temperature. This species is known to grow and reproduce in 10°C waters (Daponte et al., Reference Daponte, Capitanio, Nahabedian, Vinãs and Negri2004), considerably colder than the minimum recorded here (19.2°C). Thus, these hydrographic parameters probably are not major limiting factors for P. friderici populations in the subtropical Babitonga estuary. The relationship of juveniles with temperature probably is due to the thermal influence on reproduction, development and growth rates of chaetognaths in general (Russell, Reference Russell1932; Ghirardelli, Reference Ghirardelli1968; Terazaki, Reference Terazaki2004) and P. friderici in particular (Ramírez & Viñas, Reference Ramírez and Viñas1982; Resgalla, Reference Resgalla2010). According to our results, the optimal temperature range for juveniles is 22–23°C (Figure 6B). This agrees with the negative relationship between P. friderici and temperature in a tropical estuary with annual temperature range between 22 and 29°C (Loureiro Fernandes et al., Reference Loureiro Fernandes, Sterza and Neves2005), and with the positive relationship with temperature in temperate areas (Blanco-Bercial et al., Reference Blanco-Bercial, Alvarez-Marqués and Cabal2006). The difference in environmental preferences between juveniles and adults is similar to the related species Parasagitta setosa from the Black Sea where adults tolerate a wider temperature range than juveniles (Besiktepe and Unsal, Reference Besiktepe and Unsal2000), resulting in the higher influence of temperature on juveniles as observed here.
The observed relationship of both juveniles and adults with zooplankton biomass is probably trophic-related. All gelatinous and semi-gelatinous organisms were removed from the samples, prior to weighing, and so the bulk of the biomass estimated here was composed of crustaceans, particularly the copepods Acartia tonsa Dana, 1849, Acartia lilljeborgi Giesbrecht, 1889, Temora turbinata (Dana, 1849), Oithona hebes Giesbrecht, 1891 and Pseudodiaptomus acutus (Dahl F., 1894) (Souza, Reference Souza2013). These copepod taxa are amongst the main prey items of P. friderici, although other zooplankton such as appendicularians, molluscs, cladocerans and chaetognaths may also be eaten (Stuart & Verheye, Reference Stuart and Verheye1991; Vega-Pérez & Liang, Reference Vega-Pérez and Liang1992; Liang & Vega-Pérez, Reference Liang and Vega-Pérez1995; Liang et al., Reference Liang, Ara, Miranda, Bérgamo and Bernardes2003; Sato et al., Reference Sato, Hernández and Viñas2011). Chaetognaths may prey on a variety of planktonic organisms but their diet is typically composed mainly of copepods, reflecting the general composition of the zooplanktonic community (Feigenbaum, Reference Feigenbaum, Bone, Kapp and Pierrot-Bults1991; Casanova, Reference Casanova and Boltovskoy1999). Although chaetognaths may impact fish larvae abundance (Casanova, Reference Casanova and Boltovskoy1999), predation on ichthyoplankton is usually smaller (Feigenbaum, Reference Feigenbaum, Bone, Kapp and Pierrot-Bults1991). Accordingly, P. friderici distribution was not explained by fish larvae abundance in the present study. Food availability has commonly been considered an important factor controlling spatial and seasonal patterns of chaetognath abundance and reproduction both on estuarine and open shelf water ecosystems (Bone et al., Reference Bone, Kapp and Pierrot-Bults1991; Gibbons & Stuart, Reference Gibbons and Stuart1994; Liang, Reference Liang2002; Terazaki, Reference Terazaki2004; Loureiro Fernandes et al., Reference Loureiro Fernandes, Sterza and Neves2005; Noblezada & Campos, Reference Noblezada and Campos2012). This may be particularly true for P. friderici (e.g. Liang et al., Reference Liang, Ara, Miranda, Bérgamo and Bernardes2003) which has a wide tolerance for hydrographic conditions (see above) and thus food availability and quality appears as the main driver of its spatial and temporal dynamics as suggested here.
Acknowledgements
Dr José Maria Souza-Conceição is greatly acknowledged for inviting MNJ to join the sampling campaigns and for accessibility to the plankton samples. Dr Henry L. Spach (CEM-UFPR) helped with logistic support. Drs Ciro C. Vilar, José M. Souza-Conceição and Lilyane de O. Santos helped in the field trips. Dra. Maria A. Haddad (Zoologia-UFPR) allowed the use of her laboratory facilities to analyse part of the samples. Thanks also to the Centro de Estudos do Mar (CEM-UFPR, MPL) where this study was developed between 2007 and 2010.
Financial support
MNJ and EGT were supported by Conselho Nacional do Desenvolvimento Científico e Tecnológico (CNPq, grant no. 140945/2007-5 and 140897/2017-8 respectively) and MNJ by the ‘Fundação de Amparo à Pesquisa do Estado de São Paulo’ (FAPESP, grant no. 2011/09880-8; Project no 11/21290-1).