INTRODUCTION
Studies on gelatinous zooplankton, particularly cnidarian siphonophores, have increased in recent years. However, our knowledge of the biology, ecology and systematics of these animals, particularly in the Antarctic region, is still poor. Traditional net studies have often ignored Siphonophora in favour of more robust invertebrates, such as crustaceans. Siphonophores can be abundant and ecologically important oceanic hydrozoans (Totton & Bargmann, Reference Totton and Bargmann1965; Kirkpatrick & Pugh, Reference Kirkpatrick and Pugh1984; Mills, Reference Mills2001; Boero et al., Reference Boero, Bouillon, Gravili, Miglietta, Parsons and Piraino2008). There are approximately 180 described species; the group has the highest division of labour between zooids and the most precise organization of all colonial animals (Mapstone, Reference Mapstone2014). Siphonophores are among the most abundant carnivores in the oceanic macroplankton (Pugh, Reference Pugh1984), and include the longest animals in the world, with colonies of some species exceeding 40 m in length (Dunn, Reference Dunn2009).
The identification of all parts of the fragile colonies which are usually separated during net sampling is the greatest challenge in the study of Siphonophora. Past descriptions of many species were based solely on damaged and often incomplete colonies. Species identity within the group is typically based on the morphology of at least one swimming bell. For other zooids of the colony, identification can be more problematic, however the sexual eudoxid stage is known in a number of calycophorans (Pugh, Reference Pugh and Boltovskoy1999b). But the evidence of a link between a eudoxid and an adult colony formerly treated as separate taxa using molecular markers has so far been shown in only one case. Using DNA barcoding techniques, Grossmann et al. (Reference Grossmann, Lindsay and Collins2013a) showed that Eudoxia macra (Totton, Reference Totton1954), is the sexual stage of the small diphyomorph calycophoran Lensia cossack (Totton, Reference Totton1941).
Pyrostephos vanhoeffeni Moser (Reference Moser1925) was first identified by Moser (Reference Moser1925) as a large colourful species with unusually modified palpons on the siphosome, later termed oleocysts (Totton & Bargmann, Reference Totton and Bargmann1965). Totton provided the first accurate description and figures of the nectophores and tentilla of this species, fragments of which were first taken by the German Southpolar Expedition in 1902, just off the Antarctic Continent (in the Indian sector of the Southern Ocean) (Totton & Bargmann, Reference Totton and Bargmann1965). Pyrostephos vanhoeffeni, although not abundant, is widely distributed throughout the Southern Ocean, as well as in sub-Antarctic waters and also further north (but only as far as 33°S to 40°S in the Pacific and Atlantic Oceans respectively) (Palma, Reference Palma1986, Reference Palma, Silva and Palma2006; Pagès & Kurbjeweit, Reference Pagès and Kurbjeweit1994; Pagès et al., Reference Pagès, Pugh and Gili1994; Pagès & Schnack-Schiel, Reference Pagès and Schnack-Schiel1996; Panasiuk-Chodnicka & Żmijewska, Reference Panasiuk-Chodnicka and Żmijewska2010; Guerrero et al., Reference Guerrero, Gili, Rodriguez, Araujo, Canepa, Calbet, Genzano, Mianzan and Gonzalez2013; Lindsay et al., Reference Lindsay, Guerrero, Grossmann, Fuentes, De Broyer, Koubi, Griffiths, Raymond, d'Udekem d'Acoz, Van de Putte, Danis, David, Grant, Gutt, Held, Hosie, Huettmann, Post and Ropert-Coudert2014; Panasiuk-Chodnicka et al., Reference Panasiuk-Chodnicka, Żmijewska and Mańko2014; Palma et al., Reference Palma, Retamal, Silva and Canepa2016). It should be emphasized that P. vanhoeffeni occurs exclusively in the southern hemisphere, in contrast to all four Bargmannia species whose records come mostly from the North Atlantic (Pugh, Reference Pugh1999a).
Small colonies comprising a single nectophore (2 mm in length) and stem were collected by Margulis (Reference Margulis1982) from Antarctic waters and introduced as Mica micula. Further specimens were described later by Pagès & Gili (Reference Pagès and Gili1989). Mica micula colonies showed some characteristics associated with the family Pyrostephidae, such as the presence of stenoteles and a smaller spherical kind of nematocyst on the tentilla of the tentacles (Pugh, Reference Pugh1999a; Mapstone, Reference Mapstone2009), but stenoteles also occur in a range of other siphonophore tentilla (Mapstone, Reference Mapstone2014). Other morphological characters were imprecise. The colonies collected so far indicate that this species is limited to Antarctic waters. The ill-defined nature of the pneumatophore (suggesting it is still developing), the simple structure and singularity of the nectophore and the presence of a single gastrozooid without any other distinguishable siphosomal structures suggest that this taxon is the post-larval or siphonula stage of a physonect (Pagès & Gili, Reference Pagès and Gili1989). These authors suggested that M. micula might be a post-larvae of Bargmannia elongata, another representative of Pyrostephidae in Antarctic waters, although recent studies undermined this hypothesis (Grossmann et al., Reference Grossmann, Lindsay and Fuentes2013b).
Recently, Grossman et al. (Reference Grossmann, Lindsay and Fuentes2013b) published a redescription of Mica micula with notes on its distribution and identity. These samples were obtained during the 2008 Collaborative East-Antarctic MARine Census (CEAMARC), and all 18 specimens were collected in the area of Mertz Glacier, within the limits of the Antarctic Convergence. However, no Bargmannia nectophores or bracts were found amongst these samples, thus it was concluded that it is much more likely to be the post-larval form of the physonect Pyrostephos vanhoeffeni, which is very common in both Antarctic and sub-Antarctic waters.
In recent years the importance of molecular studies application to resolve taxonomical challenges has grown significantly. The idea of DNA barcoding, first proposed by Hebert et al. (Reference Hebert, Ratsingham and de Waard2003), is now widely used across many animal phyla (e.g. Heimeier et al., Reference Heimeier, Lavery and Sewell2010; Jinbo et al., Reference Jinbo, Kato and Ito2011 and references therein). The mitochondrial cytochrome c oxidase subunit 1 (COI), for which several protocols as well as universal and also specific primers already exist, is the most commonly used gene (e.g. Folmer et al., Reference Folmer, Black, Hoen, Lutz and Vrijenhoek1994; Hoareau & Boissau, Reference Hoareau and Boissin2010; Geller et al., Reference Geller, Meyer, Parker and Hawk2013 and references therein). Several investigations of Hydrozoa using the COI gene have aided in species identifications and indicated cryptic diversity in some taxa (e.g. Bucklin et al., Reference Bucklin, Ortman, Jennings, Nigro, Sweetman, Copley, Sutton and Wiebe2010; Ortman et al., Reference Ortman, Bucklin, Pagès and Youngbluth2010; Laakmann & Holst, Reference Laakmann and Holst2014). However, other authors suggested that the mutation rate of this gene is too slow for hydrozoans (Shearer et al., Reference Shearer, Van Oppen, Romano and Wörheide2002). Moreover, Lindsay et al. (Reference Lindsay, Grossmann, Nishikawa, Bentlage and Collins2015b) pointed out that two COI GenBank siphonophore sequences published by Ortman et al. (Reference Ortman, Bucklin, Pagès and Youngbluth2010) actually represent ostracod or protist contaminants so they are misleading. As a result, another mitochondrial gene 16S rRNA is more frequently used, works well for most pelagic hydrozoans, and many more sequences are available for this gene for hydrozoans on GenBank (Zheng et al., Reference Zheng, He, Lin, Cao and Zhang2014; Lindsay et al., Reference Lindsay, Grossmann, Nishikawa, Bentlage and Collins2015b). Dunn et al. (Reference Dunn, Pugh and Haddock2005) used the 16S rRNA gene to study phylogenetics within the order Siphonophora, and this mitochondrial gene also allowed for positive identification of Eudoxia macra as the eudoxid stage of Lensia cossack (Grossmann et al., Reference Grossmann, Lindsay and Collins2013a).
Grossmann et al. (Reference Grossmann, Lindsay and Fuentes2013b) studied the morphology of Mica micula colonies and assumed that this siphonophore is most probably the post-larval stage of Pyrostephos vanhoeffeni, in contrast to the suppositions of some other authors (Margulis, Reference Margulis1982; Pugh, Reference Pugh1999a; Mapstone, Reference Mapstone2009). However, Grossmann et al. (Reference Grossmann, Lindsay and Fuentes2013b) also stated that further research applying genetics to the problem is needed and could give the final answer to this question. The aim of the present study therefore is to use the molecular methods to check the genetic affinity of M. micula with P. vanhoeffeni.
MATERIALS AND METHODS
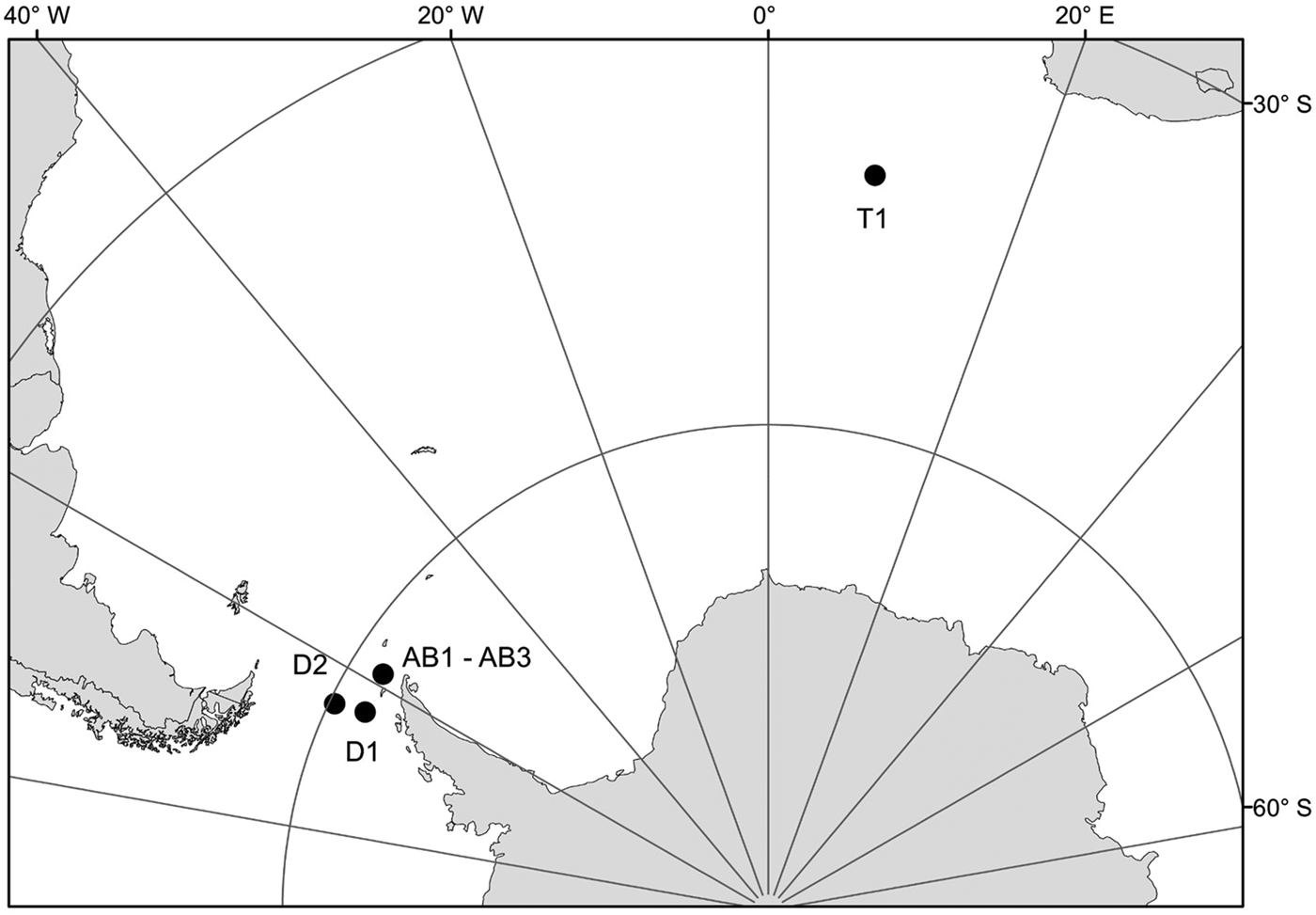
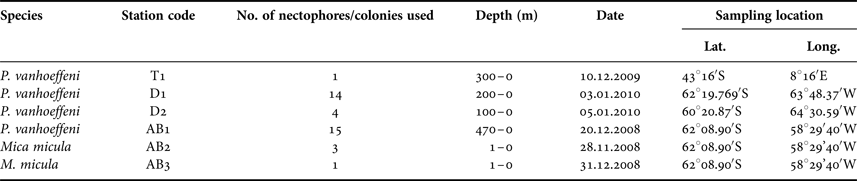
Samples for this study were collected from three areas in the Southern Ocean: on a transect from Cape Town (South Africa) to the Weddell Sea (1 station – T1), on a transect from the Antarctic Peninsula to South America through Drake Passage (2 stations – D1, D2) and in Admiralty Bay, King George Island, South Shetland Islands (3 stations – AB1–AB3) (Table 1, Figure 1). Samples from the transects Cape Town (South Africa) – Weddell Sea and Drake Passage were collected between December 2009 and January 2010, during the cruise on RV ‘Akademik Ioffe’, while those from Admiralty Bay were collected during the 33rd Polish Antarctic Expedition (Austral summer 2008/2009). Sampling was performed with a WP2 plankton net (200 µm mesh size) and a Neuston net (500 µm). Thirty-four nectophores of Pyrostephos vanhoeffeni, and four colonies of Mica micula after identification to species level were preserved in 99.5% ethanol (Table 1, Table S1). DNA extraction from all specimens was performed according to a standard phenol-chloroform method after Hillis et al. (Reference Hillis, Mable, Moritz, Hillis, Moritz and Mable1996). The initial digestion with proteinase K was performed for one hour. Air-dried DNA pellets were eluted in 100 µl of TE buffer, pH 8.00, stored at 4°C until amplification, and subsequently at −20°C for long-term storage. A fragment of 16S ribosomal RNA (16S rRNA; ~580 bp fragment) was amplified using ‘primer 1’ and ‘primer 2’ from Cunningham & Buss (Reference Cunningham and Buss1993) with DreamTaq Green PCR Mastermix (Thermo Scientific). The protocol for the PCR reaction was 94°C for 5 min, 35 cycles (94°C for 60 s, 51°C for 60 s, 72°C for 90 s); finally fragments were elongated at 72°C for 5 min. Sequences were obtained using the BigDye sequencing protocol (Applied Biosystems 3730xl) by Macrogen Inc., Korea. The sequences were aligned with MAFFT v7.308 algorithm (Katoh et al., Reference Katoh, Misawa, Kuma and Miyata2002) in Geneious 10.1.2, leading to 38 sequences of 561 bp each.
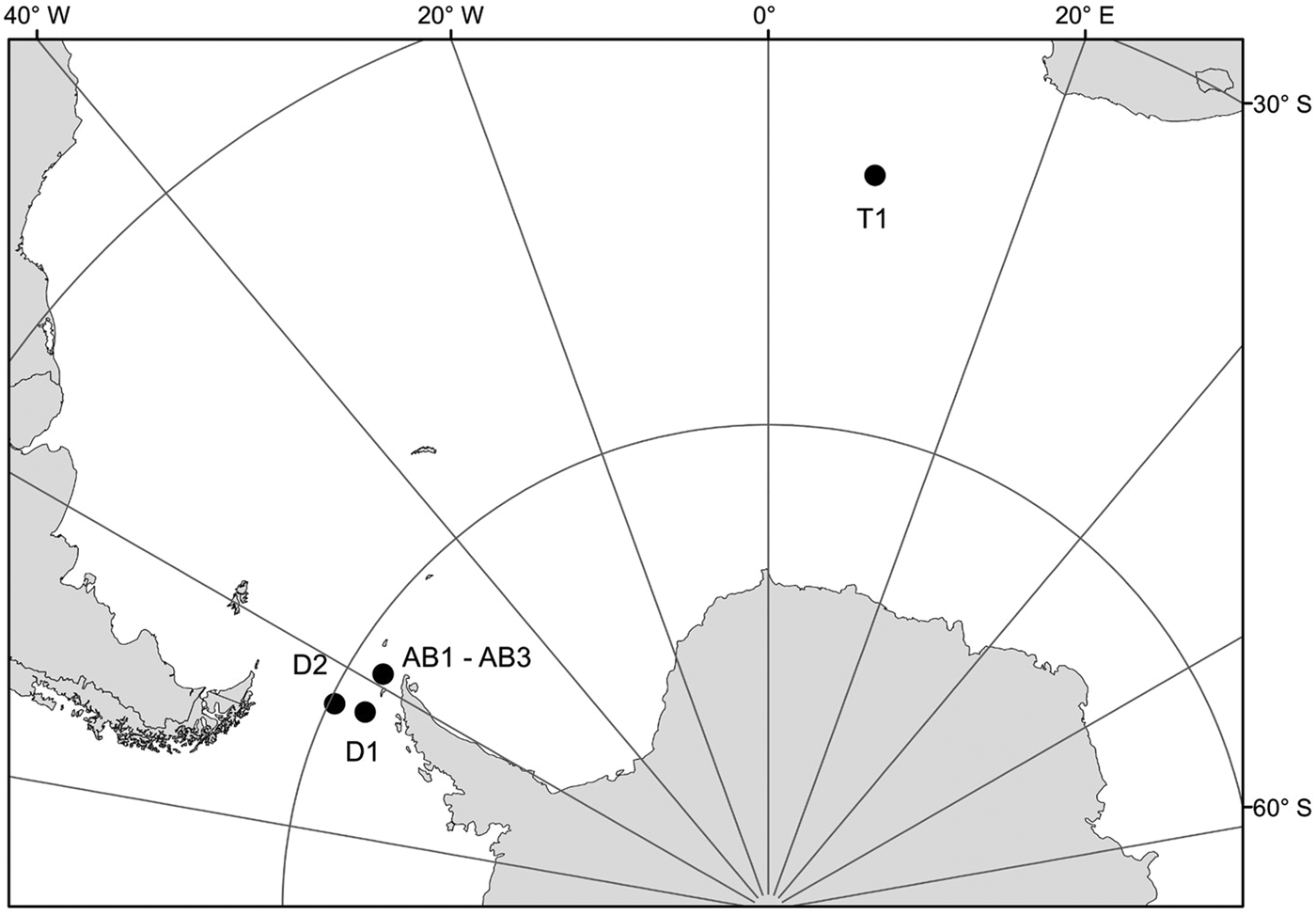
Fig. 1. Sampling points. T1, D1, D2, AB1–AB3 – station codes; see Table 1 for details.
Table 1. Characteristics of the samples used for the present work.
The uncorrected p-distance and the Kimura 2-parameter (K2P) model (Kimura, Reference Kimura1980) were used to determine sequence divergence in MEGA V7.0.18 (Kumar et al., Reference Kumar, Stecher and Tamura2016). A Neighbour-joining (NJ) tree was built based on the p-distance with both transition and transversion substitutions included and pairwise deletion chosen. Node support was inferred with a bootstrap analysis (1000 replicates). The sequences of Bargmannia amoena and B. elongata, the only representatives of the family Pyrostephidae with available 16S data, were also used in the analysis (GenBank accession numbers AY935292 and AY935321, respectively). The sequence of Apolemia rubriversa, another representative of Physonectae, was used to root the tree (GenBank accession number KF214713). All sequences were deposited in GenBank with the accession numbers KY370929–KY370966 (Table S1). Relevant voucher information, taxonomic classifications, and sequences are accessible through the public data set ‘DS-PVSO’ on the Barcode of Life Data Systems (BOLD; http://www.boldsystems.org) (Ratnasingham & Hebert, Reference Ratnasingham and Hebert2007).
RESULTS
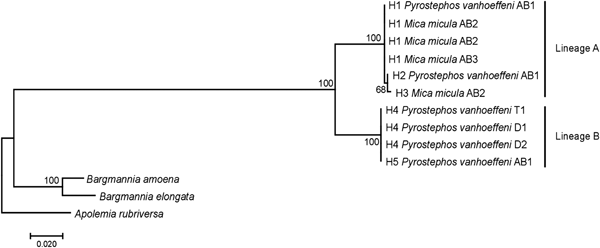
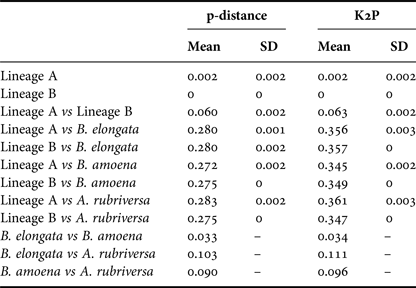
Among the 38 sequences obtained, five haplotypes were distinguished. One of them, represented by a single sequence, differed from the others by only one insertion and in the NJ tree was not treated as a separate entity. Due to the fact that some nectophores from the same samples shared haplotypes, it was assumed that they belonged to the same colony fragmented during collection. In further analyses they were not treated as separate units. This resulted in final examination of six colonies of P. vanhoeffeni and four colonies of M. micula. The values of overall uncorrected p-distance and the K2P distance between haplotypes were very similar (0.041 and 0.043, respectively). The haplotype divergence ranged from 0 to 0.063 in case of p-distance and from 0 to 0.066 for K2P distance (Table 2). The NJ tree showed that all sequences from the present study constituted a single branch further divided into two distinct clades with high support (bootstrap 100%) (lineages A, B) (Figure 2). The lineage A consisted of three haplotypes of both Mica micula and Pyrostephos vanhoeffeni, present exclusively in Admiralty Bay. The individuals belonging to the second Molecular Operational Taxonomic Unit (MOTU) identified (lineage B), represent two haplotypes of P. vanhoeffeni and were found in localities in the open sea in the Southern Ocean and in Admiralty Bay. The haplotype from Admiralty Bay differs from that in the open ocean by a single insertion. The average distance of the sequences forming lineage A was 0.002 (both p-distance and K2P), while that between the lineages A and B was 0.060 of p-distance and 0.063 of K2P (Table 3). The distance between both Bargmannia species and the two discovered clades ranged from 0.272 to 0.280 for p-distance. In the case of K2P the values were from 0.345 to 0.357. Similar values of sequence divergence were observed between the two discovered lineages and Apolemia rubriversa. Nucleotide differences between sequences from clades A and B were 6.5–6.8%.
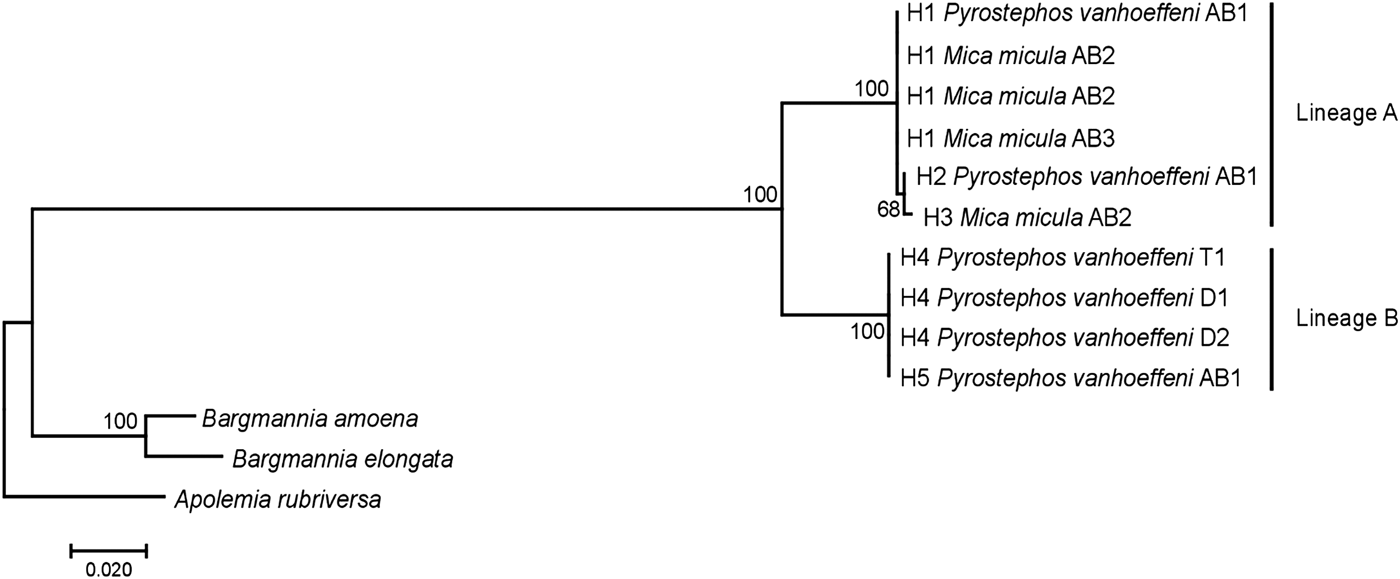
Fig. 2. Neighbour-joining (NJ) tree of 16S rRNA sequences representing each studied colony based on uncorrected p-distance; the numbers in front of the nodes indicate bootstrap support (1000 replicates, only the values higher than 50% are presented); T1, D1, D2, AB1–AB3 – station codes – see Table 1 for details. Sequences of Bargmannia amoena, B. elongata and Apolemia rubriversa retrieved from GenBank.
Table 2. Comparison of the genetic distance between haplotypes found calculated using uncorrected p-distance (above diagonal – grey tint) and Kimura 2-parameter (K2P) (below diagonal).

The distance between haplotype 4 and 5 is zero due to one insertion in the latter that is not recognized as a mutation in both measures.
Table 3. Genetic distance calculated using p-distance and Kimura 2-parameter (K2P) within and between distinguished lineages and outgroups.
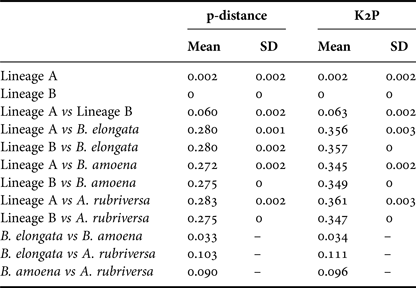
Mean - mean value, S.D. - standard deviation.
DISCUSSION
In our study molecular techniques were used to investigate two species of Southern Ocean siphonophores, namely the enigmatic taxon Mica micula (Figure 3a), and a taxon which is quite common and abundant in these waters – Pyrostephos vanhoeffeni (Figure 3b).

Fig. 3. Mica micula – young colony: a1 – from Pagès & Gili (Reference Pagès and Gili1989), a2 – A. Panasiuk, Pyrostephos vanhoeffeni – nectophore: b1–from Alvarino et al. (Reference Alvarino, Wojtan and Martinez1990), b2 – A. Panasiuk.
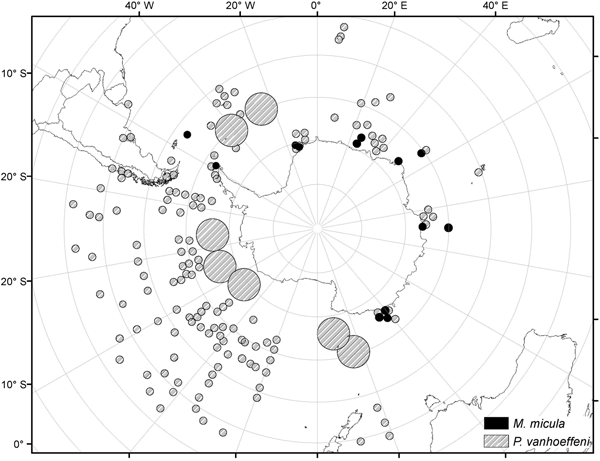
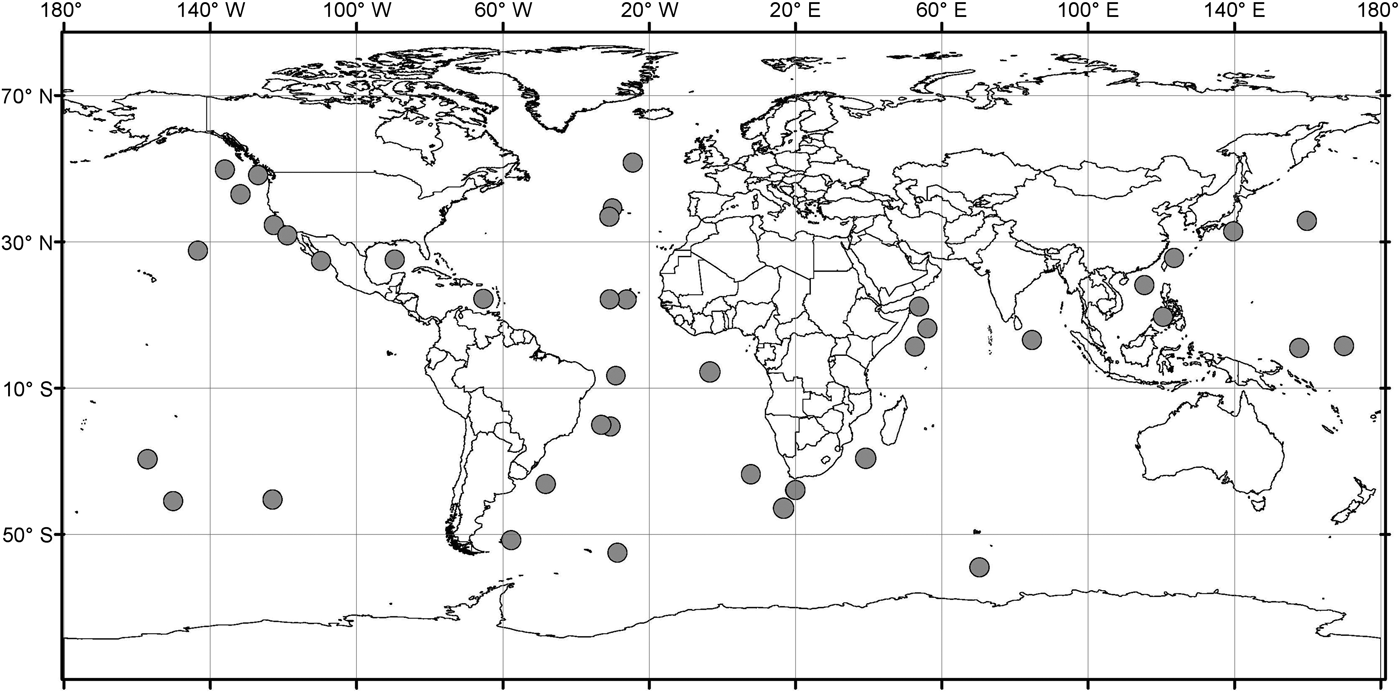
Analysis of the biogeographic distribution of Pyrostephos vanhoeffeni, Mica micula and Bargmannia elongata showed that the distribution of the two first is limited to the southern hemisphere (Figure 4). In contrast, the distribution of B. elongata is much broader, with individuals occurring in Canadian Pacific waters (Mapstone, Reference Mapstone2009), off California and San Diego and in the NE Atlantic (Pugh, Reference Pugh1999a; Dunn et al., Reference Dunn, Pugh and Haddock2005), in the Gulf of Mexico (Pugh & Gasca, Reference Pugh, Gasca, Felder and Camp2009) as well as in Japanese waters (Lindsay & Hunt, Reference Lindsay and Hunt2005; Lindsay, Reference Lindsay2006) and in the Indo-Pacific (Lindsay et al., Reference Lindsay, Umetsu, Grossmann, Miyake, Yamamoto, Ishibashi, Okino and Sunamura2015a) (Figure 5). Both P. vanhoeffeni and B. elongata have been observed in the east and west Antarctic regions (Margulis, Reference Margulis1982; Pugh et al., Reference Pugh, Pagès and Boorman1997; Toda et al., Reference Toda, Moteki, Ono, Horimoto, Tanaka and Ishimaru2010; Grossmann et al., Reference Grossmann, Lindsay and Fuentes2013b), but overall there are many more records for P. vanhoeffeni in this area than for B. elongata. Mica micula has been recorded in the East Antarctic region (Grossmann et al., Reference Grossmann, Lindsay and Fuentes2013b), Admiralty Bay (King George Island, South Shetlands Archipelago) and in the Atlantic sector of the Southern Ocean (Pagès & Gili, Reference Pagès and Gili1989) (Figures 4 and 5). The last authors collected one nectophore of B. elongata and two colonies of M. micula, but no associated specimens or nectophores of P. vanhoeffeni. Summarizing, the records for B. elongata in the Southern Ocean show very little correlation with the areas of distribution of M. micula, whereas the distribution of Pyrostephos vanhoeffeni overlaps well with that of M. micula.

Fig. 4. Distribution/records of Pyrostephos vanhoeffeni and Mica micula based on available data (from Hardy & Gunther, Reference Hardy and Gunther1935; Alvarino, Reference Alvarino1971; Pagès & Gili, Reference Pagès and Gili1989; Alvarino et al., Reference Alvarino, Wojtan and Martinez1990; Margulis, Reference Margulis1992; Pagès & Kurbjeweit, Reference Pagès and Kurbjeweit1994; Pagès et al., Reference Pagès, Pugh and Gili1994; Pakhomov et al., Reference Pakhomov, Grachev and Trotsenko1994; Pagès & Schnack-Schiel, Reference Pagès and Schnack-Schiel1996; Palma & Rosales, Reference Palma and Rosales1997; Pugh et al., Reference Pugh, Pagès and Boorman1997; Pagès & Orejas, Reference Pagès and Orejas1999; Palma & Aravena, Reference Palma and Aravena2001; Fuentes et al., Reference Fuentes, Schnack-Shiel, Schloss and Esnal2008; Panasiuk-Chodnicka & Żmijewska, Reference Panasiuk-Chodnicka and Żmijewska2010; Toda et al., Reference Toda, Moteki, Ono, Horimoto, Tanaka and Ishimaru2010, Reference Toda, Lindsay, Fuentes and Moteki2014; Grossmann et al., Reference Grossmann, Lindsay and Fuentes2013b; Guerrero et al., Reference Guerrero, Gili, Rodriguez, Araujo, Canepa, Calbet, Genzano, Mianzan and Gonzalez2013; Lindsay et al., Reference Lindsay, Guerrero, Grossmann, Fuentes, De Broyer, Koubi, Griffiths, Raymond, d'Udekem d'Acoz, Van de Putte, Danis, David, Grant, Gutt, Held, Hosie, Huettmann, Post and Ropert-Coudert2014; Panasiuk-Chodnicka et al., Reference Panasiuk-Chodnicka, Żmijewska and Mańko2014); size of the circle indicates the frequency of records.
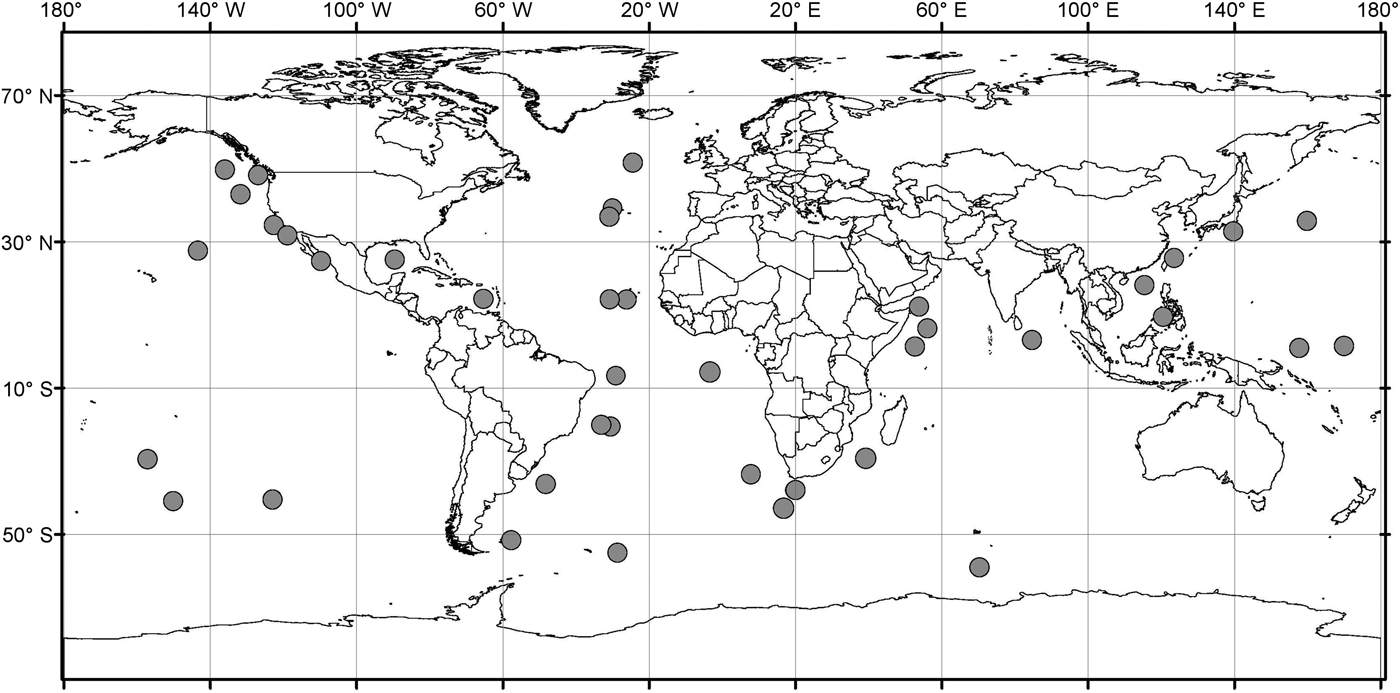
Fig. 5. Distribution/records of Bargmannia elongata based on available data (from Alvarino, Reference Alvarino1963, Reference Alvarino1971; Margulis, Reference Margulis, Naumov and Stepanjants1980, Reference Margulis1992; Alvarino et al., Reference Alvarino, Wojtan and Martinez1990; Pugh, Reference Pugh1999a; Dunn, Reference Dunn2005; Lindsay, Reference Lindsay2006; Hosia et al., Reference Hosia, Stemmann and Youngbluth2008; Mapstone, Reference Mapstone2009; Pugh & Gasca, Reference Pugh, Gasca, Felder and Camp2009; Grossmann et al., Reference Grossmann, Nishikawa and Lindsay2015); with data from GBIF (Global Biodiversity Information Facility) and OBIS (Ocean Biogeographic Information system).
A study of 16S rRNA sequences clearly show that Mica micula is the post-larval stage of Pyrostephos vanhoeffeni (Figure 2). Similar studies resulted in the recognition of another enigmatic siphonophore taxon – Eudoxia macra – as the eudoxid stage of the small diphyid Lensia cossack (Grossmann et al., Reference Grossmann, Lindsay and Collins2013a). Some authors have suggested that M. micula might be a post-larva of Bargmannia elongata, the only other representative of family Pyrostephidae identified in Antarctic waters (Pagès & Gili, Reference Pagès and Gili1989; Pugh, Reference Pugh and Boltovskoy1999b), but this has been questioned due to the non-coincidence of distribution records of these two pyrostephid taxa (Grossmann et al., Reference Grossmann, Lindsay and Fuentes2013b). Our study also shows that the genetic distance between sequences of M. micula and B. elongata is greater than the inter-family distances within some other hydrozoans (Zheng et al., Reference Zheng, He, Lin, Cao and Zhang2014).
Our results also revealed two clearly separated genetic lineages of Pyrostephos vanhoeffeni in the Antarctic (Figure 2). Lineage (A) represented by three haplotypes was restricted solely to Admiralty Bay, whereas specimens assigned to the other lineage (B) came from several regions including Drake Passage, Admiralty Bay, and also the South African region of the Atlantic Ocean. Apart from the sequence from Admiralty Bay (differing by one insertion), this widespread lineage (B) is represented by a single haplotype, which suggests constant gene flow. What is more, the colony from the South African region was collected north of the Antarctic Convergence. That indicates that the differences in water temperature observed north and south of it do not prevent mixing of the populations. This is in contrast to the findings by Grossmann et al. (Reference Grossmann, Lindsay and Collins2013a) who recorded the existence of two genetically distinct populations of another siphonophore, Lensia achilles associated with different water masses. It is also worth noting that genetic diversity observed in Admiralty Bay is noticeable as four out of five haplotypes recorded in this study were present only in this small embayment. The diversity expressed by K2P within both lineages was very low, whereas between them it amounted to 0.063 (Table 3). This value falls well within the intra-species distances observed in different Lensia species (Lindsay et al., Reference Lindsay, Grossmann, Nishikawa, Bentlage and Collins2015b). Grossmann et al. (Reference Grossmann, Lindsay and Collins2013a, Reference Grossmann, Nishikawa and Lindsay2015) have observed also the cryptic diversity within this genus. In this case, the genetic distances between populations of several morphospecies were distinctly higher (up to 0.25), compared with usually recorded intraspecific values of 0.01 to 0.16 (Lindsay et al., Reference Lindsay, Grossmann, Nishikawa, Bentlage and Collins2015b). However, one must take into account that in the case for Lensia, the nominal species with large intra-species genetic distances were sampled in different geographic locations that are not expected to exhibit gene flow in modern times, namely Japan and Antarctica, so the geographic distance between sampling localities is much greater than in the present study (Grossmann et al., Reference Grossmann, Lindsay and Collins2013a). Higher genetic diversity (0.12) was also recorded for L. achilles specimens from two different water masses – one sub-arctic and one sub-tropical (Grossmann et al., Reference Grossmann, Lindsay and Collins2013a). On the other hand, it is worth noting that the genetic distance between sequences of two species of Bargmannia used in the present study is considerably lower than the one between the two lineages of P. vanhoeffeni (Table 3). Also Zheng et al. (Reference Zheng, He, Lin, Cao and Zhang2014) who studied pelagic Hydrozoa from the order Leptothecata found that the intra-specific variation of the 16S rRNA gene was considerably lower in these cnidarians than in the siphonophores studied here (with a maximum value of K2P reaching 0.016). At the same time the interspecies distances of this parameter observed by these authors varied from 0.062 to 0.642.
The phylogeography of the recognized lineages remains an open issue. Lineage B has a wide geographic range and may represent the population of circum-Antarctic distribution, extending also north of the Antarctic Convergence. On the other hand lineage A may indicate a population of limited distribution. The study of further material from additional Antarctic localities, including both detailed morphological investigations and additional molecular analyses, could address this question.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315418000218
ACKNOWLEDGEMENTS
We wish to thank the Shirshov Institute of Oceanology of the Russian Academy of Sciences and the Polish Academy of Sciences for the opportunity to collect zooplankton samples, and the crew of RV ‘Akademik Ioffe’. The data used in the paper were also collected while based on the Henryk Arctowski Polish Antarctic Station. We would like to thank Dr Luiza Bielecka and Prof. Maciej Wołowicz for their assistance in collection of samples. Especially, we would like to thank Dr Gillian Mapstone and two anonymous reviewers whose comments and suggestions significantly improved the manuscript.
FINANCIAL SUPPORT
This work was partially supported by research grant No. N306 445 638 (2010–2012) awarded to Institute of Oceanography (University of Gdańsk) by Ministry of Science and Higher Education (Poland), and from the internal funds of the University of Lodz.